Abstract
Background: Hepatocellular carcinoma (HCC) is one of the most prevalent fatal cancers in the world. Despite advances in early diagnosis and improvements in surgical techniques, the survival of patients with HCC even after resection is poor because of the high incidence of recurrences. Therefore, the identification of prognostic factors may be helpful in the development of new treatment protocols.
Aims: To investigate HER-2/neu status in HCC by immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH), and to explore the possibility of using trastuzumab in the treatment of HCC.
Methods: Eight hundred and sixty eight surgical samples from patients with primary HCC were examined for their HER-2/neu status. IHC for HER-2/neu was performed with the HercepTest kit; FISH analysis was performed with the PathVysion HER-2 DNA probe kit. The correlations between HER-2/neu overexpression and clinicopathological characteristics were analysed statistically.
Results: HER-2/neu overexpression was detected in 21 (2.42%) of the 868 primary HCCs. Only one specimen showed HER-2/neu gene amplification by FISH. No significant associations were found between HER-2/neu overexpression and the clinicopathological parameters.
Conclusions: There is a low frequency of HER-2/neu overexpression/amplification in HCC. There appears to be no role for HER-2/neu as a prognostic marker and no benefit of anti-HER-2/neu trastuzumab treatment in patients with HCC.
Keywords: HER-2/neu, hepatocellular carcinoma, fluorescence in situ hybridisation, immunohistochemistry
Hepatocellular carcinoma (HCC) is one of the most frequent human cancers worldwide, and has been the second most lethal cancer in China since the 1990s.1,2 Many cases remain inoperable because the disease presents at an advanced stage. Despite improvements in surgical techniques, most patients with advanced disease die within a relatively short period of time.
“One of the molecular factors implicated in many neoplasms is the HER-2/neu protooncogene, which encodes a growth factor receptor of the tyrosine kinase receptor family”
Recently, some newer molecular factors such as tumour proliferative indices, nuclear DNA ploidy, and expression of genes, growth factors, and hormone receptors have been used to predict clinical outcome in patients with HCC.3 One of the molecular factors implicated in many neoplasms is the HER-2/neu protooncogene, which encodes a growth factor receptor of the tyrosine kinase receptor family. Its overexpression has been established in breast, ovarian, endometrial, and pancreatic carcinomas, and has been shown to correlate with aggressive tumour behaviour, advanced stages of disease, and a worse outcome.4–8 A humanised form of anti-HER-2/neu antibody, trastuzumab, is important in the treatment of breast carcinomas expressing high levels of HER-2/neu.9–11 However, the role of HER-2/neu overexpression/amplification in HCC remains unclear.12–17 Our study examined HER-2/neu overexpression/amplification in HCC and explored the possibility of using trastuzumab in the treatment of HCC.
MATERIALS AND METHODS
Patients and samples
Eight hundred and sixty eight liver cancer samples were obtained from surgical resections from eastern hepatobiliary surgery hospital, Second Military Medical University, Shanghai, China. No patients had received prior treatment. Standard 5 μm tissue sections were cut and stained with haematoxylin and eosin and Alcian-periodic acid Schiff and examined by light microscopy. Histopathological diagnosis was carried out according to the World Health Organisation histological classification of tumours of the liver and intrahepatic bile ducts (2000).18 Each slide was independently evaluated by two pathologists unaware of the clinicopathological characteristics of the tumour. The samples were from 735 men and 133 women. The age ranged from 25–76 years (median, 50.3). Five hundred and thirty five of the 868 patients had serum α fetoprotein concentrations ⩾ 20 μg/litre. Hepatitis B surface antigen was positive in 610 patients. Forty eight tumours were small (< 3 cm) and 820 were advanced (⩾ 3 cm). Twenty six HCCs were well differentiated, 798 were moderately differentiated, and 44 were poorly differentiated. Of the 868 patients, 844 had evidence of intrahepatic metastasis (portal vein invasion and/or intrahepatic dissemination). In 717 HCC samples liver cirrhosis was also detected.
Immunohistochemistry
The tissues had been fixed in 10% neutral buffered formalin for 12 hours and routinely processed. Paraffin wax embedded tissue blocks were cut into 4 μm thick sections, which were placed in antigen retrieval citrate buffer (pH 6.8), then brought up to the boil in a microwave. The Dako HercepTest (Dako, Carpinteria, California, USA) was performed according to the manufacturer’s instructions. The evaluation of HER-2/neu immunoreactivity was performed according to the Dako protocol for the HerceptTest, with minor modifications. Only membrane staining was considered positive, whereas cytoplasmic staining was considered non-specific. In each case, the intensity (weak, moderate, or strong) and pattern (incomplete or complete) of membrane staining and percentage of neoplastic immunoreactive cells (cutoff at 10%) were evaluated. Tumours were scored as follows: 0, no appreciable staining or staining in < 10% neoplastic cells; 1+, tumours with faint/barely appreciable incomplete membranous staining in > 10% neoplastic cells; 2+, tumours with weak to moderate complete membrane staining or containing > 10% neoplastic cells with moderate incomplete basolateral membrane immunostaining; 3+, tumours with strong immunoreactivity of the entire membrane in > 10% neoplastic cells or containing > 10% neoplastic cells with strong basolateral incomplete membrane immunoreactivity. Tumours classified as 0 or 1+ were considered negative, and those scored as 2+ or 3+ were classified as positive for HER-2/neu overexpression.19,20 For each batch, a breast carcinoma with an amplified HER-2/neu gene and strong immunoreactivity for HER-2/neu was used as a positive control.
Fluorescence in situ hybridisation
HER-2/neu gene amplification was assessed by fluorescence in situ hybridisation using the PathVysion HER-2 DNA probe kit and paraffin wax pretreatment kit (Vysis Inc, Downers Grove, Illinois, USA), according to the procedure specified by the manufacturer. Briefly, formalin fixed, paraffin wax embedded tissues were cut into 4 μm thick sections and dewaxed. Subsequently, the sections were subjected to pretreatment including protease digestion for 50 minutes. The slides were then denatured at 72°C (± 1°C) for five minutes. After a buffer wash, the sections were hybridised with prewarmed probe—the HER-2/neu specific sequence probe (LSI HER-2/neu SpectrumOrange) and a probe for the α satellite sequence on chromosome 17 (CEP 17 SpectrumGreen) overnight at 37°C. The slides were then washed with posthybridisation wash buffer at 72°C and counterstained with DAPI (4′,6-diamidino-2-phenylindole). The slides were coverslipped using mounting medium and stored at −20°C in the dark before signal enumeration with an Olympus BX60 microscope using ×100 magnification (oil immersion) and appropriate multiband pass filters. Appropriate positive (HER-2/neu gene amplification) and negative (non-amplification) control slides were included in the staining run. Analysis of the fluorescence in situ hybridisation score was carried out by comparing the ratio of the average copy number of the HER-2/neu gene with that of the chromosome 17 centromere in 60 nuclei/case. Specimens with a signal ratio of more than 2 were scored as positive (HER-2/neu amplified).21–25
Statistical analysis
Statistical analysis was performed using an IBM computer with SPSS.11 software. The differences between the frequency of HER-2/neu and clinicopathological features were analysed using the χ2 test. Significance was defined as p < 0.05 and high significance as p < 0.01.
RESULTS
Frequency of HER-2/neu overexpression/amplification
Table 1 summarises the immunohistochemistry results. Overall, HER-2/neu immunolabelling was present in 62 of 868 cases (7.14%). Forty one cases showed faint perceptible membrane staining in more than 10% of the neoplastic cells (fig 1A). Strong focal or weak diffuse labelling of more than 10% of the neoplastic cells was present in 20 cases (fig 1B). One case showed strong diffuse labelling in more than 70% of the cells (fig 1C). In the 806 remaining cases, there was no HER-2/neu labelling. Adjacent benign liver parenchyma was also uniformly negative. All positive controls stained appropriately.
Table 1.
Clinicopathological features of HER-2/neu labelling in HCC
| Parameter | HER-2/neu labelling | |||
| 0 (n = 806) | 1+ (n = 41) | 2+ (n = 20) | 3+ (n = 1) | |
| Age | ||||
| Less than median | 394 | 19 | 10 | 1 |
| Greater than median | 412 | 22 | 10 | 0 |
| Sex | ||||
| Male | 682 | 35 | 18 | 0 |
| Female | 124 | 6 | 2 | 1 |
| HBsAg | ||||
| Positive | 557 | 37 | 15 | 1 |
| Negative | 249 | 4 | 5 | 0 |
| AFP (μg/litre) | ||||
| <20 | 309 | 13 | 10 | 1 |
| ⩾20 | 497 | 28 | 10 | 0 |
| Liver cirrhosis | ||||
| Present | 673 | 27 | 17 | 0 |
| Absent | 133 | 14 | 3 | 1 |
| Tumour size | ||||
| <3 cm | 49 | 7 | 2 | 0 |
| ⩾3 cm | 757 | 34 | 18 | 1 |
| Histological grade | ||||
| Well differentiated | 20 | 4 | 1 | 1 |
| Moderately differentiated | 745 | 35 | 18 | 0 |
| Poorly differentiated | 41 | 2 | 1 | 0 |
| Tumour capsule | ||||
| Absent or not intact | 619 | 36 | 13 | 0 |
| Intact | 187 | 5 | 7 | 1 |
| Intrahepatic metastasis | ||||
| Not present | 20 | 3 | 1 | 0 |
| Present | 786 | 38 | 19 | 1 |
AFP, α fetoprotein; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma.
Figure 1.
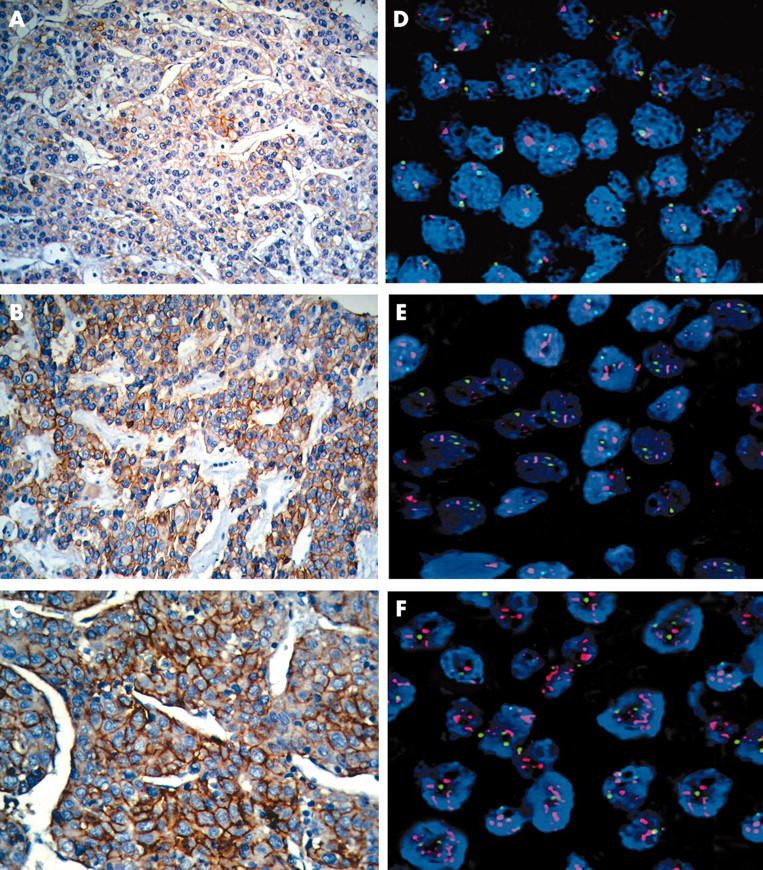
(A–C) Membrane staining in neoplastic cells with the anti-HER-2/neu antibody. (D–F) Fluorescence in situ hybridisation results. Red and green signals represent HER-2/neu and chromosome 17 hybridisations, respectively, and the blue DAPI stain demonstrates the nuclei.
Cases that scored 3+ and 2+ were also analysed by FISH. HER-2/neu gene amplification was identified in only one case. It had a Pathvysion ratio of 2.45 signals/nucleus (fig 1F). This sample had scored 3+ on IHC (fig 1C).
Association between HER-2/neu overexpression and clinicopathological characteristics
To determine whether HER-2/neu expression was associated with clinicopathological features and to reveal its biological role in HCC development and/or progression, we compared HER-2/neu expression with clinicopathological findings, including hepatitis B virus infection, serum α fetoprotein concentration, tumour size, cirrhosis, histological grade, tumour capsule integrity, and tumour intrahepatic metastasis. No significant association was found between HER-2/neu expression and clinicopathological findings (table 1).
DISCUSSION
The HER-2/neu (also known as c-erbB-2) oncogene is activated in breast cancer, correlates with neoplastic transformation and progression, and is linked to shortened patient survival and chemoresistance. The c-erbB2 gene encodes a transmembrane tyrosine kinase with 40% sequence homology to the epidermal growth factor receptor. A physiological ligand for this presumed receptor has not been identified. HER-2/neu overexpression in breast cancer is associated with other poor prognostic factors, including advanced stage, poor histological differentiation, and resistance to drug treatment.26–29
“Searching for other overexpressed proteins in hepatocellular carcinoma might lead to the discovery of new targets that could be used in specific treatments for this disease”
Previous studies of HER-2/neu expression in HCC have yielded discrepant results. In a few studies, the reported immunoreactivity with the anti-c-erbB2 antibody has ranged from 8% to 29.5%.12–14 Nakopoulou et al found HER-2/neu expression in 21 of 71 (29.6%) HCC tissues analysed by immunohistochemical staining.14 Heinze et al analysed the cytosolic HER-2/neu content and reported that increased HER-2/neu content was associated with a poor prognosis.15 Vlasoff et al found that HER-2/neu was not overexpressed or amplified in HCC, hepatocellular adenoma, or normal liver.16 Hsu et al also found that the only patient whose HCC tissue showed 1+ HER-2/neu expression was a 57 year old man who presented with an extremely large hepatic tumour and multiple lung metastases at diagnosis.17 Our study used a new visualisation technique and standardised scoring criteria that are used in the Federal Drug Administration approved HercepTest to avoid these problems. Our findings indicate that HER-2/neu overexpression occurs in 2.42% of patients with HCC, whereas HER-2/neu gene amplification was identified in only one tumour. These findings indicate that HER-2/neu overexpression/amplification is not an important prognostic factor for patients with advanced or metastatic HCC.
Take home messages.
The HER-2/neu oncogene, which is overexpressed in many cancers, is overexpressed/amplified only at a low frequency in hepatocellular carcinoma (HCC)
HER-2/neu has no role as a prognostic marker in HCC
There would be no benefit of anti-HER-2/neu trastuzumab treatment in patients with HCC
Despite advances in early diagnosis and improvements in surgical techniques, the survival of patients with HCC even after resection is still poor because of the high incidence of recurrences. New treatments are needed to improve the poor prognosis of this disease. Trastuzumab is a recombinant DNA derived humanised monoclonal antibody that selectively binds to the extracellular domain of HER-2/neu. Trastuzumab is a potential therapeutic agent for patients with breast cancer showing HER-2/neu overexpression and gene amplification.27–29 However, our results indicate that it is unlikely that patients with HCC would benefit from this treatment.
In conclusion, we found overexpression of HER-2/neu in 2.42% of HCCs. This result suggests that there is little indication for using HER-2/neu as a prognostic marker or for the use of anti-HER-2/neu trastuzumab treatment in most patients with HCC. Searching for other overexpressed proteins in HCC might lead to the discovery of new targets that could be used in specific treatments for this disease.
Acknowledgments
This study was supported by the National Natural Science Foundation of China, No.30370645 and the Hundred Leading Scientists Program of the Public Health Sector of Shanghai, No.98BR007.
Abbreviations
HCC, hepatocellular carcinoma
REFERENCES
- 1.Wu MC, Shen F. Progress in research of liver surgery in China. World J Gastroenterol 2000;6:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang ZY. Treatment of hepatocellular carcinoma. Digestion 1998;59:556–62. [DOI] [PubMed] [Google Scholar]
- 3.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol 2002;8:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaziji H, Gown AM. Accuracy and precision in HER2/neu testing in breast cancer: are we there yet? Hum Pathol 2004;35:143–6. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Norton L, Albanell J, et al. Recombinant humanized anti-HER-2 antibody (Herceptin) enhances the antitumour activity of Paclitaxel and Doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998;58:2825–31. [PubMed] [Google Scholar]
- 6.Pietras RJ, Fendly BM, Chazin VR, et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 1994;9:1829–38. [PubMed] [Google Scholar]
- 7.Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 2003;8:307–25. [DOI] [PubMed] [Google Scholar]
- 8.Schlieman MG, Fahy BN, Ramsamooj R, et al. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer 2003;89:2110–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther 1999;21:309–18. [DOI] [PubMed] [Google Scholar]
- 10.Bunn PA Jr, Helfrich B, Soriano AF, et al. Expression of Her-2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res 2001;7:3239–50. [PubMed] [Google Scholar]
- 11.Menard S, Pupa SM, Campiglio M, et al. Biologic and therapeutic role of HER2 in cancer. Oncogene 2003;22:6570–8. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Takeda T, Sakon M, et al. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer 2001;84:1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier JD, Guo K, Mathew J, et al. c-erbB-2 oncogene expression in hepatocellular carcinoma and cholangiocarcinoma. J Hepatol 1992;14:377–80. [DOI] [PubMed] [Google Scholar]
- 14.Nakopoulou L, Stefanaki K, Filaktopoulos D, et al. C-erb-B-2 oncoprotein and epidermal growth factor receptor in human hepatocellular carcinoma: an immunohistochemical study. Histol Histopathol 1994;9:677–82. [PubMed] [Google Scholar]
- 15.Heinze T, Jonas S, Karsten A, et al. Determination of the oncogenes p53 and C-erb B2 in the tumour cytosols of advanced hepatocellular carcinoma (HCC) and correlation to survival time. Anticancer Res 1999;19:2501–3. [PubMed] [Google Scholar]
- 16.Vlasoff DM, Baschinsky DY, De Young BR, et al. C-erb B2 (Her2/neu) is neither overexpressed nor amplified in hepatic neoplasms. Appl Immunohistochem Mol Morphol 2002;10:237–41. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C, Huang CL, Hsu HC, et al. HER-2/neu overexpression is rare in hepatocellular carcinoma and not predictive of anti-HER-2/neu regulation of cell growth and chemosensitivity. Cancer 2002;94:415–20. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press, 2000.
- 19.Rhodes A, Jasani B, Anderson E, et al. Evaluation of HER-2/neu immunohistochemical assay sensitivity and scoring on formalin-fixed and paraffin-processed cell lines and breast tumors: a comparative study involving results from laboratories in 21 countries. Am J Clin Pathol 2002;118:408–17. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs TW, Gown AM, Yaziji H, et al. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol 1999;17:1983–7. [DOI] [PubMed] [Google Scholar]
- 21.Ellis IO, Bartlett J, Dowsett M, et al. Best Practice No 176. Updated recommendations for HER2 testing in the UK. J Clin Pathol 2004;57:233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Saboorian MH, Frenkel E, et al. Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays. J Clin Pathol 2000;53:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay E, O’Grady A, Morgan JM, et al. Use of tissue microarray for interlaboratory validation of HER2 immunocytochemical and FISH testing. J Clin Pathol 2004;57:1140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhargava R, Lal P, Chen B. Feasibility of using tissue microarrays for the assessment of HER-2 gene amplification by fluorescence in situ hybridization in breast carcinoma. Diagn Mol Pathol 2004;13:213–16. [DOI] [PubMed] [Google Scholar]
- 25.Hsi ED, Tubbs RR. Guidelines for HER2 testing in the UK. J Clin Pathol 2004;57:241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 2004;10:1706–16. [DOI] [PubMed] [Google Scholar]
- 27.Nahta R, Trent S, Yang C, et al. Epidermal growth factor receptor expression is a candidate target of the synergistic combination of trastuzumab and flavopiridol in breast cancer. Cancer Res 2003;63:3626–31. [PubMed] [Google Scholar]
- 28.Nahta R, Takahashi T, Ueno NT, et al. P27(kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res 2004;64:3981–6. [DOI] [PubMed] [Google Scholar]
- 29.Warburton C, Dragowska WH, Gelmon K, et al. Treatment of HER-2/neu overexpressing breast cancer xenograft models with trastuzumab (Herceptin) and gefitinib (ZD1839): drug combination effects on tumor growth, HER-2/neu and epidermal growth factor receptor expression, and viable hypoxic cell fraction. Clin Cancer Res 2004;10:2512–24. [DOI] [PubMed] [Google Scholar]


