Abstract
Aims: To investigate mast cell distribution in normal adult skin to provide a reference range for comparison with mastocytosis.
Methods: Mast cells (MCs) were counted in uninvolved skin adjacent to basal cell carcinomas and other dermatological disorders in adults.
Results: There was an uneven distribution of MCs in different body sites using the anti-tryptase monoclonal antibody technique. Numbers of MCs on the trunk, upper arm, and upper leg were similar, but were significantly different from those found on the lower leg and forearm. Two distinct groups were formed—proximal and distal. There were 77.0 MCs/mm2 at proximal body sites and 108.2 MCs/mm2 at distal sites. Adjusted for the adjacent diagnosis and age, this difference was consistent. The numbers of MCs in uninvolved skin adjacent to basal cell carcinomas and other dermatological disorders were not different from those in the control group. Differences in the numbers of MCs between the distal and the proximal body sites must be considered when MCs are counted for a reliable diagnosis of mastocytosis. A pilot study in patients with mastocytosis underlined the variation in the numbers of MCs in mastocytosis and normal skin, but showed a considerable overlap. The observed numbers of MCs in adults cannot be extrapolated to children.
Conclusions: MC numbers varied significantly between proximal and distal body sites and these differences must be considered when MCs are counted for a reliable diagnosis of mastocytosis. There was a considerable overlap between the numbers of MCs in mastocytosis and normal skin.
Keywords: tryptase monoclonal antibody, body sites, immunohistochemistry, mast cell, mastocytosis
Mast cells (MCs) are multifunctional cells that play an important role in inflammatory and allergic reactions. They attract other key players of the immune system by releasing cytokines. Skin MCs are easily recognised by their metachromatic granules, which release their contents after activation by surface antigen or cytokine dependent events. The number of skin MCs can increase under certain conditions. In mastocytosis, the increase in the number of MCs is considered a primary event with an unknown pathogenesis.1 Cutaneous mastocytosis presents with mildly to severely pruritic macules and papules that host an increased number of MCs.2 Systemic mastocytosis affects several internal organs and presents with a wide range of symptoms, such as hypotension, seizures, skeletal pain, abdominal pain, and changes in defaecation.2,3 These symptoms may occur in various diseases and in the absence of dermatological signs, making the diagnosis of mastocytosis very difficult, so that it may even be missed by the clinician. Several criteria should be met for the diagnosis of mastocytosis. An increased serum tryptase concentration (> 13.5 ng/ml), dense infiltrates of mast cells in the cutaneous lesions or in the bone marrow, the expression of CD2 and CD25 on bone marrow mast cells, or the presence of a c-kit mutation may assist in the diagnosis.2,4 When systemic mastocytosis is suspected, a skin biopsy from lesional skin, or even from non-lesional skin, may be helpful in determining the number of MCs in the skin. In some cases, a slight increase in the number of MCs is seen, and in the absence of reliable reference values for the numbers of MCs in healthy skin, it remains unclear whether there is a pathological increase in the number of MCs or whether the values are within the normal range. Therefore, a reference value for the normal numbers of MCs in healthy skin may provide a valuable tool for improving the accuracy of diagnosis.
“Tryptase is considered to be an immunohistochemical marker for mast cells”
In previous studies, different numbers of MCs in normal skin were reported, but they could not serve as reference values because the methods that were used for counting MCs in skin biopsies were not uniform.5,6,7,8,9,10 Furthermore, researchers used different staining techniques and often the studied groups were very small.11 Staining with anti-tryptase monoclonal antibody (ATA) is now considered to be the gold standard for identifying MCs.12 The enzyme tryptase is also present in basophilic granulocytes, but its concentration is so low that they are stained very weakly with ATA. Therefore, tryptase is considered to be an immunohistochemical marker for MCs.12 The aim of our present study was to determine a reliable reference value for the numbers of MCs in healthy skin that could be used to establish the diagnosis of mastocytosis with certainty.
MATERIALS AND METHODS
Biopsies
Paraffin wax embedded blocks of skin biopsies, collected between 1996 and 2001 at the department of pathology of the Erasmus MC, Rotterdam, the Netherlands, were used. The biopsy blocks were divided into three groups. The first group consisted of perilesional skin from 25 basal cell carcinoma (BCC) biopsies. The mean age of the patients in this group was 66.8 years (range, 47–81). The second group consisted of perilesional skin from 95 biopsies taken from patients with various dermatological disorders (compound naevi, Spitz naevi, scars, dermatofibromas, and others). All biopsies were from the trunk, upper arm, forearm, upper leg, or lower leg. Exclusion criteria were inflammatory changes in the skin, massive degranulation of MCs, or other abnormalities. The mean age of the patients in this group was 49.7 years (range, 15–87). The third group consisted of skin biopsies of 21 healthy women (mean age, 37.7 years; range, 18–63) who underwent elective mamma reduction or abdominoplasty at the department of plastic and reconstructive surgery. None of these women had dermatological disorders and there was no report of systemic use of immunosuppressives or glucocorticoids. These biopsies were collected directly after the surgical intervention and were obtained and included after informed consent. This group served as the control group.
Staining procedure
Formalin fixed, paraffin wax embedded sections (4 μm) were used. Staining was performed using mouse ATA clone AA1 (Dako, Glostrup, Denmark) as the primary antibody, as described previously.12 Briefly, 141 skin slides were dewaxed in xylene three times for five minutes, washed in ethanol three times for five minutes, and then washed in phosphate buffered saline (PBS; pH 7.4) three times for five minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 minutes. To block non-specific antibody binding, the slides were preincubated with protein blocking reagent (Immunologic/Labvision, Klinipath, Duiven, the Netherlands) for five minutes. The slides were incubated with primary antibody AA1, diluted 1/ 200 in PBS/5% bovine serum albumin for 30 minutes, rinsed in PBS/0.1% Tween twice for five minutes, and incubated with polyvalent biotinylated antibody (Immunologic/Labvision) for 10 minutes. After washing with PBS three times for five minutes, the slides were incubated with streptavidin–peroxidase (Immunologic/Labvision) for 10 minutes. The reaction was visualised using 3,3′ diaminobenzidine (Fluka Sigma-Aldrich, Zoetermeer, the Netherlands) and 30% hydrogen peroxide (Merck, Darmstadt, Germany) in PBS for seven minutes, then rinsed with tap water for two minutes. All sections were counterstained with Mayer’s haematoxylin (Fluka AG, Buchs, Germany) for 10 seconds and rinsed in running tap water for 10 minutes. Slides were immersed three times in ethanol for three minutes after incubation in PBS for five minutes. The sections were mounted in Pertex (Histolab, Goteborg, Sweden). The negative controls consisted of omitting the staining with the primary antibody.
MC quantification
The numbers of mast cells were determined at a magnification of ×400 using a Zeiss Axioplan microscope (Carl Zeiss, Weesp, the Netherlands). Each section was photographed using a Sony colour video camera 3 CCD connected to the microscope. For accuracy of counting, a graticule with 100 fields measuring 0.22 × 0.22 mm (0.0484 mm2) under ×400 magnification was projected over each photograph. This counting method was based on the morphometric point counting technique, and the size of overlaying fields was fitted to the size of one mast cell.13,14 Ten photographs in different dermal layers were analysed for each section. To provide an overview of the dermal MCs, four photographs were taken from the stratum papillaris, three from the mid stratum reticularis, and three from the bottom stratum reticularis. Every square overlaying an MC scored one point and the sum of MCs in 10 photographs was calculated for each section. Capillaries, skin appendages, and the whole epidermis were scored in the same way. In addition to true MCs with a nucleus, clustered positively stained granules were counted as MCs and scored one point for each cluster. The numbers of MCs in each section were expressed as MCs/mm2.
MCs in all the three different biopsy groups were counted. The numbers of MCs in perilesional skin around BCCs and other skin lesions were counted and compared with those in the skin from healthy women (control group).
MC numbers, sample characteristics, and patient assessments were recorded in SPSS for Windows. Both paired and unpaired Student’s t tests were used to compare the means. A p value of < 0.05 was considered significant.
RESULTS
Numbers of MCs in healthy non-lesional and perilesional skin
In sections stained with ATA, the numbers of MCs in the sections of skin from healthy women who underwent mamma reduction or abdominoplasty (control group) were compared with those in the other two biopsy groups using an unpaired parametric Student’s t test (table 1; fig 1A). The numbers of MCs in perilesional skin in BCCs (n = 25), perilesional skin from various dermatological disorders (n = 95), and skin from the healthy group (n = 21) were not significantly different from each other. The 25 BCC biopsies and 95 other dermatological disorder biopsies were obtained from five different body sites and not exclusively from the trunk. To determine the possible influence of the body site and of the adjacent diagnosis of perilesional skin on the numbers of MCs, a multiple linear regression analysis was performed. When adjusted for the body site from which the biopsies were obtained, the numbers of MCs did not depend on the adjacent original diagnosis (p = 0.992).
Table 1.
Mean mast cell (MC) numbers in the 3 biopsy groups
| Group | Mean (SD) MC numbers | p Value* |
| Group 1: BCC (n = 25) | 91.3 (36.0) | 0.560 |
| Group 2: other dermatological disorders (n = 95) | 87.8 (41.8) | 0.560 |
| Group 3: controls (n = 21) | 79.5 (31.8) | 0.560 |
Group 1, mean number of MCs/mm2 in biopsies from perilesional skin around basal cell carcinomas (BCCs); group 2, mean number of MCs/mm2 in biopsies from perilesional skin around other dermatological disorders; group 3, mean number of MCs/mm2 in skin biopsies from healthy women.
*p Value for differences in MCs/mm2 between the three groups; Student’s t test.
Figure 1.
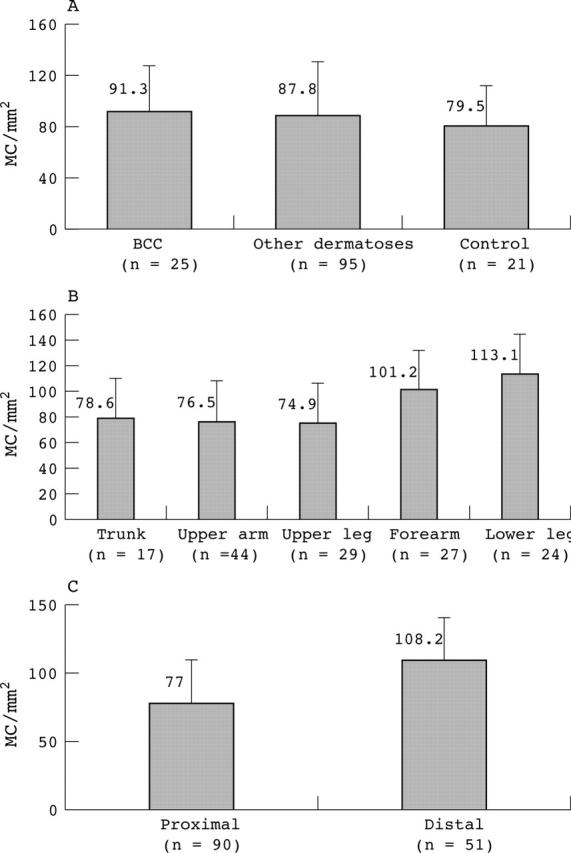
(A) The numbers of mast cells (MCs) were determined in three different biopsy groups, using anti-tryptase antibody staining and expressed as MC/mm2. No differences were found between perilesional skin biopsies around basal cell carcinomas (BCCs), perilesional skin around various dermatological disorders, and control skin (p = 0.560). (B) From these three biopsy groups, the numbers of MCs were determined in five different body sites; p values for differences in MC numbers in the trunk, upper arm, and upper leg were not significant (0.929), and neither were p values for differences between the forearm and lower leg (p = 0.240). (C) Body sites with similar MC numbers were placed into two groups. The “proximal” group was formed by the trunk, upper arm, and upper leg, whereas the “distal” group was formed by the forearm and lower leg (unpaired Student’s t test, p < 0.001).
MCs in various body sites
Perilesional skin samples stained with ATA were used for counting MCs in biopsies from different body sites. Adjusted for the diagnosis, the numbers of MCs were significantly dependent on the body site from which the skin samples were obtained (p = 0.001) (figs 2, 3A, B).
Figure 2.
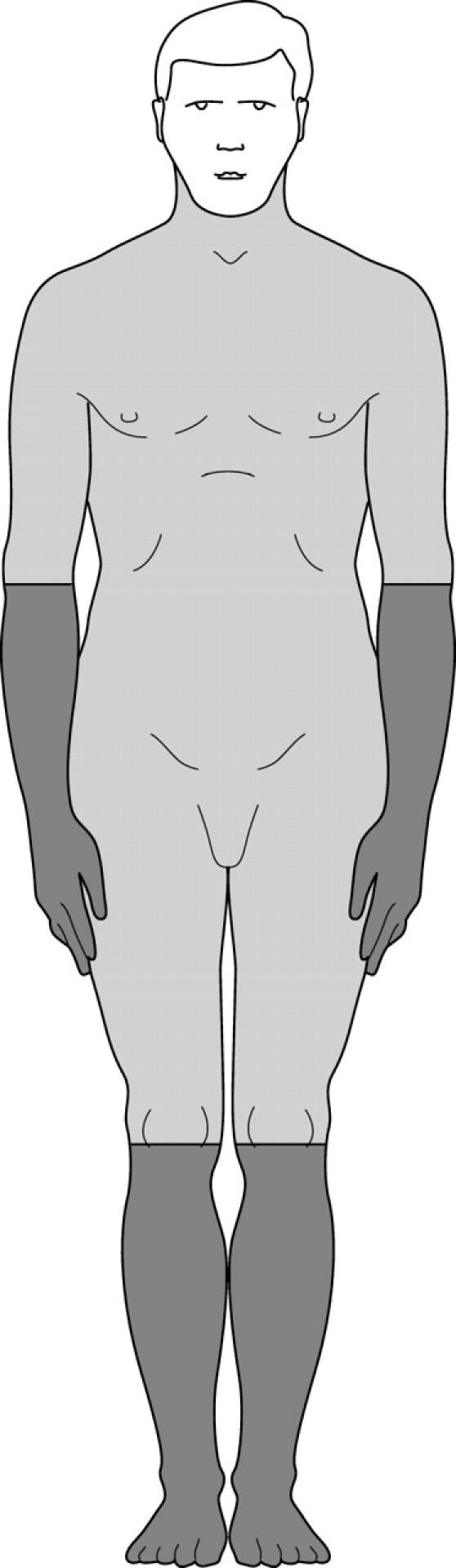
Mast cell distribution in human adult skin. Darker areas, highest numbers of mast cells in human skin (distal areas); lighter areas with the lowest numbers of mast cells in human skin (proximal areas). Mast cells in the facial area were not determined so that this area is left white.
Figure 3.
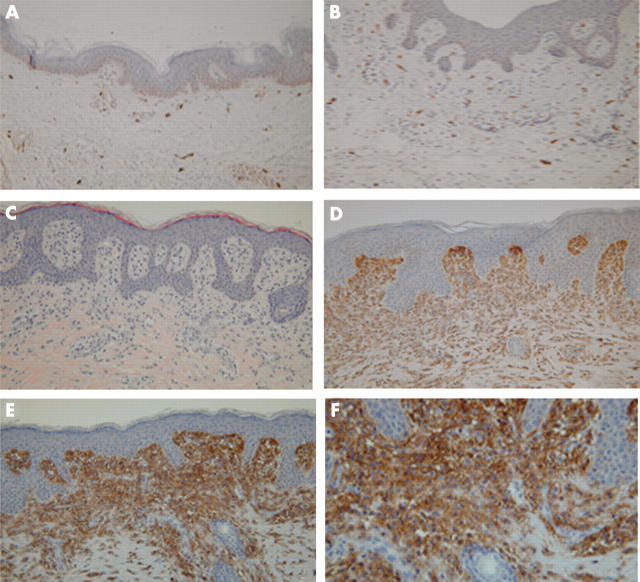
(A) Normal skin near basal cell carcinoma (original magnification, ×100). Mast cells occur in the normal dermis in small numbers as oval to spindle shaped cells. They are concentrated around the blood vessels. (B) Normal skin of mamma reduction tissue (original magnification, ×100). Mast cells occur in the normal dermis in small numbers as oval to spindle shaped cells. They are concentrated around the blood vessels. (C) Haematoxylin and eosin staining of mastocytosis (urticaria pigmentosa) (original magnification, ×100). Mast cells in the normal skin are indistinguishable from other perivascular cells. A small amount of granular cytoplasm is seen. (D) Urticaria pigmentosa, dense infiltrate of mast cells in the upper dermis located directly under the basal membrane. The infiltrate is also more concentrated around the blood vessels (original magnification, ×300). (E) Urticaria pigmentosa: positive staining of the mast cell infiltrate with CD117 (original magnification, ×300). (F) Urticaria pigmentosa: positive staining of the mast cell infiltrate with CD117 (original magnification, ×700).
Table 2 and fig 1B and C show the numbers of MCs at different body sites. The highest numbers of tryptase positive MCs were found in the lower leg and the forearm. These were significantly higher (p < 0.001) than was seen on the trunk, upper leg, and upper arm. As shown in table 2 and fig 2, two body site groups were formed according to this finding. The proximal group comprised biopsies obtained from the trunk, upper leg, and upper arm. The mean number of MCs in this group was 77.0 MCs/mm2 (SD, 33.6). The distal group comprised biopsies obtained from the lower leg and forearm. The mean number of MCs in this group was 108.2 MCs/mm2 (SD, 41.4). Adjusted for the diagnosis around which the MCs were counted (BCC or other skin lesions), a similar difference was found in the numbers of MCs at proximal and distal body sites.
Table 2.
Mast cell (MC) numbers according to the different body sites
| Body area | N | Mean (SD) MC/mm2 |
| Trunk | 17 | 78.6* (31.5) |
| Upper arm | 44 | 76.5* (32.7) |
| Upper leg | 29 | 74.9* (38.8) |
| Proximal | 90 | 77.0** (33.6) |
| Forearm | 27 | 101.2*** (32.6) |
| Lower leg | 24 | 113.1*** (46.7) |
| Distal | 51 | 108.2** (41.4) |
Perilesional skin around basal cell carcinomas, various dermatological disorders, and the control group.
*Difference in MCs/mm2 between trunk, upper arm, and upper leg: p = 0.929; **difference of MCs/mm2 between proximal and distal body areas: p = 0.000; ***difference of MCs/mm2 between forearm and lower leg: p = 0.240 (all Student’s t test).
Proximal: trunk, upper arm, and upper leg; distal: forearm and lower leg.
The number of capillaries in a biopsy does not affect MC counts
The numbers of MCs were calculated by dividing the total number of MCs by the total area of the biopsy. When the numbers of capillaries and skin appendages were subtracted from the total area of one skin biopsy, the numbers of MCs/mm2 were computed. It appeared that the numbers of capillaries and skin appendages subtracted from the total surface area had no significant influence on the accuracy of the numbers of MCs/mm2. However, the number and surface area of capillaries appeared to correlate (p < 0.001) with the number of MCs (B coefficient, 1.198). When adjusted for age, there was a significant effect of the number of capillaries on the number of MCs in proximal body sites (B coefficient, 1.5; p < 0.001). The number of capillaries had no effect on the number of MCs at distal body sites (B coefficient, −0.4; p = 0.639).
Pilot study in patients with mastocytosis (comparison)
Numerous MCs are present in mastocytosis and occur as oval to spindle shaped cells with a centrally located round to oval nucleus. They are concentrated in the upper dermis and around the blood vessels (fig 3C, D).
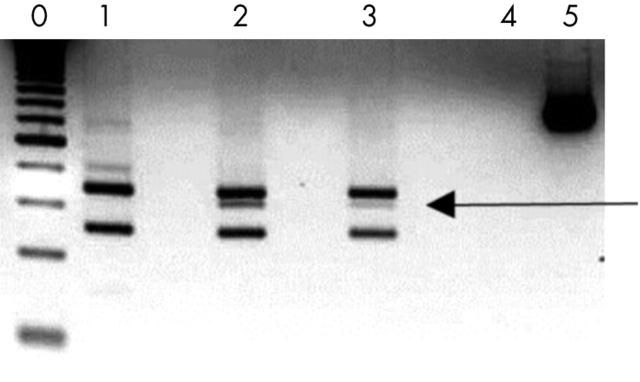
The numbers of MCs in a pilot study group of 14 patients with mastocytosis ranged from 78 to 2409 MC/mm2 (mean, 821; SD, 582). The ages of these patients ranged from 4 to 49 years, with six patients younger than 16 years. The numbers of MCs in mastocytosis were increased nearly 10 fold compared with those seen in normal skin. Figure 3E and F shows immunohistochemical staining for CD117, and an example of c-kit analysis, which is only possible in digested tissue, is shown in fig 4.
Figure 4.
Digestion fragments from polymerase chain reaction (PCR) products obtained from genomic DNA of one patient, analysed on a 4% agarose gel. Lane 0, 50 bp DNA ladder; lane 1, Asp816 (negative control); lane 2, Asp816/Val816 (positive control); lane 3, patient 43002; lane 4, blank; lane 5, PCR product. The 157 bp fragment, indicating the presence of the mutation encoding the Asp-816-Val change, is present in the tested patient (lane 3). Courtesy of R van Schdik.
DISCUSSION
In our present study, the numbers of MCs in adults were determined in biopsies obtained from five different body sites. Higher numbers of MCs were found in the forearm and lower leg (distal extremities) compared with those in the trunk, upper leg, and upper arm (centre and proximal extremities). Mast cells on the face were not counted in our study because diagnostic biopsies for mastocytosis are preferentially not taken from the face. From a pilot study in 14 patients with mastocytosis, we concluded that the lower limit for the number of MCs in mastocytosis may be as low as 78 MCs/mm2, which is also lower than the mean number of MCs in healthy skin (87.8 MCs/mm2). Thus, there was an overlap in the range of numbers of MCs in mastocytosis and normal skin.
The first reports on the measurement of MC numbers in different body sites date to 1950.15 In those early studies,5,6,10 different counting and staining techniques were used and no differences in the numbers of MCs in relation to the site of origin of the biopsy were noted. The numbers of MCs were found to be between 44 and 50 MCs/mm2. The uneven distribution in the numbers of MCs in different body sites may be the result of differences in their functional, environmental, and haemodynamic properties. Although still controversial, the numbers of MCs do not appear to be related to variations in the exposure of different body sites to ultraviolet light.16–20
We confirm the results obtained by Weber et al, who reported increased numbers of MCs on the face compared with other body sites and variations in numbers of MCs at different body sites similar to those reported here.21 The main difference between their study and ours is that they used the toluidine blue staining technique. It may be the case that more granules were stained in our study than that of Weber et al because the ATA technique is more sensitive than the toluidine blue stain used in their study. We also used a high magnification (×400) for analysing stained MCs from a picture on the computer screen. This may have magnified groups of granules that would have otherwise remained undetected.
“There was an overlap in the range of numbers of mast cells in mastocytosis and normal skin”
Collecting a large number of skin samples, using perilesional skin biopsies from BCCs or various dermatological disorders was useful, making biopsies from healthy individuals (controls) unnecessary. Cohen and Rogers emphasised the increased numbers of MCs around BCCs.22 They reported significant differences between the numbers of MCs in skin directly adjacent to a BCC compared with the surrounding skin, independent of the overall inflammatory cell response in the area. An increase in the numbers of MCs was described above and around multiple and single dermatofibromas and other benign epithelial tumours.23,24 In contrast, in other studies there was no difference in the numbers of perilesional MCs between benign and malignant skin lesions.11 By using perilesional skin biopsies without obvious signs of inflammation and determining the numbers of MCs as far away from the original lesion as possible, we avoided possible influences of inflammation on MC numbers.
Although MCs are mostly seen around dermal capillaries,6 in our study we found that adjusting for the number of capillaries does not contribute to a more precise quantification of MCs/mm2. In other studies on MC quantitation, an attempt was made to define the numbers of MCs in different dermal layers.5,7 In our study, the dermis was not divided into different dermal layers because the border between the papillary dermis and reticular dermis was not always clearly visible, which would make counting in relation to different dermal layers less accurate.
Based on our results, it is impossible to draw a strict distinguishing line between the upper limit of MC numbers in normal skin and the lower limit of MC numbers in mastocytosis. We suggest that a figure of up to 75 MCs/mm2 should be considered as normal and more than 250 MCs/mm2 as abnormal. Between 75 and 250 MCs/mm2 is the borderline area in which a diagnosis of mastocytosis should certainly be considered. These figures are crude and an individual approach still remains essential. Further studies in a large number of patients with mastocytosis are necessary to clarify the lower limit of the numbers of MCs in these patients.
Take home messages.
There was a significant difference between the numbers of mast cells (MCs) on the trunk, upper arm, and upper leg (distal location) and those found on the lower leg and forearm (proximal location)
These differences between distal and proximal locations must be considered when MCs are counted for a reliable diagnosis of mastocytosis
A pilot study in patients with mastocytosis underlined the variation in the numbers of MCs in mastocytosis and normal skin, but showed a considerable overlap, and further studies are needed to clarify the lower limit of the numbers of MCs in patients with mastocytosis
Acknowledgments
We thank R Kant, E Snijders, and F van der Ham for technical assistance. We thank A Nigg for designing macros for image analysis. We are grateful to the Department of Plastic and Reconstructive Surgery of the Erasmus MC Rotterdam for collecting skin samples from patients undergoing surgery.
Abbreviations
ATA, anti-tryptase monoclonal antibody
BCC, basal cell carcinoma
MC, mast cell
PBS, phosphate buffered saline
REFERENCES
- 1.Brockow K, Metcalfe DD. Mastocytosis. Curr Opin Allergy Clin Immunol 2001;1:449–54. [DOI] [PubMed] [Google Scholar]
- 2.Castells M, Austen KF. Mastocytosis: mediator-related signs and symptoms. Int Arch Allergy Immunol 2002;127:147–52. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann K, Henz BM. Cutaneous mastocytosis—clinical heterogeneity. Int Arch Allergy Immunol 2002;127:143–6. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Schernthaner GH, Sperr WR, et al. Variable expression of activation-linked surface antigens on human mast cells in health and disease. Immunol Rev 2001;179:74–81. [DOI] [PubMed] [Google Scholar]
- 5.Cowen T, Trigg P, Eady RA. Distribution of mast cells in human dermis: development of a mapping technique. Br J Dermatol 1979;100:635–40. [DOI] [PubMed] [Google Scholar]
- 6.Eady RA, Cowen T, Marshall TF, et al. Mast cell population density, blood vessel density and histamine content in normal human skin. Br J Dermatol 1979;100:623–33. [DOI] [PubMed] [Google Scholar]
- 7.Eady RA. The mast cells: distribution and morphology. Clin Exp Dermatol 1976;1:313–21. [DOI] [PubMed] [Google Scholar]
- 8.Garriga MM, Friedman MM, Metcalfe DD. A survey of the number and distribution of mast cells in the skin of patients with mast cell disorders. J Allergy Clin Immunol 1988;82:425–32. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Aal. Quantitative and qualitative changes in mast cells in the skin of normal Egyptians. Acta Derm Venereol 1976;56:435–9. [Google Scholar]
- 10.Mikhail GR, Miller-Milinska A. Mast cell population in human skin. J Invest Dermatol 1964;43:249–54. [PubMed] [Google Scholar]
- 11.Marshall JS, Ford GP, Bell EB. Formalin sensitivity and differential staining of mast cells in human dermis. Br J Dermatol 1987;117:29–36. [DOI] [PubMed] [Google Scholar]
- 12.Walls AF, Jones DB, Williams JH, et al. Immunohistochemical identification of mast cells in formaldehyde-fixed tissue using monoclonal antibodies specific for tryptase. J Pathol 1990;162:119–26. [DOI] [PubMed] [Google Scholar]
- 13.Kasper CS, Freeman RG, Tharp MD. Diagnosis of mastocytosis subsets using a morphometric point counting technique. Arch Dermatol 1987;123:1017–21. [PubMed] [Google Scholar]
- 14.Kasper CS, Tharp MD. Quantification of cutaneous mast cells using morphometric point counting and a conjugated avidin stain. J Am Acad Dermatol 1987;16:326–31. [DOI] [PubMed] [Google Scholar]
- 15.Helmstrom B, Holmgren H. Numerical distribution of mast cells in the human skin and heart. Acta Anat (Basel) 1950;10:81–107. [DOI] [PubMed] [Google Scholar]
- 16.Kochevar IE, Moran M, Granstein RD. Experimental photoaging in C3H/HeN, C3H/HeJ, and Balb/c mice: comparison of changes in extracellular matrix components and mast cell numbers. J Invest Dermatol 1994;103:797–800. [DOI] [PubMed] [Google Scholar]
- 17.Kligman LH, Murphy GF. Ultraviolet B radiation increases hairless mouse mast cells in a dose-dependent manner and alters distribution of UV-induced mast cell growth factor. Photochem Photobiol 1996;63:123–7. [DOI] [PubMed] [Google Scholar]
- 18.Learn DB, Moloney SJ. Numbers of murine dermal mast cells remain unchanged during chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed 1991;8:195–9. [PubMed] [Google Scholar]
- 19.Bhawan J, Andersen W, Lee J, et al. Photoaging versus intrinsic aging: a morphologic assessment of facial skin. J Cutan Pathol 1995;22:154–9. [DOI] [PubMed] [Google Scholar]
- 20.Rosen LB, Frank B. Mast cells in sun-exposed and non-sun-exposed skin. An autopsy study. Am J Dermatopathol 1987;9:208–11. [DOI] [PubMed] [Google Scholar]
- 21.Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol 2003;148:224–8. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MS, Rogers GS. The significance of mast cells in basal cell carcinoma. J Am Acad Dermatol 1995;33:514–7. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology 1995;190:9–13. [DOI] [PubMed] [Google Scholar]
- 24.Roche WR. Mast cells and tumors. The specific enhancement of tumor proliferation in vitro. Am J Pathol 1985;119:57–64. [PMC free article] [PubMed] [Google Scholar]