Abstract
Background/Aims: Merkel cell carcinoma (MCC) is a rare malignant cutaneous neuroendocrine tumour that mostly affects the elderly. It shows rapid progression of the primary tumour, together with a vertical growth pattern into the underlying subcutaneous tissue. Metastatic dissemination to regional lymph nodes is early and frequent. Tenascin-C (Tn-C) is a large extracellular matrix glycoprotein that is expressed in various benign and malignant processes. Expression of Tn-C is also associated with invasion and cellular proliferation, and is often downregulated in fully evolved advanced carcinomas. In previous studies, Tn-C expression correlated with prognosis in tumours of different origin.
Methods: Immunohistochemistry was used to investigate the expression of Tn-C in 25 MCC specimens and to evaluate the prognostic importance of this glycoprotein.
Results: Seventeen samples expressed Tn-C. Staining was mainly seen in the invasion borders and within the connective tissue septae inside the tumours. The expression of Tn-C correlated significantly with large tumour size. There was also frequent expression of Tn-C in primary tumours with metastatic dissemination. Most of the Tn-C negative samples were of small size.
Conclusions: Tn-C expression seems to increase with tumour size and malignant behaviour. Expression was slightly enhanced in tumours with high proliferative indices. Expression is seen mainly in areas of invasive growth and, in this respect, resembles that of other invasive tumours.
Keywords: Merkel cell carcinoma, tenascin-C, tumour size, prognosis, metastasis
Cells are surrounded by extracellular matrix (ECM). Apart from holding cells and organs together, the ECM serves as a mediator of receptor induced interactions between cells. In cell proliferation and morphogenesis, these interactions guide the growth and differentiation of cells.1 Members of non-collagenous ECM proteins include laminins,2 fibronectins,3 and tenascins. Tenascins are synthesised by fibroblasts and are thought to be involved in cell adhesion and motility, supporting cell growth, tissue modelling, and the formation of demarcation lines along tissue lines.4 The tenascin molecule is composed of epidermal growth factor-like repetitions that can bind to the epidermal growth factor receptors of tumour cells. Tenascin-C (Tn-C) regulates angiogenesis in tumours through the regulation of vascular endothelial growth factor.5
Tn-C is a large, modular, hexameric ECM glycoprotein with a unique six armed multidomain macromolecular structure.6 It is the founding member of the tenascin family of ECM proteins. First observed in 1983, Tn-C was named glioma mesenchymal ECM antigen.7 It is expressed transiently during organogenesis,8 and in adult tissues in regions of inflammation, in wound healing, and in neoplasia.9,10 It is absent from or present in small amounts only in fully developed organs.
“Tenascin-C regulates angiogenesis in tumours through the regulation of vascular endothelial growth factor”
In malignancies, increased expression of Tn-C has been shown during the course of tumour development.11 Earlier immunohistochemical and in vitro studies have studied the role of Tn-C in tumour progression and metastatic development.12,13 Tn-C expression correlates with prognosis in cancers of different origin, such as breast, gastric, and brain tumours.14–16 Tn-C tends to accumulate in the invasion border, a staining pattern that correlates with a worse prognosis.17 Both cancer cells and adjacent stromal cells can produce tenascin to coordinate the surrounding microenvironment.18,19 In some tumours, stromal expression of Tn-C correlates with the lack of local tumour invasion, and it may block cancer growth, thus indicating a better prognosis.19,20 However, in other tumours, such as head and neck squamous cell carcinomas, Tn-C does not predict overall survival or disease free survival.21
Merkel cell carcinoma (MCC) is a rare and often aggressive primary neuroendocrine carcinoma of the skin. Its annual incidence ranges from 0.2 to 0.45/100 000 individuals.22 It mostly affects the elderly in sun exposed areas of the body. The diagnosis is based on the characteristic histology and is validated by immunohistochemistry using cytokeratin 20.23 Thyroid transcription factor 1 (TTF-1) antibody is used to exclude small cell lung carcinoma metastasis.24 The natural course of MCC is rapid progression of the primary tumour, together with extension to underlying tissues and frequent metastasis to regional lymph nodes.25 In our previous study, we found that a tumour size larger than 2 cm was a significant prognostic marker for poor survival.26
Although the expression of Tn-C has been studied extensively in various normal and neoplastic tissues, little is known about its expression in neuroendocrine skin carcinomas. The aim of our present study was to investigate the expression of Tn-C in primary MCC and also to establish whether this expression correlates with tumour or patient characteristics.
MATERIALS AND METHODS
Our study comprised 25 patients treated for MCC between 1987 and 2003 at the department of plastic surgery, Helsinki University Central Hospital, and at Vaasa Central Hospital, Finland.
One primary tumour sample was collected from each of the 25 patients (25 samples). The diagnoses were confirmed with immunohistochemical analysis using antibodies to cytokeratin 20 and TTF-1, with TTF-1 being negative in all samples. The greatest surface dimension, tumour size, was measured from haematoxylin and eosin stained slides and documented as < 2 cm or ⩾ 2 cm. None of the patients received preoperative chemotherapy or radiotherapy. The clinical outcome was recorded from the patients’ charts. The local ethics committee approved our study protocol.
Haematoxylin and eosin staining revealed that the typical histology of our MCC samples was that of dermal involvement with repeated extension to subcutaneous tissue. The tumours consisted of small blue cells with sparse cytoplasm; nuclei were medium sized and mitoses were abundant. Three of the tumours did not extend to the underlying subcutaneous tissue and were classified as superficial carcinomas.
The mean follow up time of the patients was 3.2 years (range, seven days to 11 years). Ten of the 25 patients died during follow up. Table 1 details the patient and tumour characteristics.
Table 1.
Patient and tumour characteristics
| Characteristic | |
| Number | 25 |
| Mean age (range) in years | 76.7 (59–100) |
| Sex (M/F) | 10/15 |
| Mean tumour size (range) in cm | 2.5 (0.8–6.5) |
| Tumour size | |
| <2 cm | 11 |
| ⩾2 cm | 14 |
| Location | |
| Head and neck | 11 |
| Trunk | 3 |
| Extremities | 11 |
| Local recurrence | 8 |
| Metastasis | 11 |
| Dead | 10 |
Immunohistochemistry
One 4 μm thick section was cut for each tumour from paraffin wax embedded blocks, dewaxed in xylene, and rehydrated in a series of graded alcohols. The sections were pretreated in trypsin for 30 minutes for antigen retrieval. They were then immersed in 0.6% hydrogen peroxide in methanol for 30 minutes to block endogenous peroxidase activity, and in blocking solution (1.5/100 normal horse serum in phosphate buffered saline) for 15 minutes to block non-specific binding sites. Antihuman tenascin monoclonal antibody (clone DB7; Biohit Diagnostics Oy, Helsinki, Finland) was applied overnight at a dilution of 1/2000 in phosphate buffered saline containing 0.1% sodium azide and 0.5% bovine serum albumin at room temperature. All the sections were treated with biotinylated horse antimouse immunoglobulin (1/200 dilution; Vector Laboratories, Burlingame, California, USA); antibody binding sites were visualised with avidin–biotin–peroxidase complex (Vectastain ABComplex; Vector Laboratories) and 3-amino-9-ethylcarbazole (Sigma Chemical Co, St Louis, Missouri, USA). Counterstaining was performed with Mayer’s haematoxylin. Red/brown extracellular staining was considered positive.
The results were scored by three independent researchers (TB, TJ, and VK) unaware of the patient’s status. The positive controls used throughout the staining procedure were formalin fixed, paraffin wax embedded sections of breast cancer. The negative controls consisted of omitting the primary antibody.
One section of each tumour was analysed, and the staining pattern was recorded. For quantification, the staining area was observed, and staining intensity was scored as low (+), moderate (++), or high (+++).
Statistical analysis
Statistical analysis was performed with NCSS 2000 software. The χ2 test was used to analyse the correlation between clinical data and Tn-C staining. p Values < 0.05 were considered significant.
RESULTS
Seventeen of the 25 samples stained positive for Tn-C. The staining intensity was high in nine, moderate in four, and low in four samples.
Staining was patchy and occurred mainly in tumour borders, especially towards the subcutaneous fat. Apart from lining the vascular structure, Tn-C expression was virtually absent within the tumour tissue. In nine of the tumour samples, the connective tissue septae were stained. Staining tended to be stronger around the septae than at the tumour borders. No cytoplasmic staining was seen within the tumour cells. The staining was continuous, with a flame-like pattern, and had extensions to the surrounding tissues. Samples that contained normal skin showed moderate staining in the dermo–epidermal junction (fig 1A–C).
Figure 1.
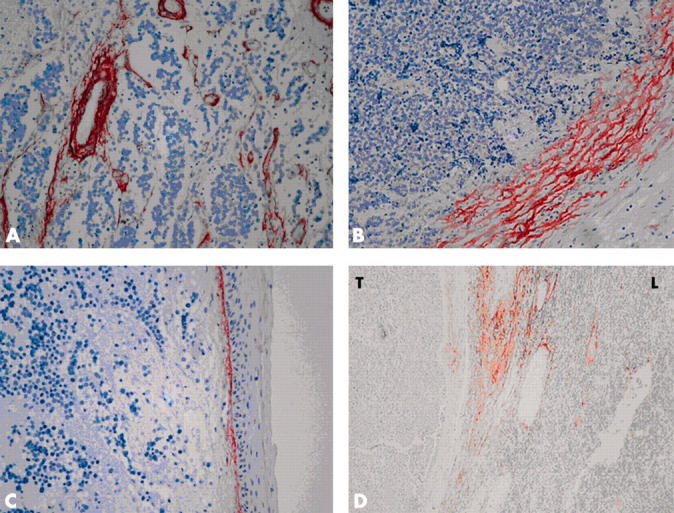
(A) Strong tenascin-C (Tn-C) expression around the vascular structures within the tumour tissue. The tumour cells show no Tn-C expression. (B) Expression of Tn-C in the invasion fronts of the tumour, with flame-like extensions. (C) Moderate Tn-C staining in the dermo–epidermal junction of the normal skin. (D) Expression of Tn-C in metastatic lymph node. Septae show moderate to strong staining intensity, whereas normal lymphatic tissue (L) and metastatic tumour tissue (T) are negative. (A–D) Original magnification, ×200.
Interestingly, six of the eight negative tumour samples were of small size. Their size varied from 1 to 2 cm. Four of the eight negative cases were only 1 cm in diameter.
There was a significant correlation between Tn-C expression and tumour size. A correlation was established with large tumour size (⩾ 2 cm; p = 0.032), but not with age, sex, tumour localisation, invasion, recurrence, or metastasis. We also found enhanced expression of Tn-C in tumours that developed metastases (82%; p = 0.2), although this was not significant.
Table 2 presents the correlations between Tn-C expression, tumour size, and tumours that metastasised.
Table 2.
Correlation between tenascin C (Tn-C) expression, tumour size, and tumours that metastasised
| Size <2 cm | Size ⩾2 cm | Non-metastatic tumours | Metastatic tumours | |
| No. of samples | 11 | 14 | 14 | 11 |
| Tn-C negative | 6 (55%) | 2 (14%) | 6 (43%) | 2 (18%) |
| Tn-C positive | 5 (45%) | 12 (86%) | 8 (57%) | 9 (82%) |
| Staining intensity | ||||
| Low | 1 | 3 | 1 | 4 |
| Moderate | 1 | 3 | 2 | 2 |
| High | 3 | 6 | 5 | 3 |
DISCUSSION
In our series, about two thirds of the MCCs studied expressed Tn-C. Of the 17 positive samples, nine showed strong staining intensity, four showed moderate intensity, and four showed low staining intensity. A significant correlation was found between large tumour size and Tn-C positivity (p = 0.032).
Eight samples in our study did not express Tn-C. Most of them were small tumours, less than 2 cm in diameter. As a result of sample collecting bias, the proportion of negative samples might be even lower. We submitted only one slide for each tumour to immunohistochemical analysis, so that because Tn-C expression was focal, and most of our tumours were ⩾ 2 cm, we may have selected a tumour segment that showed no expression.
Detection of Tn-C does not reveal the malignant potential of an individual tumour directly; rather, it shows the capacity of the tumour to invade and the capacity of the cells to proliferate. Enhanced Tn-C expression has been shown in association with wound healing and in inflammatory processes in proliferating cells in the skin.27,28
We found that Tn-C expression, whether found in the invasion fronts or fibrous septae within the tumour tissue, correlated with larger tumour size. Large tumours are inclined to metastasise, resulting in poor overall survival of patients.26 Here, Tn-C expression was not evident in over half (six of 11) of the small tumours, whereas only two of the 14 large tumours were Tn-C negative.
In our samples, Tn-C was principally expressed at the invasive tumour front and extended into the underlying subcutaneous fat, in agreement with that found in other invasive malignant tumours.
“We noted a relation between tenascin-C expression and metastasis of the primary tumour”
There was no distinct staining pattern in three superficial carcinomas, which showed high, moderate, and low expression of Tn-C, respectively. In nine samples, the expression was found in the fibrous septae, where the tumour was invading the surrounding dermal matrix. Although the stromal pattern of staining has previously been correlated with a better prognosis,19 this was not the case in our study series. We also noted a relation between Tn-C expression and metastasis of the primary tumour. This may be a common staining pattern in neuroendocrine carcinomas, such as small cell lung carcinomas.29
A correlation between cell proliferation, measured by Ki-67, and Tn-C expression has been reported in breast cancer.30 As an aggressive neoplasm, MCC shows high proliferative indices. We have shown pronounced overexpression of cyclin A in primary MCC.31 Cyclin A is a cell cycle enhancer, the immunohistochemical expression of which has been used to detect proliferative cells. Moreover, its expression is a valuable prognostic factor in different types of carcinomas and sarcomas. Although we established overexpression in individual tumours and a slight tendency towards cyclin A accumulation in large tumours and in primary tumours with metastatic potential, this was not significant. Table 3 summarises Tn-C expression in 21 MCC samples with cyclin A expression within the same patient population. Tn-C expression correlated with high cyclin A expression (16 of 21 samples) and negative Tn-C expression with low (< 20%) cyclin A expression (13 of 21 samples).
Table 3.
Correlations between tenascin C (Tn-C) and cyclin A expression in 23 primary Merkel cell carcinomas
| Cyclin A <20% | Cyclin A >20% | |
| Tn-C negative | 5 (63%) | 3 (37%) |
| Tn-C positive | 6 (37%) | 10 (63%) |
Our samples showed high intensity staining around the vascular structures within tumours. Previously, Kim et al have found enhanced immunoreactivity of Tn-C in tumour vessels and even greater expression in high grade tumours.32 Tn-C controls the process of neoangiogenesis by regulating expression of the vascular endothelial growth factor, which activates the endothelial cells in mature blood vessels to sprout new capillaries.5
Take home messages.
In our series of Merkel cell carcinomas, Tn-C expression increased with tumour size, malignant behaviour, and proliferation
Expression was highest in areas of invasive growth, similar to that seen in other invasive tumours
We performed an immunohistochemical study for Tn-C in one lymph node metastasis of MCC. In this sample, staining intensity was moderate to high and was seen only in the septae (fig 1D). Of interest was the fact that expression of Tn-C was low in the primary tumour. Our samples containing normal skin showed moderate Tn-C expression in the dermo–epidermal junction. This finding is in line with the results of Schalkwijk et al.33
In conclusion, the expression of Tn-C in this series of MCC samples appeared to increase with tumour size, malignant behaviour, and proliferation. Expression was highest in areas of invasive growth and, in this respect, resembles that of other invasive tumours.
Abbreviations
ECM, extracellular matrix
MCC, Merkel cell carcinoma
Tn-C, tenascin-C
TTF-1, thyroid transcription factor 1
REFERENCES
- 1.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development 1993;117:1183–98. [DOI] [PubMed] [Google Scholar]
- 2.Brown JC, Timpl R. The collagen superfamily. Int Arch Allergy Immunol 1995;107:484–90. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Fibronectins. Sci Am 1986;254:42–51. [DOI] [PubMed] [Google Scholar]
- 4.Inaguma Y, Kusakabe M, Mackie EJ, et al. Epithelial induction of stromal tenascin in the mouse mammary gland: from embryogenesis to carcinogenesis. Dev Biol 1988;128:245–55. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Hiraiwa N, Hashimoto H, et al. Tenascin-C regulates angiogenesis in tumors through the regulation of vascular endothelial growth factor expression. Int J Cancer 2004;108:31–40. [DOI] [PubMed] [Google Scholar]
- 6.Schenk S, Chiquet-Ehrismann R. Tenascins. Methods Enzymol 1994;245:52–61. [DOI] [PubMed] [Google Scholar]
- 7.Bourdon MA, Wikstrand CJ, Furthmayr H, et al. Human glioma–mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res 1983;43:2796–805. [PubMed] [Google Scholar]
- 8.Erickson HP, Bourdon MA. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol 1989;5:71–92. [DOI] [PubMed] [Google Scholar]
- 9.Latijnhouwers MA, Pfundt R, de Jongh GJ, et al. Tenascin-C expression in human epidermal keratinocytes is regulated by inflammatory cytokines and a stress response pathway. Matrix Biol 1998;17:305–16. [DOI] [PubMed] [Google Scholar]
- 10.Natali PG, Nicotra MR, Bigotti A, et al. Comparative analysis of the expression of the extracellular matrix protein tenascin in normal human fetal, adult and tumor tissues. Int J Cancer 1991;47:811–16. [DOI] [PubMed] [Google Scholar]
- 11.Pilch H, Schaffer U, Schlenger K, et al. Expression of tenascin in human cervical cancer—association of tenascin expression with clinicopathological parameters. Gynecol Oncol 1999;73:415–21. [DOI] [PubMed] [Google Scholar]
- 12.Goepel C, Stoerer S, Koelbl H. Tenascin in preinvasive lesions of the vulva and vulvar cancer. Anticancer Res 2003;23:4587–91. [PubMed] [Google Scholar]
- 13.Kilic T, Bayri Y, Ozduman K, et al. Tenascin in meningioma: expression is correlated with anaplasia, vascular endothelial growth factor expression, and peritumoral edema but not with tumor border shape. Neurosurgery 2002;51:183–92 discussion 192–3. [DOI] [PubMed] [Google Scholar]
- 14.Herold-Mende C, Mueller MM, Bonsanto MM, et al. Clinical impact and functional aspects of tenascin-C expression during glioma progression. Int J Cancer 2002;98:362–9. [DOI] [PubMed] [Google Scholar]
- 15.Jahkola T, Toivonen T, von Smitten K, et al. Expression of tenascin in invasion border of early breast cancer correlates with higher risk of distant metastasis. Int J Cancer 1996;69:445–7. [DOI] [PubMed] [Google Scholar]
- 16.Wiksten JP, Lundin J, Nordling S, et al. Tenascin-C expression correlates with prognosis in gastric cancer. Oncology 2003;64:245–50. [DOI] [PubMed] [Google Scholar]
- 17.Jahkola T, Toivonen T, Virtanen I, et al. Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer 1998;78:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara A, Yoshida T, Tamaki H, et al. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res 1995;1:1035–41. [PubMed] [Google Scholar]
- 19.Sakakura T, Kusakabe M. Can tenascin be redundant in cancer development? Perspect Dev Neurobiol 1994;2:111–16. [PubMed] [Google Scholar]
- 20.Sugawara I, Hirakoshi J, Masunaga A, et al. Reduced tenascin expression in colonic carcinoma with lymphogenous metastasis. Invasion Metastasis 1991;11:325–31. [PubMed] [Google Scholar]
- 21.Atula T, Hedstrom J, Finne P, et al. Tenascin-C expression and its prognostic significance in oral and pharyngeal squamous cell carcinoma. Anticancer Res 2003;23:3051–6. [PubMed] [Google Scholar]
- 22.Chuang TY, Su WP, Muller SA. Incidence of cutaneous T cell lymphoma and other rare skin cancers in a defined population. J Am Acad Dermatol 1990;23 (2 Pt 1) :254–6. [DOI] [PubMed] [Google Scholar]
- 23.Scott MP, Helm KF. Cytokeratin 20: a marker for diagnosing Merkel cell carcinoma. Am J Dermatopathol 1999;21:16–20. [DOI] [PubMed] [Google Scholar]
- 24.Byrd-Gloster AL, Khoor A, Glass LF, et al. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol 2000;31:58–62. [DOI] [PubMed] [Google Scholar]
- 25.Boyle F, Pendlebury S, Bell D. Further insights into the natural history and management of primary cutaneous neuroendocrine (Merkel cell) carcinoma. Int J Radiat Oncol Biol Phys 1995;31:315–23. [DOI] [PubMed] [Google Scholar]
- 26.Koljonen V, Bohling T, Granhroth G, et al. Merkel cell carcinoma: a clinicopathological study of 34 patients. Eur J Surg Oncol 2003;29:607–10. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz-Rey JA, Suarez-Penaranda JM, Da Silva EA, et al. Immunohistochemical detection of fibronectin and tenascin in incised human skin injuries. Forensic Sci Int 2002;126:118–22. [DOI] [PubMed] [Google Scholar]
- 28.Schalkwijk J, Steijlen PM, van Vlijmen-Willems IM, et al. Tenascin expression in human dermis is related to epidermal proliferation. Am J Pathol 1991;139:1143–50. [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med 1999;5:662–8. [DOI] [PubMed] [Google Scholar]
- 30.Jahkola T, Toivonen T, Nordling S, et al. Expression of tenascin-C in intraductal carcinoma of human breast: relationship to invasion. Eur J Cancer 1998;34:1687–92. [DOI] [PubMed] [Google Scholar]
- 31.Koljonen V, Tukiainen E, Haglund C, et al. Expression of cyclin A in Merkel cell carcinoma. APMIS 2004;112:39–44. [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Bak KH, Kim YS, et al. Expression of tenascin-C in astrocytic tumors: its relevance to proliferation and angiogenesis. Surg Neurol 2000;54:235–40. [DOI] [PubMed] [Google Scholar]
- 33.Schalkwijk J, Van Vlijmen I, Oosterling B, et al. Tenascin expression in hyperproliferative skin diseases. Br J Dermatol 1991;124:13–20. [DOI] [PubMed] [Google Scholar]


