Abstract
Aims: To examine the prognostic relevance of the expression of the Bcl-2, Bcl-xL, and Bax proteins in stage IB squamous cervical carcinoma (SCC).
Methods: In total, 220 patients who underwent radical hysterectomy and bilateral lymphadenectomy at the Norwegian Radium Hospital for stage IB SCC between 1987 and 1993 were studied. Immunohistochemistry using monoclonal antibodies against Bcl-2, Bcl-xL, and Bax was used to examine protein expression. Ten patients who underwent hysterectomy for uterine prolapse served as controls.
Results: Cytoplasmic expression of Bcl-2, Bcl-xL, and Bax was low (< 5% positive cells) in 159 of 220 (73%), 193 of 220 (87%), and 39 of 220 (18%) tumours, respectively, and high (⩾ 5% positive cells) in 61 of 220 (27%), 27 of 220 (13%), and 181 of 220 (82%) tumours, respectively. In univariate analysis, all classic clinicopathological parameters but none of the investigated proteins were associated with prognosis. In multivariate analysis, only deep stromal invasion was independently related to survival.
Conclusion: Bcl-2, Bcl-xL, and Bax were not independently associated with prognosis in stage IB SCC.
Keywords: Bcl-2, Bcl-xL, and Bax, immunohistochemistry, prognosis, cervical neoplasms, retrospective studies, biological markers
Since apoptosis, or programmed cell death, was described in 1972,1 and since it was recognised that cell proliferation and cell survival are regulated by different pathways,2 cell death has become a topic of great interest in cancer research. This discovery has made it clear that tumorigenesis not only depends on proliferation but equally on the potential of a cell to resist cell death (apoptosis). Apoptosis thus became a new target for treatment. In addition, the DNA damage caused by chemotherapy and radiotherapy leads to apoptosis. Resistance to apoptosis, present in many tumours, could possibly explain why antitumour treatment sometimes fails. Restoring apoptosis could then be a means of drastically improving the effectiveness of existing treatment.
“It would be useful to identify prognostic factors that could help target adjuvant treatment”
Members of the Bcl-2 family of proteins are key regulators of apoptosis. They are divided into prosurvival proteins that can inhibit apoptosis, such as Bcl-2 and Bcl-xL, and proapoptotic members, such as Bax. Bcl-2 has been identified as the gene activated by the t(14;18)(q32;q21) translocation in B cell follicular lymphoma and leukaemia, thereby demonstrating its oncogenic potential.3 Bcl-2 enhances cell survival by inhibiting apoptosis induced under a wide range of stimuli.4 Transfection of the Bcl-2 gene into different cell lines confers increased resistance to several cytostatic drugs.5 Alternative splicing of Bcl-x, identified in 1993, results in two distinct protein products. The larger, Bcl-xL, is similar in size and structure to Bcl-2 and is antiapoptotic. In contrast, the smaller, Bcl-xS, inhibits Bcl-2 and thus is proapoptotic. In vivo, strong expression of Bcl-xS is seen in cells with a high turnover, such as developing lymphocytes. In contrast, Bcl-xL is found in tissues containing long lived postmitotic cells, such as adult brain.6 Bax (Bcl-2 associated protein X) is probably the most important member of the proapoptotic proteins. It inhibits Bcl-2 but can also directly induce apoptosis.7 Proapoptotic and antiapoptotic proteins can homodimerise and heterodimerise and titrate one another’s function, suggesting that their relative concentrations determine cell fate.8 Protection from apoptosis can be differentially provided by Bcl-2 or Bcl-xL, depending on the cell type and type of apoptosis induction.9–11
Although the incidence of cervical cancer in industrialised countries has decreased with the advent of systematic screening, it is still the second most frequent cause of death from cancer in women aged 20–39.12 Nearly half of patients present with stage I disease and adjuvant treatment is given to about one third of patients primarily treated with surgery.13 This adjuvant treatment is accompanied by considerable morbidity,14 and treating the many for the benefit of the few is undesirable. Thus, it would be useful to identify prognostic factors that could help target adjuvant treatment. Risk grouping can be done on the basis of clinical factors,15 but biomolecular factors may improve these prognostic models.
The aim of our study was to describe the expression of the Bcl-2, Bcl-xL, and Bax proteins, and their prognostic value in early squamous cervical carcinoma (SCC).
MATERIALS AND METHODS
Materials
From January 1987 to December 1993, 242 patients with International Federation of Gynaecology and Obstetrics (FIGO) stage IB SCC (tumours clinically limited to the cervix and with invasion depth of ⩾ 5 mm or horizontal spread of ⩾ 7 mm) were treated by radical hysterectomy and bilateral pelvic lymphadenectomy at the Norwegian Radium Hospital, Oslo, Norway. All cervical carcinomas diagnosed within the catchment area of the hospital are treated solely at this institution. All patients were examined under general anaesthesia and tumour diameter was assessed by inspection and palpation. No preoperative screening for lymph node metastasis was done. Our study comprises the 220 patients for whom sufficient pathological material was available. Postoperatively, adjuvant treatment was given in the case of lymph node metastasis, large tumour diameter, or invasion into the parametria. A median of 26 lymph nodes were removed. Radiotherapy was given to 32 patients, radiation and chemotherapy to 12, and another 31 received chemotherapy only. Patients were followed until July 2001 and none was lost to follow up. During follow up, 44 (19.7%) patients had a relapse and 37 (16.6%) died of cervical cancer (another four died of other diseases, five of unknown causes after a long period without relapse, and one of postoperative complications). All patients who experienced a relapse were admitted to our institution. Median follow up for patients without relapse was 128 months (interquartile range, 112–146), and for patients still alive it was 129 months (interquartile range, 114–147). The median age was 39 years (interquartile range, 34–51). Table 1 provides a detailed description of the tumour characteristics. As normal controls we used sections from formalin fixed, paraffin wax embedded tissue from 10 normal cervices from patients who underwent hysterectomy for prolapse (age range, 31–49 years; median age, 43). The specimens were re-evaluated blindly by an experienced pathologist (KL) for histopathological diagnosis according to the World Health Organisation.16
Table 1.
Five year disease free (DFS) and disease specific (DSS) survival
| Total (n = 220) | Relapses (n = 44) | DFS | p Value | Deaths (n = 37) | DSS | p Value | |
| Bcl-2 expression | |||||||
| Low | 159 | 35 (22%) | 80% | 0.20 | 29 (18%) | 85% | 0.46 |
| High | 61 | 9 (15%) | 85% | 8 (13%) | 88% | ||
| Bcl-xL expression | |||||||
| Low | 193 | 39 (20%) | 81% | 0.68 | 34 (18%) | 85% | 0.61 |
| High | 27 | 5 (18.5%) | 85% | 3 (11%) | 89% | ||
| Bax expression | |||||||
| Low | 39 | 4 (10%) | 90% | 0.09 | 4 (10%) | 90% | 0.25 |
| High | 181 | 40 (22%) | 80% | 33 (18%) | 85% | ||
| Depth of invasion | |||||||
| ⩽10 mm | 125 | 15 (12%) | 93% | <0.0001 | 10 (8%) | 93% | <0.0001 |
| 11–15 mm | 48 | 8 (17%) | 81% | 8 (17%) | 85% | ||
| >15 mm | 47 | 21 (45%) | 65% | 19 (40%) | 68% | ||
| Tumour size | |||||||
| <2 cm | 100 | 12 (12%) | 90% | 0.0002 | 9 (9%) | 94% | 0.0003 |
| 2.0–3.9 cm | 86 | 19 (22%) | 80% | 17 (20%) | 84% | ||
| ⩾4 cm | 34 | 13 (38%) | 62% | 11 (32%) | 68% | ||
| Vascular invasion | |||||||
| Absent | 119 | 14 (12%) | 91% | 0.0006 | 10 (8%) | 92% | 0.0006 |
| Present | 101 | 30 (30%) | 76% | 27 (27%) | 79% | ||
| Parametrial invasion | |||||||
| Absent | 205 | 36 (18%) | 83% | 0.0003 | 30 (15%) | 87% | 0.0009 |
| Present | 15 | 8 (53%) | 60% | 7 (47%) | 67% | ||
| Lymph node metastasis | |||||||
| Absent | 178 | 28 (16%) | 85% | 0.0012 | 21 (12%) | 89% | <0.0001 |
| Present | 42 | 16 (38%) | 69% | 16 (38%) | 74% |
Low Bcl-2, Bcl-xL, and Bax expression: <5% of tumour cells.
Immunohistochemistry
Sections from formalin fixed, paraffin wax embedded blocks were immunostained using the biotin–streptavidin–peroxidase method (Supersensitive Immunodetection System, LP-UL; Biogenex, San Ramon, California, USA) and the OptiMax Plus automated cell staining system (Biogenex). Dewaxed sections for Bcl-2, Bcl-xL, and Bax staining were microwaved in 10mM citrate buffer (pH 6.0) to unmask the epitopes. Sections were treated with 1% H2O2 for 10 minutes to block endogenous peroxidase. The sections were then incubated with monoclonal antibodies against Bcl-2 (1/20 dilution; 10 μg IgG1/ml; Dako Cytomation, Glostrup, Denmark), Bcl-xL (1/25 dilution; 2 μg IgG2a/ml; Zymed Laboratories, South San Francisco, California, USA), and Bax (1/100 dilution; 2 μg IgG1/ml; Neomarkers, Freemont, California, USA) for 30 minutes at room temperature. The sections were then incubated with biotin labelled secondary antibody (1/30 dilution) and streptavidin–biotin–peroxidase (1/30 dilution) for 20 minutes each. Tissue was stained for five minutes with 0.05% 3,3′-diaminobenzidine tetrahydrochloride, freshly prepared in 0.05M Tris buffer (pH 7.6) containing 0.024% H2O2, and then counterstained with haematoxylin, dehydrated, and mounted in Diatex. All the dilutions of antibody, biotin labelled secondary antibody, and streptavidin–peroxidase were made with phosphate buffered saline (pH 7.4) containing 1% bovine serum albumin. All series included positive controls. Replacement of the monoclonal antibody with mouse myeloma protein of the same subclass and concentration was used as negative control. All controls gave satisfactory results. Only cytoplasmic staining was considered positive. Four classes were used to describe the number of positively stained tumour cells: none; < 5% positive; between 5% and 50% positive; > 50% positive. Protein expression was defined as low when < 5% of cells were positive for Bcl-2, Bcl-xL, and Bax. This was based on published data17 or the distribution pattern in normal squamous epithelium and cancer tissue. We later verified all other cutoff values in our data. One entire section was screened independently by two investigators (RH and GVdP), unaware of the outcome. All discordant scores were reassessed and a single score agreed upon.
STATISTICAL ANALYSIS
The association between the expressed proteins and between protein expression and clinicopathological parameters was tested by means of the χ2 test with the Yates continuity correction for 2 × 2 tables or Fisher’s exact test. For continuous data, the Mann–Whitney test was used. Disease free survival and disease specific survival rates were calculated from the date of first admission to relapse or death, respectively, or to 17 July 2001 using the method of Kaplan and Meier. The log rank test with a test for trend in case of ordered variables was used for univariate analysis, and a Cox proportional hazards regression model was used for multivariate analysis of survival. All variables with p < 0.1 in the univariate analysis were entered into the regression model and a backward stepwise procedure was used. The hazard proportionality was verified by computing the log minus log against time. The SPSS® statistical package was used for statistical analysis and significance was defined as p < 0.05.
Power calculation: we needed 42 events to detect a difference of 15% (90% v 75%) in disease free survival at the 5% level with 90% power.
RESULTS
Immunohistochemistry
Table 2 summarises the staining results in SCC. Cytoplasmic expression of Bcl-2, Bcl-xL, and Bax was low (< 5% positive cells) in 159 of 220 (73%), 193 of 220 (87%), and 39 of 220 (18%) tumours, respectively, and high (⩾ 5% positive cells) in 61 of 220 (27%), 27 of 220 (13%), and 181 of 220 (82%) tumours, respectively (fig 1A–C). In normal squamous epithelium, Bcl-2 expression was absent in two cases and present in less than 5% of cells in eight cases. Bcl-2 staining was limited to the basal layers. Bcl-xL and Bax were not expressed (fig 1D–F).
Table 2.
Staining results for squamous cervical carcinoma
| Per cent of cells stained | Bcl-2 | Bcl-xL | Bax | |||
| N | % | N | % | N | % | |
| 0 | 131 | (60%) | 168 | (76%) | 14 | (6%) |
| <5% | 28 | (13%) | 25 | (11%) | 25 | (11%) |
| 5–50% | 47 | (21%) | 21 | (10%) | 91 | (41%) |
| >50% | 14 | (6%) | 6 | (3%) | 90 | (41%) |
| Total | 220 | (100%) | 220 | (100%) | 220 | (100%) |
Figure 1.
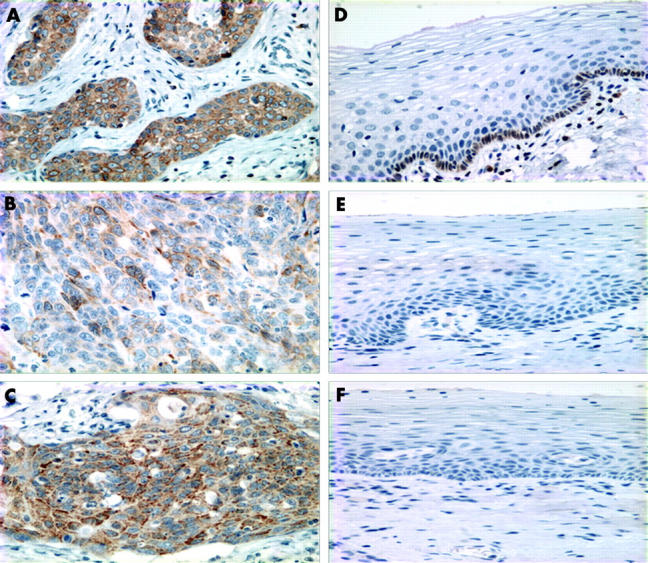
Immunohistochemical staining for (A) Bcl-2, (B) Bcl-xL, and (C) Bax in early squamous cervical carcinomas; staining for (D) Bcl-2, (E) Bcl-xL, and (F) Bax in normal squamous epithelium.
Relation between the apoptotic markers
Bcl-2 was significantly related to Bcl-xL (Spearman ρ = 0.284; p < 0.0001) and Bax (Spearman ρ = 0.145; p = 0.032).
Protein expression in relation to clinicopathological parameters
High Bax expression was associated with large tumour size (p = 0.03) and there was a trend to an association with deep invasion (p = 0.051). Bcl-2 and Bcl-xL expression were not associated with these parameters.
Relation to survival
Table 1 shows the relation between the clinicopathological and immunohistochemical data and survival in univariate analysis.
None of the markers for apoptosis showed a correlation with survival. In addition, other cutoff values for the single proteins did not reach significance. We then combined proapoptotic and antiapoptotic proteins, namely: high Bcl-2 or Bcl-xL with low Bax; low Bcl-2 or Bcl-xL with high Bax; high Bcl-2 and Bcl-xL with low Bax; and low Bcl-2 and Bcl-xL with high Bax. None of these combinations reached significance. Depth of invasion, pelvic lymph node metastasis, tumour diameter, parametrial invasion, and vascular invasion were significantly associated with survival. In the multivariate analysis, all variables with p < 0.1 were entered into the model except for tumour size because of the strong correlation with stromal invasion. Deep stromal invasion was the only independent predictor for disease free survival (p < 0.0001) and overall survival (p < 0.0001).
DISCUSSION
The relation of the clinicopathological parameters to survival, and the possible explanations for the mere trend to significance of lymph node metastasis have been discussed in a previous study.18
Bcl-2 expression is increased in SCC compared with normal cervical epithelium, and using 5% as a cutoff, 27% of tumours showed Bcl-2 expression. The rate of Bcl-2 expression in early stage SCC in the literature ranges from 20% to 61%.17,19–23
The prognostic value of Bcl-2 in SCC is controversial, with reports describing Bcl-2 as a prognostic factor for improved survival,17,22,24,25 decreased survival,26 or as having no prognostic relevance.19–21,27,28 This applies both for early stages with surgery as the primary treatment and for advanced stages, treated by radiotherapy. Although one could focus on the differences between the techniques used (Ferrandina et al used western blotting28) or the differences in cutoff values used (only the 30% cutoff value used by Graflund and colleagues19 and Pillai and colleagues25 really differs), the explanation for the differences is probably the lack of the validation of prognostic factors in general, which would have disclosed the “data derived” nature of these studies.29 Comparing the most significant study by Tjalma and colleagues17 with ours, a very similar technique and the same cutoff values were used. In that study, Bcl-2 looked very promising, but the study was explorative and the cutoff values were data derived. Such an approach is not objectionable, as long as one bears in mind that it is only hypothesis generating, which means that the results might be true only for the examined dataset. In this context, it is imperative that such studies should be repeated in an independent population. We could not confirm these results in our independent population, providing strong evidence against the usefulness of Bcl-2 as an independent prognostic factor in stage IB SCC. Other differences between these studies are the low survival rates and the omission of tumour size or invasion depth in the multivariate analysis in the study by Tjalma et al.17
We did not find prognostic significance for Bcl-xL. Bcl-xL protein expression has not previously been analysed by means of immunohistochemistry. No prognostic significance was found in two studies using western blotting.28,30 In these studies, Bcl-xL expression in SCC was not different from that seen in normal tissue.
In our study, 82% of tumours showed Bax expression, which is similar to the 83% and 72% found in two other studies24,31 using a cutoff value of 10%. In agreement with most previous studies,24,28,30,31 we did not find Bax expression to be of prognostic value. As mentioned, Ferrandina et al and Mozzetti et al used western blotting.28,30
Because proapoptotic proteins mainly act through heterodimerisation with antiapoptotic proteins, it is their relative concentration that might act as a rheostat for apoptosis.8 Therefore, we examined the proapoptotic to antiapoptotic ratio for its prognostic value; however, no significant association with survival was found. In addition, measuring the whole process of apoptosis by surface morphology and composition, nuclear events and DNA cleavage, cytoplasmic biochemical activation events (such as caspase activity), cell dissolution, or mitochondrial function has been disappointing in determining prognosis.20,32
Take home messages.
The antiapoptotic proteins, Bcl-2 and Bcl-xL, and the proapoptotic protein Bax were not independently associated with prognosis in stage IB squamous cervical carcinoma
These apoptosis regulatory proteins have no prognostic value in this disease
In conclusion, in this adequately powered study, none of the investigated proteins—Bcl-2, Bcl-xL and Bax—was independently associated with prognosis.
Acknowledgments
We thank M T P Nguyen for excellent technical assistance. Supported in part by grants from the Norwegian Cancer Society.
Abbreviations
SCC, squamous cervical carcinoma
REFERENCES
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988;335:440–2. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto Y, Finger LR, Yunis J, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t (14; 18) chromosome translocation. Science 1984;226:1097–9. [DOI] [PubMed] [Google Scholar]
- 4.White E. Life, death, and the pursuit of apoptosis. Genes Dev 1996;10:1–15. [DOI] [PubMed] [Google Scholar]
- 5.Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood 1993;81:151–7. [PubMed] [Google Scholar]
- 6.Boise LH, Gonzalez-Garcia M, Postema CE, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993;74:597–608. [DOI] [PubMed] [Google Scholar]
- 7.Jurgensmeier JM, Xie Z, Deveraux Q, et al. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A 1998;95:4997–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993;74:609–19. [DOI] [PubMed] [Google Scholar]
- 9.Findley HW, Gu L, Yeager AM, et al. Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood 1997;89:2986–93. [PubMed] [Google Scholar]
- 10.Park JR, Bernstein ID, Hockenbery DM. Primitive human hematopoietic precursors express Bcl-x but not Bcl-2. Blood 1995;86:868–76. [PubMed] [Google Scholar]
- 11.Ma A, Pena JC, Chang B, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci U S A 1995;92:4763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5–26. [DOI] [PubMed] [Google Scholar]
- 13.Benedet JL, Odicino F, Maisonneuve P, et al. Carcinoma of the cervix uteri. J Epidemiol Biostat 2001;6:7–43. [PubMed] [Google Scholar]
- 14.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- 15.Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study. Gynecol Oncol 1999;73:177–83. [DOI] [PubMed] [Google Scholar]
- 16.Poulsen HE, Taylor CW, Sobin LH. Histological typing of female genital tract tumours. International histological classification of tumours. Geneva: World Health Organisation, 1975.
- 17.Tjalma W, Weyler J, Goovaerts G, et al. Prognostic value of bcl-2 expression in patients with operable carcinoma of the uterine cervix. J Clin Pathol 1997;50:33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Putte G, Holm R, Lie AK, et al. Expression of p27, p21 and p16 protein in early squamous cervical cancer and its relation to prognosis. Gynecol Oncol 2003;89:140–7. [DOI] [PubMed] [Google Scholar]
- 19.Graflund M, Sorbe B, Karlsson M. Immunohistochemical expression of p53, bcl-2, and p21(WAF1/CIP1) in early cervical carcinoma: correlation with clinical outcome. Int J Gynecol Cancer 2002;12:290–8. [DOI] [PubMed] [Google Scholar]
- 20.Jain D, Srinivasan R, Patel FD, et al. Evaluation of p53 and Bcl-2 expression as prognostic markers in invasive cervical carcinoma stage IIb/III patients treated by radiotherapy. Gynecol Oncol 2003;88:22–8. [DOI] [PubMed] [Google Scholar]
- 21.Uehara T, Kuwashima Y, Izumo T, et al. Expression of the proto-oncogene bcl-2 in uterine cervical squamous cell carcinoma: its relationship to clinical outcome. Eur J Gynaecol Oncol 1995;16:453–60. [PubMed] [Google Scholar]
- 22.Dimitrakakis C, Kymionis G, Diakomanolis E, et al. The possible role of p53 and bcl-2 expression in cervical carcinomas and their premalignant lesions. Gynecol Oncol 2000;77:129–36. [DOI] [PubMed] [Google Scholar]
- 23.Saegusa M, Takano Y, Hashimura M, et al. The possible role of bcl-2 expression in the progression of tumors of the uterine cervix. Cancer 1995;76:2297–303. [DOI] [PubMed] [Google Scholar]
- 24.Crawford RA, Caldwell C, Iles RK, et al. Prognostic significance of the bcl-2 apoptotic family of proteins in primary and recurrent cervical cancer. Br J Cancer 1998;78:210–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillai MR, Jayaprakash PG, Nair MK. Bcl-2 immunoreactivity but not p53 accumulation associated with tumour response to radiotherapy in cervical carcinoma. J Cancer Res Clin Oncol 1999;125:55–60. [DOI] [PubMed] [Google Scholar]
- 26.Rajkumar T, Rajan S, Baruah RK, et al. Prognostic significance of Bcl-2 and p53 protein expression in stage IIB and IIIB squamous cell carcinoma of the cervix. Eur J Gynaecol Oncol 1998;19:556–60. [PubMed] [Google Scholar]
- 27.Chung TK, Cheung TH, Lo WK, et al. Expression of apoptotic regulators and their significance in cervical cancer. Cancer Lett 2002;180:63–8. [DOI] [PubMed] [Google Scholar]
- 28.Ferrandina G, Mozzetti S, Marone M, et al. Bcl-2, bax, bcl-x(L) and bcl-x(S) expression in neoplastic and normal cervical tissue. Cancer Lett 2000;155:19–27. [DOI] [PubMed] [Google Scholar]
- 29.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med 2000;19:453–73. [DOI] [PubMed] [Google Scholar]
- 30.Mozzetti S, Ferrandina G, Marone M, et al. Expression of bcl-2, bax-xL, and bcl-xS in endometrial and cervical tissues. Cancer Detect Prev 2000;24:536–41. [PubMed] [Google Scholar]
- 31.Tjalma WA, Weyler JJ, Bogers JJ, et al. The importance of biological factors (bcl-2, bax, p53, PCNA, MI, HPV and angiogenesis) in invasive cervical cancer. Eur J Obstet Gynecol Reprod Biol 2001;97:223–30. [DOI] [PubMed] [Google Scholar]
- 32.Paxton JR, Bolger BS, Armour A, et al. Apoptosis in cervical squamous carcinoma: predictive value for survival following radiotherapy. J Clin Pathol 2000;53:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]


