Abstract
Background: Dominant negative inhibition of nuclear factor κB (NFκB) signalling activity in a human osteosarcoma cell line (Saos2) results in malignant reversion and the induction of the osteoblast differentiating transcription factor, Runx2/Cbfa1. This observation suggests that there is an inverse relation between a transcription factor associated with malignant progression and chemoresistance (NFκB) and an osteoblast differentiating transcription factor (Runx2/Cbfa1).
Aims: To assess and correlate Runx2/Cbfa1 and NFκB (p65) immunoreactivity in human osteosarcoma.
Methods: Runx2/Cbfa1 and NFκB (p65) immunoreactivity was assessed on 11 paraffin wax embedded archival specimens of human primary osteosarcoma by standard immunohistochemical methods and scored on a scale of 0–3. A Pearson correlation analysis between Runx2/Cbfa1 and NFκB (p65) scores was established.
Results: Runx2/Cbfa1 was expressed constitutively in all pathology specimens of human osteosarcoma. Of note, a chondroblastic osteosarcoma showed the highest Runx2/Cbfa1 immunoreactivity. A Pearson correlation did not support an inverse correlation between Runx2/Cbfa1 and NFκB (p65) scores (r = 0.57) in human osteosarcoma.
Conclusion: Runx2/Cbfa1 immunoreactivity does not inversely correlate with NFκB immunoreactivity, and thus cannot serve as an indirect measure of NFκB activity or an independent predictive or prognostic indicator.
Keywords: differentiation, nuclear factor κB, osteosarcoma, Runx2/Cbfa1, transformation
Osteosarcoma is the most common primary bone malignancy, with a propensity for pulmonary metastasis and a predilection for paediatric and geriatric age groups. The cornerstone of current clinical management involves high dose neoadjuvant chemotherapy, to which 40–60% of cases are non-responsive. Thus, prognosticating, stratifying, and individualising treatment is crucial for optimal and cost effective management.1
“Although the NFκB activation status of a tumour has predictive and prognostic value, it cannot be measured by routine laboratory investigations”
Histopathologically, osteosarcomas are characterised by atypical cells (osteoblasts, mesenchymal cells, fibroblasts, and chondrocytes) enmeshed in a disorganised bony matrix (immature osteoid). We recently showed that dominant negative inhibition of the promalignant transcription factor, nuclear factor κB (NFκB), in a human osteosarcoma cell line (Saos2) results in malignant reversion and the induction of the osteoblast differentiating transcription factor, Runx2/Cbfa1.2,3 This finding was interesting for two reasons:
It validated the concept that cellular differentiation and transformation are mutually exclusive processes.
It suggested an inverse relation between a transcription factor associated with malignant progression4 and chemoresistance (NFκB)5 and an osteoblast differentiating transcription factor (Runx2/Cbfa1).
Although the NFκB activation status of a tumour has predictive and prognostic value, it cannot be measured by routine laboratory investigations. Our present study was designed to assess whether Runx2/Cbfa1 expression could serve as an indirect measure of NFκB activation status and potential resistance/responsiveness to chemotherapy.
MATERIALS AND METHODS
Thirty five paraffin wax embedded archival specimens of primary osteosarcoma, diagnosed and treated at the University of Rochester Medical Center between 1980 and 2002, were identified from the surgical pathology database. Corresponding clinical information was obtained from medical records. All specimens that were surgical resections of the tumour before neoadjuvant chemotherapy were identified and enrolled in our study. To ascertain sample immunoreactivity, specimens were stained with anti-collagen I antibody (Santa Cruz Biotechnology; Santa Cruz, California, USA) by indirect immunohistochemistry with avidin–biotin peroxidase, as described previously.6 Eleven specimens were found to be immunoreactive and subsequently stained with Runx2/Cbfa1 and NFκB (p65) specific antibodies (Santa Cruz). Immunoreactive specimens were reviewed and graded by an orthopaedic oncologist (RNR) using a previously established grading system.6 A Pearson correlation analysis between Runx2/Cbfa1 and NFκB (p65) scores was established.
RESULTS
Although immunoreactivity is not a quantitative measure, we have previously demonstrated significant correlations between tumour subtypes and the immunoreactivity of specific proteins in sample groups as small as six, using the same immunohistochemical staining and analyses techniques.6 Our results show that Runx2/Cbfa1 is expressed constitutively in all 11 pathology specimens of human osteosarcoma. Of note, a chondroblastic osteosarcoma showed the highest Runx2/Cbfa1 immunoreactivity (fig 1). A Pearson correlation analysis did not establish an inverse correlation between Runx2/Cbfa1 and NFκB (p65) scores (r = 0.57).
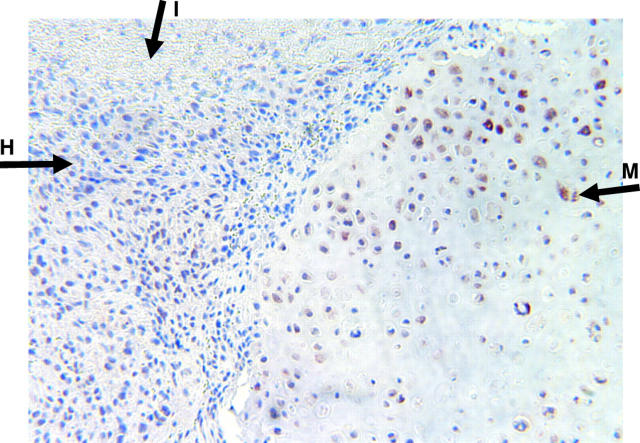
Figure 1.
Photomicrograph (original magnification, ×100) of a high grade chondroblastic osteosarcoma stained with anti-Runx2/Cbfa1 antibody (brown staining; 3+ score). Note the hyperchromatic neoplastic mesenchymal cells typical of high grade osteosarcoma (H) and the mature hypertrophic chondrocytes in cartilaginous matrix (M). I, immature osteoid. The implication of Runx2/Cbfa1 in late chondrocyte differentiation and maturation concurs with its high expression in chondroblastic variants of osteosarcoma at the bone–cartilage interface.
DISCUSSION
Runx2/Cbfa1 is a central regulator of skeletal development. In this respect, Runx2/Cbfa1 coordinately induces cell cycle arrest and bone specific gene expression, leading to osteoblast and chondrocyte differentiation and maturation.7,8 The genotypic, phenotypic, and morphological changes associated with Runx2/Cbfa1 gene manipulations in vivo reveal a distinct temporal relation between osteoblast and chondrocyte differentiation. Whereas Runx2/Cbfa1 is implicated in early osteoblast differentiation, it plays a role in the later stages of chondrocyte differentiation, maturation, and possibly, endochondral ossification.8 It follows that although Runx2/Cbfa1 expression is essential for the commitment of preosteoblasts to the osteoblast lineage, additional signals/factors are required to attain full osteoblast differentiation and maturation. The cooperative interactions between Runx2/Cbfa1 and the retinoblastoma (Rb) tumour suppressor protein is a case in point, which reconciles with the molecular pathogenesis of osteosarcoma.9 In an Rb−/− context, such as in 60% of human osteosarcomas, preosteoblasts (committed osteoblasts expressing Runx2/Cbfa1) do not exit the cell cycle, mature, and elaborate organised osteoid. We previously demonstrated that NFκB is involved in the decision matrix that sanctions osteoblast proliferation/transformation and differentiation, given the inverse modulation of Runx2/Cbfa1 expression and responsiveness in a human osteosarcoma cell line.2 Although this inverse relation was not verified by our clinicopathological study, its validity cannot be ruled out. There is indeed compelling evidence that only a subset of cells within a tumour, so called “cancer stem cells”, are tumorigenic and possess the metastatic phenotype.10,11 Thus, it is conceivable that the inverse correlation between Runx2/Cbfa1 and NFκB is only found in the “cancer stem cells”, which represent a small subset of the tumour mass.
Take home messages.
Runx2/Cbfa1 was expressed constitutively in all pathology specimens of human osteosarcoma and was not inverse correlated with NFκB (p65) expression
Thus, Runx2/Cbfa1 immunoreactivity in human osteosarcoma cannot serve as an indirect measure of NFκB activation status, or an independent predictor of resistance/responsiveness to chemotherapy
Of note, however, a chondroblastic osteosarcoma showed the highest Runx2/Cbfa1 immunoreactivity
“Although Runx2/Cbfa1 expression is essential for the commitment of preosteoblasts to the osteoblast lineage, additional signals/factors are required to attain full osteoblast differentiation and maturation”
Thus, we conclude that Runx2/Cbfa1 immunoreactivity in human osteosarcomas cannot serve as an indirect measure of NFκB activation status, or an independent predictor of resistance/responsiveness to chemotherapy.
Table 1.
Runx2/Cbfa1 and NFκB (p65) immunoreactivity scores in human osteosarcoma
| Pathology report | Runx2/Cbfa1 | NFκB (p65) |
| High grade osteosarcoma (pleomorphic) | 2+ | 3+ |
| Chondroblastic osteosarcoma | 3+ | 3+ |
| Low grade parosteal osteosarcoma | 2+ | 3+ |
| Low grade chondroblastic osteosarcoma | 2+ | 3+ |
| High grade osteosarcoma | 1+ | 3+ |
| Chondroblastic osteosarcoma | 2+ | 2+ |
| High grade osteosarcoma | 2+ | 2+ |
| Osteosarcoma, fibroblastic variant | 1+ | 2+ |
| High grade osteosarcoma (pleomorphic) | 2+ | 3+ |
| High grade osteosarcoma (pleomorphic) | 2+ | 3+ |
| High grade osteosarcoma | 2+ | 3+ |
Acknowledgments
VBA is supported by the Wilmot Foundation of the JP Wilmot Cancer Center. The authors acknowledge the excellent technical assistance of B Stroyer and L Gehan.
Abbreviations
NFκB, nuclear factor κB
Rb, retinoblastoma protein
REFERENCES
- 1.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol 1994;12:423–31. [DOI] [PubMed] [Google Scholar]
- 2.Andela VB, Sheu TJ, Puzas EJ, et al. Malignant reversion of a human osteosarcoma cell line, Saos-2, by inhibition of NFkappaB. Biochem Biophys Res Commun 2002;297:237–41. [DOI] [PubMed] [Google Scholar]
- 3.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997;89:747–54. [DOI] [PubMed] [Google Scholar]
- 4.Andela VB, Schwarz EM, Puzas JE, et al. Tumor metastasis and the reciprocal regulation of pro-metastatic and anti-metastatic factors by nuclear factor kappaB. Cancer Res 2000;60:6557–62. [PubMed] [Google Scholar]
- 5.Wang CY, Cusack JC Jr, Liu R, et al. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 1999;5:412–17. [DOI] [PubMed] [Google Scholar]
- 6.Rosier RN, O’Keefe RJ, Teot LA, et al. P-glycoprotein expression in cartilaginous tumors. Surg Oncol 1997;65:95–105. [DOI] [PubMed] [Google Scholar]
- 7.Stricker S, Fundele R, Vortkamp A, et al. Role of Runx genes in chondrocyte differentiation. Dev Biol 2002;245:95–108. [DOI] [PubMed] [Google Scholar]
- 8.Pratap J, Galindo M, Zaidi SK, et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 2003;63:5357–62. [PubMed] [Google Scholar]
- 9.Thomas DM, Carty SA, Piscopo DM, et al. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell 2001;8:303–16. [DOI] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–11. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Becker MW, Wicha M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 2004;14:43–7. [DOI] [PubMed] [Google Scholar]