Abstract
An extremely high alkaline phosphatase (AP) concentration (3609 IU/litre) was found in a 20 year old primigravida at 37 week’s gestation, prompting an examination of its histological and cellular origin. Immunohistochemistry and western blots using antibodies against AP, Ki-67, phospho-protein kinase B (Akt), phospho-p44/42 mitogen activated protein kinase/extracellular signal regulated kinase 1/2 (MAPK/Erk1/2), phospho-glycogen synthase kinase-3β (GSK-3β), phospho-stress activated protein kinase/c-Jun N-terminal kinase, total-Akt, total-GSK-3β, and phospho-p38-MAPK were carried out on index and control placental samples of the same gestational age. Compared with controls, staining of the index placenta showed minimal AP labelling of the brush border and remarkable positivity of the intervillous space. Cytotrophoblastic proliferation was 8–10% in the index placenta compared with 1–2% in controls. The index placenta also had raised concentrations of protein kinases with important roles in cell differentiation. The proliferation and differentiation rates of the cytotrophoblasts were found to be five times higher in index samples than in controls. It is hypothesised that loss of syncytial membranes in immature villi led to increased AP concentrations in the maternal circulation and decreased AP staining of the placenta. Loss of the syncytium might also stimulate increased proliferation of villous cytotrophoblasts, which would then fuse and maintain the syncytium.
Alkaline phosphatase (AP) is known to be produced by the liver, bones, small intestine, and kidneys, and different AP isoforms are also expressed by the placenta during pregnancy.1 The average amount of AP in one human term placenta amounts to 40 mg.2 The placental isoforms are known as heat stable AP, because they are heat resistant at 60°C, a property that is the main criterion for distinguishing them from the other isoenzymes.3 In early pregnancy, the tissue non-specific AP isoenzyme is mainly expressed in the placenta, and reaches a peak value around 10 weeks of pregnancy. At the end of the second trimester, most of the AP activity comprises term placental AP isoenzymes1 (90% of which are the P1 type, 10% the P2 type) produced by the syncytiotrophoblasts, and these isoenzymes appear in maternal serum between the 15th and 26th weeks of pregnancy.4 Their plasma concentrations increase exponentially during gestation—they are present at concentrations three times greater than those seen in non-pregnant women—and have a long half life (seven days) postpartum.5 Extremely high AP concentrations should arouse a suspicion of bone, hepatic, endocrine, and renal diseases, malignancy, and drug treatment, but can also be associated with heavy smoking and pregnancy (table 1).3,4,6 The aetiology of increased AP is still unknown, and because we could find no literature dealing with its cellular background we decided to investigate the biochemical and pathophysiological aspects of this phenomenon.
Table 1.
Differential diagnosis of raised serum alkaline phosphatase concentrations with regard to the origin of isoenzymes
| Tissue non-specific isoenzyme | Placental isoenzyme | Intestinal isoenzyme | |
| Normal tissue origin | Early placenta (<10 weeks) | Term placenta | Intestine |
| Liver | Uterus | ||
| Bone | Testis | ||
| Kidney | |||
| Bone diseases | Fracture | ||
| Paget’s disease | |||
| Rachitis | |||
| Osteomalacia | |||
| D-Hypervitaminosis | |||
| Osteogenesis inperfecta | |||
| Osteoporosis | |||
| Hepatic diseases | Hepatitis | Cholestasis | Cirrhosis |
| Obstructive icterus | Gilbert syndrome | ||
| Primary biliary cirrhosis | |||
| Cholestasis | |||
| Renal disease | Renal failure | Renal failure | |
| Chronic renal disease | |||
| Endocrine diseases | Hyperthyroidism | Diabetes mellitus | |
| Hyperparathyroidism | |||
| Acromegaly | |||
| Cushing’s syndrome | |||
| Malignant tumours | Hepatocellular carcinoma | Ovarian carcinoma | |
| Bone sarcoma | Pancreas carcinoma | ||
| Lymphoma | Gastric carcinoma | ||
| Bone and liver metastasis | |||
| Pregnancy related diseases | IUGR Down’s syndrome | ||
| Pre-eclampsia | |||
| Drug treatment | Antiepileptic treatment | Steroid treatment | |
| Others | Heavy smoking |
IUGR, intrauterine growth restriction.
“Extremely high alkaline phosphatase concentrations should arouse a suspicion of bone, hepatic, endocrine, and renal diseases, malignancy, and drug treatment, but can also be associated with heavy smoking and pregnancy”
METHODS
Clinical data
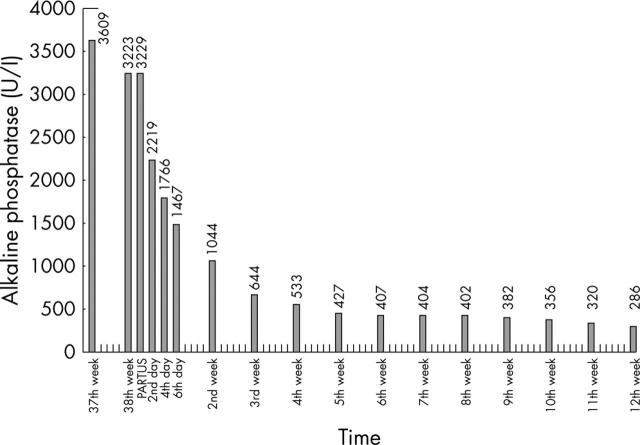
A 20 year old primigravida was admitted to the clinic because of oedema and suspicion of pre-eclampsia during the 37th gestational week. After a general examination and precise observation, an extremely high serum AP concentration was detected (3609 U/litre). Blood pressure was normal, there was no proteinuria, and the patient had no complaints. Laboratory tests showed that her blood parameters and renal, hepatic, and endocrine functions were normal. No systemic immune disease was detected. Ovarian tumour markers (CA-125, carcinoembryonic antigen, and CA-19-9) were non-pathological. The bone associated AP fraction was determined to be only 97 U/litre (normal range, 100–350). Electrophoresis of total AP using the Hydragel Protein kit (Sebia, Issy-les-Moulineaux, France) showed the presence of 65.9% (2128 U/litre) P1 and 30.1% (972 U/litre) P2 placental isozymes. On the 38th week of pregnancy, a mature girl was born by vaginal labour with a weight of 3200 g and an Apgar score of 9/10. The 730 g placenta had no macroscopic abnormalities or infarction. The human chorionic gonadotrophin concentration declined quickly after birth, whereas the concentration of AP decreased exponentially during the following weeks and reached normal values at 12 weeks postpartum (fig 1).
Figure 1.
Changes in total serum alkaline phosphatase (AP) concentrations during pregnancy and postpartum. The AP concentration reached its peak at the 37th gestational week. After birth, it decreased exponentially during the following weeks, and became normal 12 weeks later.
Histopathology and immunohistochemistry
Samples were obtained from the index case and from normal term placentas (n = 5). Tissue blocks were routinely fixed in formalin and embedded in paraffin wax. Full thickness representative blocks were taken from three different central-paracentral areas of each placenta and an en face block of the maternal surface was cut. Further blocks from the umbilical cord and membranes were sampled for routine examination only. Consecutive serial sections (4 μm thick) of the same blocks were also cut and prepared for immunostaining. Tissue samples were stained with haematoxylin and eosin for histopathological examination, or with monoclonal IgG antibodies against cell proliferation marker Ki-67 (Histopathology, Pecs, Hungary) and placental AP (Lab Vision, Freemont, California, USA) for immunohistochemical evaluation. Immunostaining was performed by the streptavidin–biotin–immunoperoxidase technique, with H2O2/3-amino-9-ethylcarbazole development using the Universal kit (Immunotech, Marseille, France). Index and control placentas were examined blindly according to a standard protocol by two of the authors (BH and AB) independently.
SDS-PAGE and chemiluminescent western blot analysis
Explants (100 mg each) were taken from five different areas of normal and index placental tissues, and were homogenised on ice in 10 ml of lysate buffer (pH 7.5, 50mM Tris, 1mM PMSF). Homogenates were centrifuged at 4000 ×g for five minutes, supernatants were collected, and their protein contents were measured using the BioRad assay and equalised in Laemmli sample buffer. Protein extracts (10 μg each) were loaded and separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Western blot analyses were performed by applying rabbit polyclonal IgGs to the following antigens: phospho-protein kinase B (phospho-Akt), phospho-p44/42 mitogen activated protein kinase/extracellular signal regulated kinase 1/2 (phospho-p44/42 MAPK/Erk1/2), phospho-glycogen synthase kinase-3β (phospho-GSK-3β), phospho-stress activated protein kinase/c-Jun N-terminal kinase (phospho-SAPK/JNK), total-Akt, and total-GSK-3, in addition to mouse monoclonal IgG to phospho-p38-MAPK (Cell Signaling Technology Inc, Beverly, Massachusetts, USA). Horseradish peroxidase labelled goat antirabbit and antimouse IgGs (Sigma-Aldrich Co, St Louis, Missouri, USA) were used as secondary antibodies. Protein bands were revealed by the ECL chemiluminescence system (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). This was followed by quantitative densitometric analysis of the bands with Scion Image for Windows and ImageJ software.
RESULTS
Histopathology
In the index case, there were increased numbers of syncytial knots on the surface of the chorionic villi (fig 2A). Several groups of avascular tertiary villi with homogenous hyaline-like stroma were also found but fetal vessel thrombosis was not revealed. “Proliferation centres”7 were frequent in the immature villi, unlike in other term placentas (fig 2B). Several areas showed villous crowding, where the intervillous space was nearly obliterated by the large, poorly vascularised villi (fig 2C). Histopathology also showed asymmetrical intimal fibrin cushions in the fetal chorionic vessels. The maternal vessels showed no specific changes. Control placentas showed none of the above listed lesions.
Figure 2.
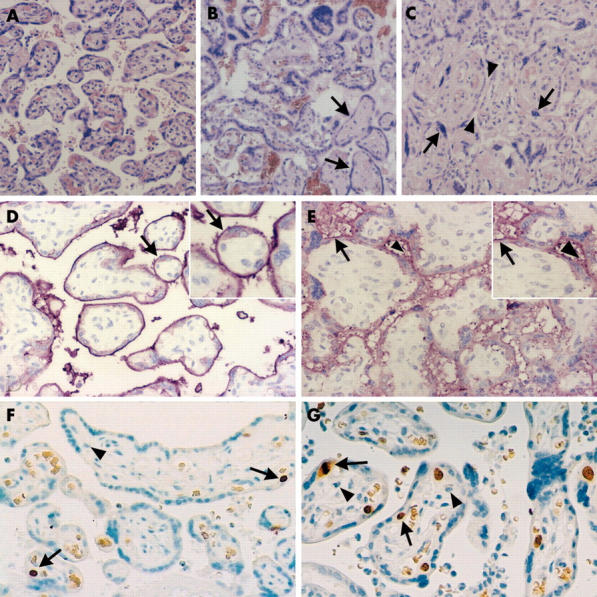
Histopathological and immunohistochemical examination of the index and control placentas. (A–C) Haematoxylin and eosin staining of placental tissue sections (original magnification, ×100). (A) Normal term placenta, 38 weeks of gestation; (B) index placenta containing immature villi organised into proliferation centres and fibrotic, avascular villi (arrows); (C) villous crowding in the index placenta with pinched intervillous space (arrowheads) and syncytial knotting (arrows). (D, E) Immunohistochemical staining for alkaline phosphatase (AP) in placental tissue sections with inserts of higher magnification (original magnification, ×200). (D) In normal placentas, intensive linear positivity of the brush border membrane was detected (arrow), whereas in the index placenta, weak, diffuse, interrupted granular positivity of the membrane was seen (the arrow shows a normal part, whereas the arrowhead points to AP staining of the intervillous space). (F, G) Immunohistochemical staining for Ki-67 in placental tissue sections (original magnification, ×100). The nuclei of proliferating cytotrophoblasts were stained (one of the arrows shows a nucleus in the mitotic phase). (G) The ratio of proliferating cells in the index placenta was 8–10%, whereas it was approximately 1–2% in control placentas of the same gestational age (F). No Ki-67 staining was seen in either the syncytiotrophoblast layers or the villous stromal cells (arrowhead).
Immunohistochemistry
Similar to the data in the literature,3 in control placentas AP was normally found on the brush border membranes of the syncytiotrophoblast layer of the villus surface (fig 2D). No staining could be seen in other villous cells, such as cytotrophoblasts. Staining of the index placenta resulted in minimal AP labelling of the brush border, and yielded a remarkable diffuse AP positivity in the intervillous space (fig 2E). We used anti-Ki-67 IgG and counted 10 fields in every case to examine the average ratio of proliferating cells, which was found to be approximately 1–2% in control placentas (fig 2F), whereas the index placenta showed significantly increased positivity of the cytotrophoblastic cells underlying the syncytium so that the average ratio of proliferating cells was 8–10% (fig 2G).
SDS-PAGE and chemiluminescent western blot analysis
Five different proteins involved in cellular signal transduction pathways were investigated in the term placental tissue extracts. As shown in fig 3, compared with normal placentas, some markers showed remarkable overexpression in the index sample. Phospho-GSK-3β (fig 3A) showed a similar increase to that of phospho-Akt (fig 3C), whereas there were no differences in total-GSK-3 (fig 3B) and total-Akt (fig 3D) expression. Three other basic signal transduction proteins—phospho-p38-MAPK (fig 3E), phospho-SAPK/JNK (fig 3F), and phosphorylated p44/42 MAPK/Erk1/2—were found to be overexpressed in the index placenta compared with controls (fig 3G). Densitometric analyses were performed, and the following differences in protein content were found in the index case compared with controls (100%): phospho-GSK 3β, 152%; total-GSK-3, 106%; phospho-Akt, 174%; total-Akt, 103%; p38MAPK, 249%; phospho-p44/42 MAPK/Erk1/2, 561%; and phospho-SAPK/JNK, 202% (table 2).
Figure 3.
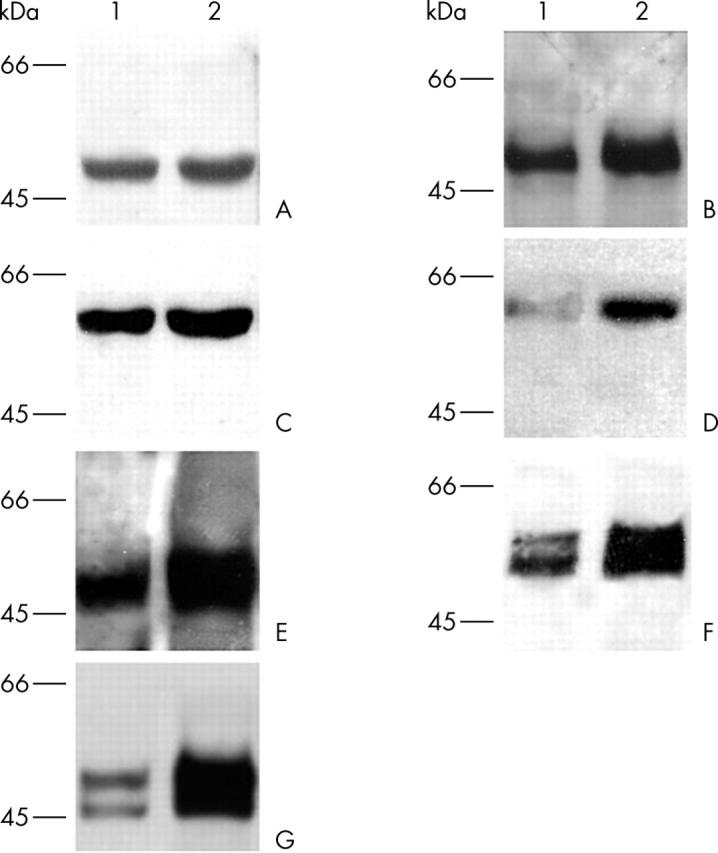
Chemiluminescent western blot analyses of control (1) and index (2) placentas. (A, B) Expression of total glycogen synthase kinase-3β (GSK-3β) was the same in both samples (A1, A2), whereas significantly increased expression of phospho-GSK-3β was found in the index placenta (B2) compared with the control placenta (B1). (C, D) The expression of total protein kinase B (Akt) was similar in both control and index placentas (C1, C2), whereas the expression of phospho-Akt was considerably higher in the index sample (D2) than in the normal placentas (D1). (E–G) Expression of (E) phospho-p38-mitogen activated protein kinase (MAPK), (F) stress activated protein kinase/c-Jun N-terminal kinase, and (G) p44/42 MAPK/extracellular regulated kinase 1/2 was greatly increased in the index placental tissue samples (E2, F2, G2) compared with controls (E1, F1, G1).
Table 2.
Comparison of immunohistochemistry and western blot results in the index and control placentas
| Method | Index placenta | Control placentas |
| Immunohistochemistry | Slight and discontinuous AP staining in syncytiotrophoblast brush border membrane | Intensive continuous AP staining in syncytiotrophoblast brush-border membrane |
| 8–10% Ki-67 positive syncytiotrophoblast cells/field on average | 1–2% Ki-67 positive syncytiotrophoblast cells/field on average | |
| Western blot | Total-Akt 103% | Total-Akt 100% |
| Total-GSK-3β 106% | Total-GSK-3β 100% | |
| Phospho-Akt 174% | Phospho-Akt 100% | |
| Phospho-GSK-3β 152% | Phospho-GSK-3β 100% | |
| Phospho-Erk 1/2 561% | Phospho-Erk 1/2 100% | |
| Phospho-SAPK/JNK 202% | Phospho-SAPK/JNK 100% | |
| p38MAPK 249% | p38MAPK 100% |
Akt, protein kinase B; AP, alkaline phosphatase; GSK-3β, glycogen synthase kinase-3β; p38-MAPK, p38 mitogen activated protein kinase; Erk1/2, extracellular signal regulated kinase1/2; SAPK/JNK, stress activated protein kinase/c-Jun N-terminal kinase.
DISCUSSION
Only a few publications could be found that dealt with isolated extreme rises in AP during pregnancy,8,9 although numerous diseases are known to be related to high AP concentrations (table 1).3,4,6 It has been reported10 that raised AP concentrations can predict premature birth in the second trimester, but this parameter has no clinical relevance because of its low sensitivity and specificity. Placental infarcts or abruption could also be the reason for increased AP concentrations in the maternal serum. In non-pregnant women with high AP, it is necessary to exclude malignancy. Fishman et al were the first to report on malignancy derived placental-like alkaline phosphatase (PLAP) production.4 Increased concentrations of PLAP have been found in 15–64% of ovarian cancers, 54% of colorectal carcinomas, and 40% of lung cancers.6,11 PLAP activity was found to constitute less than 1% of total AP activity in non-pregnant, non-smoking women, and its connection to numerous malignant diseases has been shown.
With regard to our case, we could exclude liver, kidney, bone, and immunological diseases, in addition to thyroid dysfunction. Intrauterine growth retardation, pre-eclampsia, and Down’s syndrome were also excluded. The mother did not smoke and was not undergoing drug treatment. Because the concentration of β human chorionic gonadotrophin was within the normal range at the time of hospitalisation (17 706 IU/ml; reference range, 94–60 000 IU/ml at the 29–40th week of pregnancy) and did not change appreciably, a syncytiotrophoblastic disorder was not suspected originally. To gain a better insight into the morphological and functional changes leading to this phenomenon, several types of pathological and biochemical examinations were carried out. Numerous cellular signal transduction pathways via well established protein kinases that might lead to proliferation were also investigated.
In our study, histopathological examination found several avascular villi in one block, intimal fibrin cushions in fetal vessels, and diffuse syncytial knot formation on villous surfaces. In several areas, proliferation of the cytotrophoblasts was detected and persistence of embryonal villi was present.
“When a raised serum alkaline phosphatase concentration is present during pregnancy, differential diagnostically important diseases must be systematically excluded”
Immunohistochemical and western blot results also pointed to an increased rate of cytotrophoblast proliferation. Numbers of Ki-67 positive proliferating cytotrophoblastic cells were found to be five times greater in the index sample than in the controls. A remarkable increase in phosphorylated p44/42 MAPK/Erk1/2, known to be a signal transduction factor involved in proliferation and differentiation, was detected, indicating an increased tendency for cell differentiation and syncytiotrophoblast formation. Increases in other protein kinases, p38MAPK and SAPK/JNK, also known to take part in the signal transduction necessary for cell activation were detected. Increased amounts of phospho-Akt (active form) and phospho-GSK-3β (inactive form), together with a slight difference between the total-Akt and total-GSK-3β concentrations, suggested a possible inhibition of the apoptotic pathway. As published previously, Akt is known to regulate cellular processes in response to phosphatidylinositol-3 kinase activation. Phospho-Akt transduces signals to activate cell growth, survival, proliferation, and differentiation, at the same time inhibiting proapoptotic signals via phosphorylation and thus inactivation of GSK-3β.12 Summarising our observations, we suggest that changes in the expression of phosphorylated p44/42 MAPK/Erk1/2 taken together with the immunohistochemical findings of increased Ki-67 expression indicate an intensive cytotrophoblastic proliferation. The inhibition of apoptosis is mediated by at least two pathways. Phospho-Akt inactivates GSK-3β, a proapoptotic factor, whereas p38MAPK, an important factor for cell survival, might also indicate the activation of an alternative antiapoptotic process. According to the data in the literature, the cell activating effect of p38MAPK is mediated via several transcription factors (Stat-1, Atf-2, etc), similar to the JNK/JUN pathway.13
Take home messages.
We report a patient with an extremely high serum alkaline phosphatase (AP) concentration at 37 week’s gestation
Compared with controls, staining of the index placenta showed minimal AP labelling of the brush border and remarkable positivity of the intervillous spaces
Cytotrophoblastic proliferation was greater in the index placenta than in the controls, and the index placenta also had raised concentrations of protein kinases with important roles in cell differentiation
The proliferation and differentiation rates of the cytotrophoblasts were found to be five times higher in the index samples than in the controls
We suggest that loss of syncytial membranes in immature villi led to increased AP concentrations in the maternal circulation and decreased AP staining of the placenta
Loss of the syncytium might also have stimulated increased proliferation of the villous cytotrophoblasts, which would then fuse and maintain the syncytium
In control placentas, we detected strong AP labelling of the brush border membrane of the syncytiotrophoblasts, whereas minimal AP staining of the brush border was found in the immature villi of the index placenta, and a remarkable diffuse AP positivity was seen in the intervillous spaces. We hypothesise that loss of the syncytial membranes in immature villi led to increased AP concentrations in the maternal circulation and decreased AP staining of the placenta. Loss of the syncytium might also have stimulated increased proliferation of the villous cytotrophoblasts, which would then fuse and maintain the syncytium.
Although cytotrophoblast proliferation and the increased AP concentration did not affect fetal development in our present case, attention should be drawn to the pathophysiology of this phenomenon. Our investigations shed new light on a significant change in placental function, the exact cause of which is still to be elucidated. In conclusion, when a raised serum AP concentration is present during pregnancy, differential diagnostically important diseases must be systematically excluded. In such cases, we would also recommend precise monitoring of fetal and maternal conditions, histopathological examination of the placenta, and more attention to the follow up of declining AP concentrations after delivery.
Acknowledgments
The authors would like to thank Dr G Szekeres for technical assistance in immunostaining and S Starkey for critical reading of the manuscript. This work was supported by Hungarian Grants ETT T-09 163/01, 149/2003; FKFP 0166/2001; OMFB-BIO 00201/2002; and OTKA T/020622, T/023076, T/029824, T/046473, M/36996.
Abbreviations
Akt, protein kinase B
AP, alkaline phosphatase
GSK-3β, glycogen synthase kinase-3β
p38-MAPK, p38 mitogen activated protein kinase
p44/42 MAPK/Erk1/2, p44/42 mitogen activated protein kinase/extracellular signal regulated kinase1/2
PLAP, placental-like alkaline phosphatase
SAPK/JNK, stress activated protein kinase/c-Jun N-terminal kinase
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
REFERENCES
- 1.Nozawa S , Arai H, Jeng C, et al. Shift of placental alkaline phosphatase isoenzymes in the course of pregnancy. Nippon Sanka Fujinka Gakkai Zasshi 1984;36:1145–54. [PubMed] [Google Scholar]
- 2.Than GN, Bohn H, Szabó DG. Advances in pregnancy-related protein research. Boston, USA: CRC Press, 1993.
- 3.Nozawa S , Fishman WH. Heat-stable alkaline phosphatase: chemistry and biology. In: Grudzinskas JG, Teisner B, Seppala M, eds. Pregnancy proteins, biology, chemistry, clinical application. Australia: Academic Press, 1982:121–53.
- 4.Fishman WH, Ignis NI, Stolbach LI, et al. A serum alkaline phosphatase isoenzyme of human neoplastic cell origin. Cancer Res 1968;28:150–4. [PubMed] [Google Scholar]
- 5.Blom E , Ali MM, Mortensen B, et al. Elimination of alkaline phosphatases from circulation by the galactose receptor: different isoforms are cleared at various rates. J Clin Chim Acta 1998;270:125–37. [DOI] [PubMed] [Google Scholar]
- 6.Muensch HA, Maslow WC, Azama F, et al. Placental-like alkaline phosphatase. Cancer 1986;58:1689–94. [DOI] [PubMed] [Google Scholar]
- 7.Benirschlike K , Kaufmann P. Pathology of the human placenta. 3rd ed. New York: Springer Verlag, 1995.
- 8.Hatzmann W , Becker R, Maeso M, et al. Excessiver Anstieg der alkalischen Plasmaphosphatase während des letzten Trimenon der Schwangerschaft. Geburtsh Frauenheilk 1994;54:378–80. [DOI] [PubMed] [Google Scholar]
- 9.Vongthavaravat V , Nurnberger MM, Balodimos N, et al. Isolated elevation of serum alkaline phosphatase level in an uncomplicated pregnancy: a case report. Am J Obstet Gynecol 2000;183:505–6. [DOI] [PubMed] [Google Scholar]
- 10.Meyer R , Thompson S, Addy C, et al. Maternal serum placental alkaline phosphatase level and risk for preterm delivery. Am J Obstet Gynecol 1995;173:181–6. [DOI] [PubMed] [Google Scholar]
- 11.Nozawa S , Udagawa Y, Ohkura H, et al. Serum placental alkaline phosphatase (PLAP) in gynaecologic malignancies—with special reference to the combination of PLAP and CA54/61 assay. Clin Chim Acta 1990;186:275–84. [DOI] [PubMed] [Google Scholar]
- 12.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated protein kinase B. Nature 1995;378:785–9. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W , Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002;12:9–18. [DOI] [PubMed] [Google Scholar]