Abstract
Background: Astrocytic tumours, the most common gliomas, are often classified intraoperatively using standard morphological staining. The final diagnosis and grading of gliomas on paraffin wax sections is often assisted by Ki-67 immunohistochemistry, but standard immunostaining protocols take too long to be used intraoperatively.
Aims: To investigate a new rapid Ki-67 immunohistochemical test for its use in an intraoperative setting.
Methods: The new Ki-67 immunostaining (Ultrarapid-Ki67®) method on frozen sections can be carried out in 10 minutes. Thirty four pilocytic and diffuse astrocytomas were immunostained by rapid Ki-67 and results were compared with corresponding MIB-1 staining, histological grading, and prognosis.
Results: The staining protocol was practical to perform and the results were morphologically and quantitatively indistinguishable from those after immunostaining with MIB-1, an antibody recognising Ki-67 in paraffin wax embedded tissue. A comparison of Ultrarapid-Ki67 and MIB-1 immunostaining of paraffin wax sections showed almost identical quantitative correlation in astrocytic gliomas (r = 0.916; p<0.001). The Ultrarapid-Ki67 indices (percentage of positive cells) of low grade (I/II) astrocytomas ranged from 0% to 6.1%, whereas those of representative high grade (III/IV) tumours were significantly higher (range, 5.6–45%; p<0.001). The best prognostic cutoff point for Ultrarapid-Ki67 was 7.5%, which divided diffuse grade II–IV astrocytomas into significantly differing subsets (p = 0.0008).
Conclusion: Ultrarapid-Ki67 immunostaining is a useful adjunct to morphological diagnosis and grading of astrocytic tumours, and as a fast test (∼10 minutes for staining plus three to four minutes for scoring), it could be used in routine intraoperative diagnosis of gliomas and other neoplastic diseases.
Intraoperative frozen section diagnosis of astrocytic tumours is valuable to neurosurgeons for several reasons. First, the neoplastic nature of the removed tissue needs to be confirmed, because surgery is adjusted according to the histopathological nature of the tissue. For example, glioblastomas, metastases, and granulomas may resemble each other macroscopically, but their surgical treatments differ from each other. Second, it is necessary to distinguish benign neoplasms and gliotic tissue from anaplastic and grade IV gliomas. Neurosurgeons may adjust the extent of the resection, and in cases of malignant tumours, apply BCNU (carmustine) wafers in the resected tumour bed or use gene therapeutic approaches in clinical trials.
The interpretation of intraoperative frozen sections of gliomas is demanding and requires the evaluation of subtle histological features not always familiar to the general pathologist, whose daily diagnostic routine rarely includes central nervous system tumours. In some cases, the nature of an astrocytic tumour may remain uncertain even when investigated by an experienced neuropathologist. It has been estimated that intraoperative diagnostic accuracy with regard to central nervous system tumours is approximately 90%.1 The World Health Organisation (WHO) 20002 grading criteria of the most common gliomas—diffuse astrocytomas—include the presence or absence of atypia, mitoses, endothelial proliferation, and necrosis. However, strict interpretation of these criteria may not always be straightforward in frozen sections. For example, diffuse astrocytomas without necrosis or endothelial proliferation are divided into grade II and III tumours according to the absence or presence of mitotic figures, respectively. However, solitary mitotic figures may be seen in grade III astrocytomas prognostically resembling grade II astrocytomas.3 Furthermore, as a result of the relatively poor morphology of frozen sections, mitotic figures are often difficult to identify with certainty.
“Unfortunately, standard immunohistochemical procedures typically take two to four hours to perform, precluding their use during intraoperative frozen section diagnosis”
The proliferation activity of tumours can also be assessed by means of Ki-67 immunohistochemistry, which is now widely used in clinical pathology and neuropathology diagnostics. Burger and co-workers were the first to show the tight association between Ki-67 immunopositivity and glioma grade in freshly frozen samples of astrocytomas.4 We and others have described the prognostic value of immunostaining with MIB-1,5–7 an antibody recognising the Ki-67 antigen in paraffin wax embedded tissue.8 Based on a large number of studies, the added prognostic information provided by Ki-67 immunostaining is now well established with regard to astrocytic tumours. The latest WHO classification of central nervous system tumours2 includes Ki-67 as an additional tool in histological typing and grading, although it cannot be regarded as being entirely prognostic in individual cases.
On the basis of abundant data and the widespread clinical use of paraffin wax embedded material, Ki-67 immunostaining could also be applied to the intraoperative diagnosis of astrocytic tumours. Unfortunately, standard immunohistochemical (IHC) procedures typically take two to four hours to perform, precluding their use during intraoperative frozen section diagnosis. Rapid or semirapid immunostaining protocols have been described,9,10 but so far they have not gained wide acceptance in routine practice. In our study, we introduce an ultrarapid immunostaining protocol, which makes it possible to perform Ki-67 immunostaining intraoperatively. We compared rapid frozen section Ki-67 immunostaining with the corresponding MIB-1 staining of formalin fixed, paraffin wax embedded samples. Proliferation indices were also compared with regard to their ability to predict the final histological diagnosis and prognosis of patients with grade I–IV astrocytomas.
METHODS
Patients and tumours
Tumour material for our study was collected from 34 patients surgically treated for astrocytoma at Tampere University Hospital, Finland from 1996 to 2000. At the time of intraoperative consultation, frozen sections (6–8 μm thick) were cut from a sample selected by the neurosurgeon and stained with haematoxylin and eosin (HE). After confirming the intraoperative diagnosis, additional sections were cut, sealed airtight, and stored unfixed at −70°C. Histopathological typing and grading were performed by an expert neuropathologist according to the criteria presented by the WHO.2 The material from the 34 patients (14 male and 20 female) consisted of nine pilocytic astrocytomas (grade I) and 25 diffuse astrocytomas (nine grade II astrocytomas, four grade III anaplastic astrocytomas, and 12 grade IV glioblastomas). The age of the patients ranged from 2 to 81 years (median, 48). The tumours were radically resected and most patients with high grade gliomas also received radiotherapy. Follow up data were available for at least 36 months in all cases.
Ultrarapid Ki-67 immunostaining
Slides were immunostained using the Ultrarapid-Ki67® IHC kit (Immuno Diagnostic Inc, Hämeenlinna, Finland), according to the manufacturer’s instructions. Briefly, the slides were first fixed in 100% acetone/0.03% hydrogen peroxide for one minute at room temperature and air dried. Tissue sections were encircled with a hydrophobic pen (PAP-pen) to help keep antibody droplets on the sections. The sections were first incubated with ready to use mouse Ki-67 antibody for three minutes, followed by a short rinse in phosphate buffered saline (10 seconds with continuous slide agitation). The detection antibody (antimouse–horseradish peroxidase polymer) was applied and the sections were incubated for three minutes before rinsing with phosphate buffered saline for 10 seconds. Diaminobenzidine (DAB) was used as chromogenic substrate and was prepared by adding one drop of reagent A and one drop of reagent B to 500 μl of DAB diluent. The slides were incubated in DAB solution for one minute and washed with distilled water. Antibody and DAB incubations were carried out on a thermal plate set at 41°C. The slides were counterstained with haematoxylin (10 seconds), dehydrated, and embedded according to routine frozen section protocols.
Control Ki-67 (MIB-1) immunostaining
After intraoperative consultation, the remaining frozen tissue block was fixed in 10% buffered formalin overnight and embedded in paraffin wax. Sections cut from this block were immunostained with MIB-1 antibody (DakoCytomation, Glostrup, Denmark). Heat induced epitope retrieval (Tris/EDTA buffer (pH 9.0) 2 × 7 minutes in an microwave oven) and an automated immunostaining protocol (TechMate immunostainer) were used. These slides were counterstained with haematoxylin and analysed visually. This staining was termed “control MIB-1”. MIB-1 staining was also carried out using a tissue block showing the highest mitotic activity and cellularity, as described previously.11 These sections were identified from all the HE stained sections by a neuropathologist. These tissue sections were stained with MIB-1, as described above, and counterstained with methyl green. In the comparisons this staining was termed “highest MIB-1”.
Scoring of immunopositivity
Scoring of Ultrarapid-Ki67 and control MIB-1 slides was carried out in the area of highest immunopositivity by two observers (I and II). In total, 300–500 neoplastic tumour cells were analysed. Necrotic and haemorrhagic tumour areas were ignored. In the visual estimation only definitely brown nuclei were recorded as positive. The results were expressed as percentage of immunoreactive tumour cell nuclei. Analysis of immunopositivity of the highest MIB-1 slides was carried out by observer II using a CAS-200 image analysis system (CAS-200 Software; Becton Dickinson, Mountain View, California, USA), as described previously.5 The proliferation index obtained by computer assisted image analysis was also reported as the percentage of immunopositive nuclei.
Statistical methods
Pearson’s correlation coefficient (r), and Mann-Whitney and Kruskal-Wallis tests were used to study concordance of the IHC tests. Optimal cutoffs for proliferation indices in survival analyses were defined using receiver operating characteristics (ROC) curves, as described previously.5 The significance of survival differences was determined by the log rank test. Statistical analyses were carried out using SPSS for Windows software (SPSS Inc, Chicago, Illinois, USA).
RESULTS
The staining pattern of Ultrarapid-Ki67 frozen sections of gliomas was similar to that of MIB-1 staining in paraffin wax embedded tumour sections. Ultrarapid-Ki67 reagents revealed proliferating astrocytoma nuclei (fig 1), which were scarce in low grade tumours and abundant in glioblastoma (fig 1A, B). During the preparation of our article intraoperative IHC was used in the clinical setting. A clinical case demonstrating the usefulness of intraoperative Ki-67 is shown in fig 1C–E. HE staining of a cortical tumour was inconclusive, leaving the differential diagnosis of primary brain tumour (preferentially meningioma) and metastatic carcinoma. A high Ki-67 labelling index (30–40% Ki-67 positive tumour cells) ruled out low grade and benign tumours. Additional rapid pan-cytokeratin staining (1E) defined the tumour finally as a metastatic carcinoma.
Figure 1.
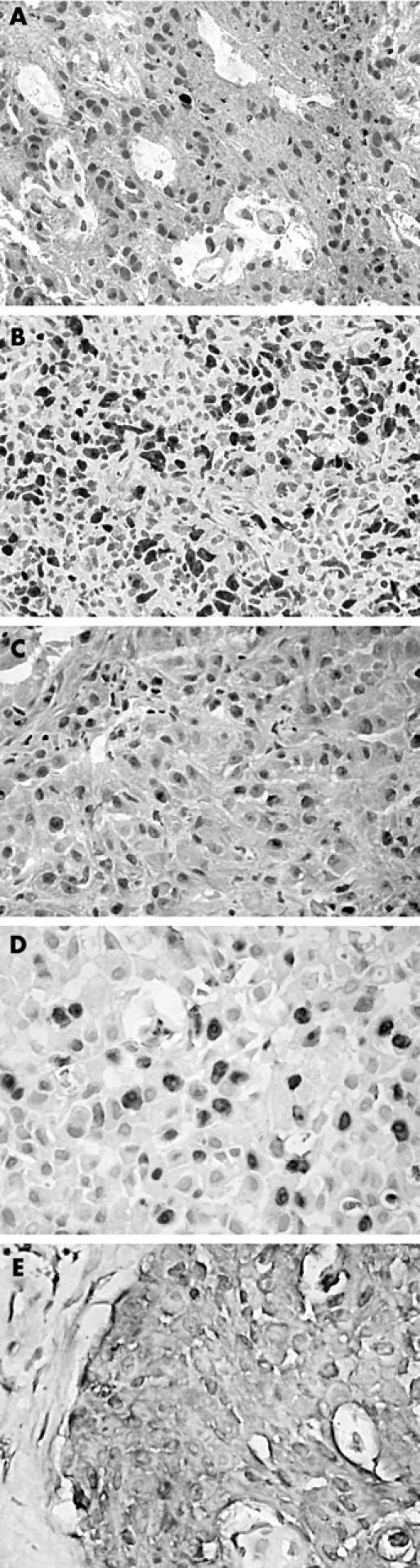
Immunostaining in a frozen section of (A) low grade and (B) high grade glioma using the Ultrarapid-Ki67 kit. An additional clinical case demonstrating the usefulness of intraoperative Ki-67 is shown in (C–E). (C) Haematoxylin and eosin staining of a cortical tumour was inconclusive, leaving the differential diagnosis of primary brain tumour (preferentially meningioma, one mitosis/10 high power fields) and metastatic carcinoma. (D) A high Ki-67 labelling index ruled out low grade and benign tumours. (E) Additional rapid intraoperative pan-cytokeratin staining defined the tumour finally as a metastatic carcinoma.
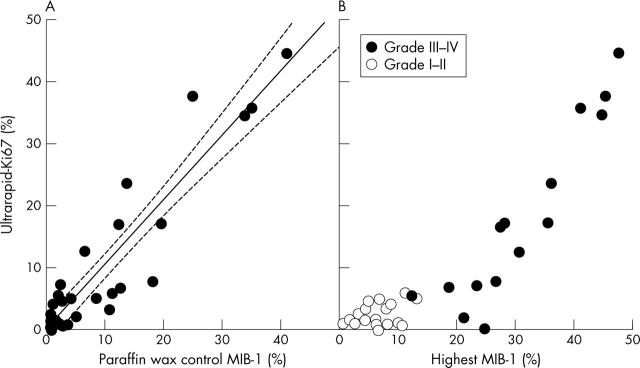
Figure 2A shows the proliferation indices of Ultrarapid-Ki67 frozen sections and corresponding MIB-1 staining in paraffin wax embedded sections (control MIB-1). The correlation between the two methods was nearly perfect (r = 0.916; p < 0.001). When the highest proliferation indices in paraffin wax embedded sections (highest MIB-1) were used for comparison, the correlation remained substantial (r = 0.790; p < 0.001; fig 2B). Interobserver variation between observers I and II was minimal, as indicated by a correlation coefficient of r = 0.946 (p < 0.001, data not shown).
Figure 2.
Growth fractions (percentage of Ki-67 positive cells) by rapid frozen section and standard paraffin wax embedded section Ki-67 immunostaining in matched pairs of human astrocytoma samples. (A) Comparison between Ultrarapid-Ki67 values of frozen sections and corresponding MIB-1 values of paraffin wax embedded control sections analysed by the same observer (r = 0.916; p < 0.001). The regression line and its 95% confidence intervals are plotted in solid and dashed lines, respectively. (B) Comparison between Ultrarapid-Ki67 index of frozen sections and selected tumour areas showing highest MIB-1 index of paraffin wax embedded tumour material analysed by computer assisted image analysis (r = 0.790 p < 0.001). Open symbols, low grade tumour in final diagnosis; filled symbols, high grade tumour in final diagnosis.
Table 1 shows the mean and median Ultrarapid-Ki67 and MIB-1 proliferation indices by final histological grade. Proliferation indices by all three methods differed significantly with histological grade (Ultrarapid-Ki67: p < 0.001; control MIB-1: p = 0.001; highest MIB-1: p < 0.001, Kruskal-Wallis test). The mean and median values for Ultrarapid-Ki67 and control MIB-1 were comparable. The analysis concentrating in the most proliferative areas (highest MIB-1) yielded higher proliferation indices.
Table 1.
Ki-67 indices by histological grade
| Grade | N | Ultrarapid-Ki67 | Control MIB-1 | Highest MIB-1 | ||
| Observer 1 | Observer 2 | Observer 1 | Observer 2 | |||
| I | 9 | Mean | 2.69 | 2.59 | 3.1 | 6.92 |
| SD | 2.20 | 3.00 | 3.92 | 3.87 | ||
| Median | 1.50 | 1.00 | 1.05 | 6.20 | ||
| Range | 0–6.10 | 0–9.40 | 0.30–10.50 | 0.40–13.00 | ||
| II | 9 | Mean | 2.41 | 2.68 | 1.74 | 5.47 |
| SD | 1.52 | 1.55 | 1.24 | 3.07 | ||
| Median | 1.70 | 2.30 | 1.35 | 5.80 | ||
| Range | 0.90–5.10 | 1.20–5.3 | 0.30–3.80 | 1.30–10.30 | ||
| III | 4 | Mean | 3.73 | 5.05 | 4.78 | 19.08 |
| SD | 3.05 | 3.62 | 5.37 | 5.22 | ||
| Median | 3.85 | 5.40 | 3.3 | 19.75 | ||
| Range | 0.30–6.90 | 0.30–9.10 | 0.20–12.30 | 12.20–24.60 | ||
| IV | 12 | Mean | 21.92 | 19.33 | 18.29 | 36.71 |
| SD | 13.43 | 10.89 | 12.64 | 10.10 | ||
| Median | 17.25 | 17.55 | 15.65 | 35.65 | ||
| Range | 5.90–45.00 | 7.20–41.20 | 2.10–40.60 | 23.20–55.50 | ||
| Total material | 34 | Mean | 9.52 | 8.81 | 8.67 | 18.48 |
| SD | 12.21 | 10.31 | 11.02 | 15.68 | ||
| Median | 4.90 | 5.10 | 3.55 | 11.60 | ||
| Range | 0–45.00 | 0–41.20 | 0.20–40.60 | 0.40–55.50 | ||
Table 2 shows a comparison of intraoperative tumour grade, Ki-67 indices, and final tumour grade. The tumours were divided into low grade (grades I and II) and high grade (grades III and IV) astrocytomas according to the original intraoperative frozen section interpretation based on HE staining. A significant difference (p < 0.001, Mann-Whitney test) and only a minor overlap were found when the proliferation indices (Ultrarapid-Ki67) were compared between these groups (table 2).
Table 2.
Comparison of intraoperative frozen section grading, Ki-67 indices, and final grading using paraffin wax embedded sections
| Intraoperative frozen section grading | p Value* | |||
| Low grade (N = 20) | High grade (N = 13) | Uncertain (N = 1) | ||
| Ultrarapid-Ki67 | ||||
| Mean | 2.4 | 19.9 | 16.9 | |
| Median | 1.7 | 17.2 | 16.9 | <0.001 |
| Range | 0–6.1 | 5.6–45.0 | ||
| Control MIB-1 | ||||
| Mean | 2.5 | 17.6 | † | |
| Median | 1.1 | 13.4 | <0.001 | |
| Range | 0.2–10.5 | 1.8–40.6 | ||
| Highest MIB-1 | ||||
| Mean | 7.9 | 34.1 | 27.3 | |
| Median | 6.3 | 35.3 | 27.3 | <0.001 |
| Range | 0.4–24.6 | 12.2–55.5 | ||
| Final grade | ||||
| I | 9 | 0 | ||
| II | 9 | 0 | 0.004 | |
| III | 2‡ | 2 | ||
| IV | 0 | 11 | 1 | |
*Mann-Whitney test for proliferation indices and χ2 test for grades; †insufficient tumour tissue for control MIB-1 staining; ‡two tumours with Ultrarapid-Ki67 indices of 0.3 and 2.1, control MIB-1 of 0.2 and 4.8, and highest MIB-1 of 24.6 and 21.0, respectively.
Histopathological differential diagnosis of grading (low grade versus high grade) could not be performed in one case intraoperatively (uncertain frozen section grading, mostly necrotic sample) (table 2). The final diagnosis in this patient, after thorough investigation of all paraffin wax embedded tissue, was glioblastoma (grade IV). Interestingly, the Ultrarapid-Ki67 index of 300–400 non-necrotic cells present in this frozen section was 16.9%, which would have indicated a high grade tumour. There were two cases misinterpreted as low grade tumour intraoperatively, although the final diagnosis was anaplastic astrocytoma (table 2). The original intraoperative diagnoses were based on the lack of mitoses. In concordance with mitotic counting, the Ultrarapid-Ki67 and control MIB-1 data showed low proliferation indices in both cases (0.3% and 2.1%, 0.2% and 4.8%, respectively; table 2). More representative and extensive postoperative analysis using all the resected material revealed tissue areas with mitotic figures and higher MIB-1 indices (highest MIB-1: 24.6% and 21.0%, respectively), resulting in the diagnosis of grade III anaplastic astrocytoma in both cases.
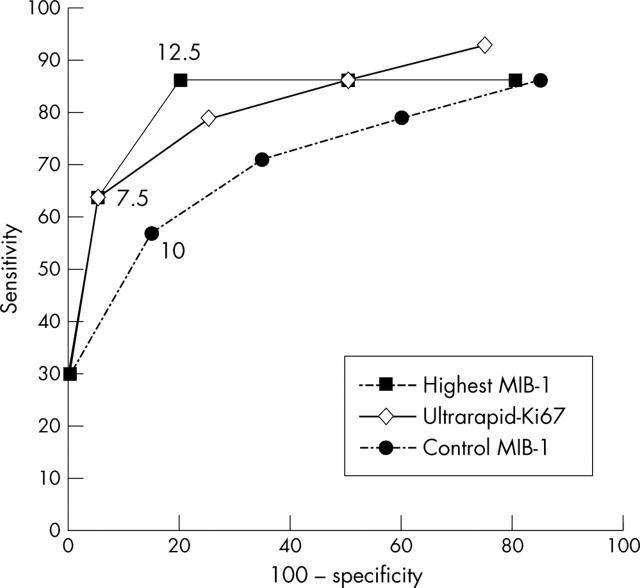
The prognostic value of Ultrarapid-Ki67 and the optimal cutoff points were studied by means of ROC analysis. Figure 3 shows the prognostic sensitivity (true positive; that is, deceased patient with Ki-67 index above cutoff point) and specificity (100 false positive; that is, a living patient with Ki-67 index above cutoff point) during a three year follow up period for Ultrarapid-Ki67 and the two MIB-1 assays. The almost superimposed ROC curves indicate that Ultrarapid-Ki67, control MIB-1, and highest MIB-1 were of similar prognostic value. Optimal cutoffs yielding the best sensitivity and specificity for the three tests were 7.5%, 10%, and 12.5 %, respectively (fig 3). Using these cutoffs, a highly significant distinction was found in survival analysis (Ultrarapid-Ki67: p < 0.0001; control MIB-1: p = 0.0047; highest MIB-1: p = 0.0001; log rank test). Prognostic significance was also seen when only diffuse astrocytomas (grades II–IV) were included in the analysis (Ultrarapid-Ki67: p = 0.0008; control MIB-1: p = 0.0167; highest MIB-1: p = 0.005).
Figure 3.
Receiver operating characteristics curve showing prognostic true and false positivity rates of the Ki-67 indices. The predictive properties (%) are calculated on the basis of three year survival of the 34 patients divided into six subgroups of approximately equal size. The patient groups are placed in the curve according to decreasing proliferation: patients with the highest tumour proliferation are at the bottom left corner of the curve, and patients with the lowest tumour proliferation are at the top right corner of the curve. The most efficient cutoff point of each Ki-67 index is shown.
DISCUSSION
It is well known that intraoperative grading of astrocytomas is a demanding task when based on frozen section HE staining alone. In our study, we evaluated an ultrarapid immunostaining protocol for Ki-67, which can be used intraoperatively to aid frozen section interpretation—typing and grading of gliomas. Using material from 34 patients, we found an almost perfect correlation between Ultrarapid-Ki67 and MIB-1 staining, with MIB-1 staining being carried out in matched paraffin wax embedded samples. The prognostic value of Ultrarapid-Ki67 was as good as that of the paraffin wax embedded section MIB-1 procedure. The mean and median values of the Ultrarapid-Ki67 data in each glioma grade matched well with the cutoff points suggested in the WHO grading scheme,2 further indicating the validity of the method. Thus, the results indicate that estimation of the tumour proliferation rate could aid pathologists not only postoperatively, but also in the intraoperative differential diagnosis of borderline cases.
“Using material from 34 patients, we found an almost perfect correlation between Ultrarapid-Ki67 and MIB-1 staining, with MIB-1 staining being carried out in matched paraffin wax embedded samples”
When considering the usefulness of immunohistochemical tumour proliferation assessment, overlapping Ki-67 indices in different histopathological categories and considerable intratumorous regional variation of proliferation are well known problems. The advantage of Ki-67 comes from its wider coverage of the cell cycle, resulting in higher percentages of positively labelled proliferating cells when compared with proliferating cell nuclear antigen or mitotic indices. This provides a better statistical distinction between different histological grades.5,11,12 At the practical level, another important issue to consider is sampling. Variation in tumour proliferation indices is much smaller when evaluation is systematically performed in the area of highest proliferation.11 Unfortunately, samples from these tumour areas are not always sent for intraoperative diagnostic assessment. This fact was illustrated by the two discordant cases showing very low indices in the Ultrarapid-Ki67 and control MIB-1 tests, and absence of mitotic figures in histology. However, postoperative analysis using all the formalin fixed and paraffin wax embedded material revealed mitoses and high MIB-1 indices, resulting in a final diagnosis of grade III anaplastic astrocytoma. These false negative cases show that frozen section based grading is always preliminary only. Regional intratumorous heterogeneity may result in underestimation of malignancy intraoperatively, irrespective of which method of tumour proliferation assessment is used.
The usefulness of Ultrarapid-Ki67 immunostaining was highlighted in one case in which intraoperative diagnosis remained uncertain as a result of extensive tissue necrosis. If available at the time of intraoperative consultation, a high Ultrarapid-Ki67® index (16.9%) would have suggested a high grade astrocytoma. The final diagnosis in this case was glioblastoma with the highest MIB-1 index of 27.3%. As presented with the clinical case example, we believe that intraoperative Ki-67 assay could also be of great help in cases where the distinction between low grade glioma and non-neoplastic tissue processes (gliosis) or primary and metastatic malignancy remains uncertain.
Take home messages.
We describe a rapid method for the immunohistochemical staining of Ki-67 in frozen glioma sections intraoperatively
This method was found to be practical to perform and the results were morphologically and quantitatively indistinguishable from those after immunostaining with MIB-1, an antibody recognising Ki-67 in paraffin wax embedded tissue
Thus, Ultrarapid-Ki67 immunostaining is a useful adjunct to the morphological diagnosis and grading of astrocytic tumours, and because of its speed could be used in the routine intraoperative diagnosis of gliomas and other neoplastic diseases
The present ultrarapid immunostaining method for Ki-67 follows the same methodology as the one in which a pan-cytokeratin antibody is used in the intraoperative evaluation of sentinel lymph nodes removed in connection with breast cancer (CytoNel; Immunodiagnostic Inc). Other techniques for rapid Ki-67 and other rapid methods in IHC have been presented previously,9,10,13–15 but further reports on their clinical usefulness are lacking. In our study, we showed that the novel Ultrarapid-Ki67 immunostaining method can be carried out in 10 minutes and visual scoring of one slide takes an additional three to four minutes. In our series, Ultrarapid-Ki67 IHC was found to be highly concordant, both quantitatively and qualitatively, with conventional MIB-1 immunostaining of paraffin wax embedded sections of the same tumours.
Technical precautions in Ultrarapid-Ki67 immunostaining are similar to those used in any technique involving frozen sections. Tissue material is often fragmented and can be difficult to cut with a cryomicrotome. Therefore, an experienced histotechnologist is needed to prepare and cut high quality frozen sections. The most important technical detail in the staining protocol is the immediate acetone fixation of the slide. In other respects, intraoperative Ki-67 staining is relatively simple to perform, and does not require special equipment other than a thermal plate.
The results of the histopathological examination of tumours must be reported in a timely fashion to be clinically useful at the time of surgery. Immunohistochemical methods that can be performed in 10 minutes or less clearly fulfil this criterion, and will probably be used in other areas of tumour pathology as soon as immunostaining protocols for other diagnostically useful antibodies are developed and optimised. In our study, we describe a method for Ki-67 immunostaining that is likely to become a useful adjunct to the intraoperative frozen section evaluation of astrocytic tumours.
Acknowledgments
We thank Ms R Randen, Ms E Riikonen, and Mr J Lampinen for skilful technical assistance. Ms K Nordfors is acknowledged for her help in the revision of the manuscript. This work was supported by grants from the Research Fund of Tampere University Hospital and the Cancer Society of Finland. J Haapasalo is a fellow of the Graduate School, Biomedical Sciences, University of Turku. Other sources of support are the Finnish Cancer Foundation, Scientific Foundation of TAUH, and Juselius Foundation
Abbreviations
DAB, diaminobenzidine
HE, haematoxylin and eosin
IHC, immunohistochemistry
ROC, receiver operating characteristics
WHO, World Health Organisation
REFERENCES
- 1.Shah A, Muzumdar G, Chitale A, et al. Squash preparation and frozen section in intraoperative diagnosis of central nervous system tumors. Acta Cytol 1998;42:1149–54. [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Cavenee WK, eds. Pathology and genetics of tumours of the nervous system. WHO classification of tumours. Lyon: IARC Press, 2000.
- 3.Giannini C, Scheithauer PW, Burger PC, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol 1999;58:46–53. [DOI] [PubMed] [Google Scholar]
- 4.Burger PC, Shibata T, Kleihues P. The use of monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol 1986;10:611–17. [DOI] [PubMed] [Google Scholar]
- 5.Sallinen P, Haapasalo H, Visakorpi T, et al. Relation of Ki-67 (MIB-1), PCNA and S-phase fraction with patient survival in formalin-fixed, paraffin-embedded astrocytoma material. J Pathol 1994;174:275–82. [DOI] [PubMed] [Google Scholar]
- 6.Onda K, Davis RL, Shibuya M, et al. Correlation between the bromodeoxyuridine labeling index and the MIB-1 and Ki-67 proliferating cell indices in cerebral gliomas. Cancer 1994;74:1921–6. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham JM, Kimmel DW, Scheithauer BW, et al. Analysis of proliferation markers and p53 expression in gliomas of astrocytic origin: relationships and prognostic value. J Neurosurg 1997;86:121–30. [DOI] [PubMed] [Google Scholar]
- 8.Cattoretti G, Becker MHG, Key G, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave-processed formalin-fixed paraffin-embedded sections. J Pathol 1992;168:357–63. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumi Y, Serizawa A, Kawai K. Enhanced polymer one-step staining (EPOS) for proliferating cell nuclear antigen (PCNA) and Ki-67 antigen: application to intra-operative frozen diagnosis. Pathol Int 1995;45:108–15. [DOI] [PubMed] [Google Scholar]
- 10.Kämmerer U, Kapp M, Gassel AM, et al. A new rapid immunohistochemical staining technique using the EnVision antibody complex. J Histochem Cytochem 2001;49:623–30. [DOI] [PubMed] [Google Scholar]
- 11.Sallinen P, Sallinen S-L, Helén P, et al. Grading of diffusely infiltrating astrocytomas by quantitative histopathology, cell proliferation and image cytometric DNA analysis. Neuropathol Appl Neurobiol 2000;26:319–31. [DOI] [PubMed] [Google Scholar]
- 12.Rautiainen E, Haapasalo H, Sallinen P, et al. Histone mRNA in situ hybridization in astrocytomas—a comparison with PCNA, MIB-1 and mitoses in paraffin-embedded material. Histopathology 1998;32:43–50. [DOI] [PubMed] [Google Scholar]
- 13.Ichihara T, Nakao A, Suzuki Y, et al. Improvement of the rapid immunoperoxidase staining method for intraoperative pathological diagnosis of pancreatic cancer using microwave irradiation. J Surg Oncol 1989;42:209–14. [DOI] [PubMed] [Google Scholar]
- 14.Tabibzadeh SS, Shah KD. Application of a quick immunoenzymatic labeling as an adjunct to frozen-section diagnosis. Am J Clin Pathol 1989;91:63–6. [DOI] [PubMed] [Google Scholar]
- 15.Richter T, Nahrig J, Komminoth P, et al. Protocol for ultrarapid immunostaining of frozen sections. J Clin Pathol 1999;52:461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]