Abstract
Small cell neuroendocrine carcinoma of the breast is a rare tumour with less than 30 cases reported in the literature. The clinicopathological findings of three cases of primary neuroendocrine carcinoma of the breast and a review of the pertinent literature are presented. The morphological and immunohistochemical patterns of this tumour are similar to its pulmonary counterpart. Expression of neuroendocrine markers is inconsistent, so morphology is the mainstay of diagnosis. Size is a very important prognostic factor in this tumour, as in breast carcinomas of the usual type.
Keywords: breast, carcinoma, histopathology, neuroendocrine, primary
Small cell neuroendocrine carcinoma (SCNC) has been described in many extrapulmonary sites including breast, larynx, gastrointestinal tract, prostate, bladder, ovary, and cervix.1–5 The histological appearances of these tumours in all sites are similar. Reports also suggest that the clinical course of extrapulmonary SCNC is as aggressive as its pulmonary counterpart.1,5,6 Primary SCNC of the breast is as rare as it is in other extrapulmonary sites. Fewer than 30 cases have been reported in the literature, with the largest series of nine cases reported by Shin et al.4 This tumour is thought to be distinct from tumours of the usual type with neuroendocrine differentiation.6 The distinction is particularly important in view of the perceived more aggressive behaviour of SCNC.1,5,6 We report three cases of primary SCNC of the breast on our file, one of which has been reported previously.7
CASE REPORT
Case 1
A 46 year old, para 0+1, woman presented with a right breast lump. Clinical examination revealed a painless, firm mobile mass with no palpable axillary lymph nodes. She had no relevant past medical history. Her father had carcinoma of the thyroid gland but there was no family history of breast cancer. Clinical and radiological investigations (computerised tomography and positron electron tomography) did not reveal tumour elsewhere in the body. The patient had simple excision of the tumour, the histology of which showed SCNC, 1.0 cm in size. No axillary clearance was performed. She was further treated with radiation to the chest wall and local lymph nodes, followed by six courses of cisplatin and VP16. She is alive and free of disease 48 months after lumpectomy.
Case 2
A 60 year old woman presented with a subareolar mass in her right breast of short duration. She was a smoker and known asthmatic. She had ovarian cystectomy 30 years before the breast lump, the histological diagnosis of which is not known. There was no relevant family history. Clinical examination revealed a firm subareolar mass with no axillary lymphadenopathy. Radiological and clinical examination failed to reveal tumour elsewhere in the body. She had a simple lumpectomy, which was diagnosed as SCNC with foci of an in situ ductal component. The size of the tumour was 1.7 cm. Axillary clearance was not performed. She then had radiotherapy and six courses of cisplatin and VP16. The patient died of disease 20 months after surgery.
Case 3
A 61 year old woman presented with left breast and left axillary masses measuring 1.7 cm and 4 cm, respectively. There was no relevant medical history or family history of breast cancer. The two lumps were excised and both diagnosed as SCNC. The axillary mass was a lymph node completely replaced by tumour. No axillary dissection was carried out, but there was no clinical evidence of another node being involved. She was further treated by radiation to the chest wall and regional lymph nodes and six courses of VP16 and cisplatin. A recent positron electron tomography scan showed a small lesion in the left lung. She is alive with metastatic disease six months after excision.
MATERIALS AND METHODS
The archival haematoxylin and eosin stained slides from the three cases of primary breast SCNC were reviewed and fresh sections cut where indicated. Immunohistochemical analysis was performed as a batch on 4 μm sections of each tumour using the avidin–biotin peroxidase technique (table 1). Using the antigen retrieval methods, antibodies, and dilutions shown in table 1, expression of the following was assessed: CAM5.2, cytokeratin 7 (CK7), CK20, oestrogen receptor, progesterone receptor, HER2, and neuroendocrine markers (neurone specific enolase, PGP 9.5, chromogranin, and synaptophysin). The clinical charts were also reviewed.
Table 1.
Immunohistochemical procedure and antibodies
| Antibody | Source | Dilution | Pretreatment |
| CAM5.2 | Becton Dickinson, Oxford, UK | Neat | None |
| CK7 | BioGenex, San Ramon, California, USA | 1/50 | Protease |
| CK20 | Dako, Glostrup, Denmark | 1/100 | Protease |
| NSE | Dako | 1/200 | Pressure cooker |
| PGP9.5 | Dako | 1/100 | Pressure cooker |
| Synaptophysin | Dako | 1/100 | None |
| Chromogranin | BioGenex | 1/50 | None |
| ER/PR | Dako | 1/50 | Pressure cooker |
| HER2 | Dako | Neat | Boil to 97°C |
CK, cytokeratin; ER, oestrogen receptor; NSE, neurone specific enolase; PR, progesterone receptor.
CLINICAL FINDINGS
All the patients were female, and their ages, clinical findings, treatments, and outcomes are outlined in table 2. None of the patients had a history of previous carcinoma or family history of breast cancer. One patient was a smoker. They all presented within one to two months of noticing the tumour. All the patients had lumpectomy followed by irradiation to the chest wall and regional lymph nodes, in addition to six courses of chemotherapy (cisplatin and VP16).
Table 2.
Clinical summary of the cases
| Case 1 | Case 2 | Case 3 | |
| Age (years) | 46 | 60 | 61 |
| Location | Right | Right | Left |
| Size (cm) | 1.0 | 1.7 | 1.7 |
| Node | Clinically negative | Clinically negative | Positive ipsilateral node |
| Treatment | Lumpectomy, irradiation, and chemotherapy | Lumpectomy, irradiation, and chemotherapy | Lumpectomy, irradiation, and chemotherapy |
| Outcome | Free of disease (48 months) | Dead within 20 months | Alive with disease (6 months) |
PATHOLOGY FINDINGS
Gross
The tumours were 1.0 cm, 1.7 cm, and 1.7 cm, respectively, with poorly circumscribed fleshy white to tan cut surfaces and focal areas of necrosis. The 4.0 cm axillary lymph node in the third patient was completely replaced by tumour.
Microscopy
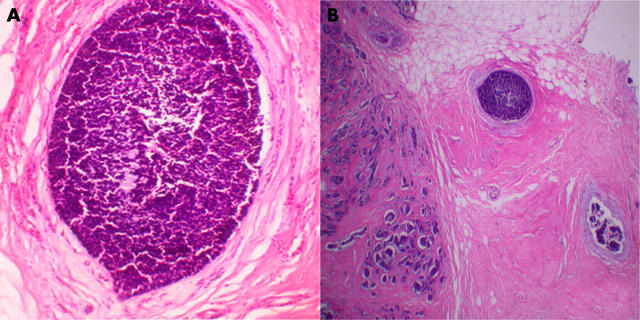
All three tumours had similar morphology. They were composed of fairly uniform small dark cells disposed in nests and trabecular patterns separated by bands of fibrous tissue. The cells had a high nucleocytoplasmic ratio, small hyperchromatic nuclei with inconspicuous nucleoli, scanty cytoplasm, and poorly defined cytoplasmic borders. There were areas of dirty necrosis and the mitotic count ranged from 10 to 20/10 high power fields. Foci of an in situ component similar to the infiltrating tumour were identified in case 2 (fig 1). No in situ or invasive ductal or lobular carcinoma of the usual type was seen.
Figure 1.
(A) Section showing the in situ component in one of the carcinomas. (B) Another in situ component.
Immunohistochemistry
Table 3 shows the results of immunoperoxidase staining. The tumours were negative for CD45 and HMB45. The in situ components in case 2 showed a similar immunohistochemical profile to the invasive component.
Table 3.
Immunohistochemical pattern of the tumours
| Case 1 | Case 2 | Case 3 | |
| CAM5.2 | ++ | ++ | ++ |
| CK7 | ++ | ++ | ++ |
| CK20 | – | – | – |
| NSE | +++ | +++ | +++ |
| PGP9.5 | +++ | +++ | +++ |
| Chromogranin | + | + | + |
| Synaptophysin | + | + | – |
| ER | – | – | – |
| PR | – | – | – |
| HER2 | – | – | – |
Staining: +, weak or focal; ++, moderately strong; +++, strong diffuse staining.
CK, cytokeratin; ER, oestrogen receptor; NSE, neurone specific enolase; PR, progesterone receptor.
DISCUSSION
Primary SCNC of the breast is a rare tumour with less than 30 cases reported in the literature. Most cases are found in women, as is the case with breast carcinoma of the usual type. Only one case occurring in a 52 year old man has been reported in the literature.8 The reported age of incidence varies from 40 to 70 years, with a higher incidence in women greater than 60 years. There is considerable similarity between the morphological and histochemical features of these tumours and pulmonary small cell carcinomas.4–6,8
The histogenesis is still unclear, because the presence of neuroendocrine cells in normal breast has not been proved conclusively.9,10 It has been suggested that SCNC is a variant of metaplastic carcinoma arising from usual lobular or ductal carcinoma.4 This position is strengthened by the dimorphic appearance of the tumour in a large number of reported cases.4,11 However, some believe that SCNC is a distinct type of breast carcinoma different from the usual types of carcinoma, with variable degrees of neuroendocrine differentiation and carrying a worse prognosis.1,6 The presence of an intraductal component with a morphological and immunohistochemical profile similar to the invasive component, as in one of our cases (case 2), lends support to the hypothesis of a primary small cell carcinoma in its own right. Primary SCNC also occurs, as already stated, in many other sites where neuroendocrine cells are normally absent or not readily identifiable, including the ovary and prostate.
The prognostic relevance of neuroendocrine differentiation in breast carcinoma is a subject of debate. Although most studies reported an appreciably worse prognosis,1,5,6 a few did not.4,9 This discrepancy may result from non-separation of pure neuroendocrine carcinoma from carcinoma of the usual type with areas of neuroendocrine differentiation.6,11 There is no mention of the degree of differentiation in some of the reported cases.6,11 In addition, the neuroendocrine component in most of the tumours of the usual type falls into the moderately to well differentiated World Health Organisation (WHO) category. Most of the reported pure SCNC cases show an appreciably worse prognosis. Using the WHO criteria, all our cases fall into the poorly differentiated SCNC category. A uniform standard for diagnosis of neuroendocrine carcinoma as is the case with mucinous carcinoma should be set, and the degree of differentiation according to the current WHO classification of neuroendocrine carcinoma should be clearly stated for reasonable comparison.
Take home messages.
We report three rare cases of small cell neuroendocrine carcinoma of the breast
The morphological and immunohistochemical patterns of this tumour are similar to its pulmonary counterpart
Expression of neuroendocrine markers is inconsistent, so morphology is the mainstay of diagnosis
Size is a very important prognostic factor in this tumour, as in breast carcinomas of the usual type
The prognosis may not be as poor as previously thought, particularly for early stage disease
“Positive neuroendocrine markers will give strong support to the diagnosis, and this should be carefully searched for”
Size is an important prognostic factor in breast carcinoma in general.4,9 Shin et al found that patients with a mean tumour size of 5.2 cm did appreciably worse than those with a mean tumour size of 2.6 cm. The second woman (case 2) in our series with a tumour size of 1.7 cm died within 20 months of diagnosis. The woman with a 1.0 cm tumour (case 1) is alive and free of disease 48 months after diagnosis.
The immunoprofile of epithelial markers in our series is similar to most reported cases.1,4,5,8,11 All the tumours showed at least focal positivity for CAM 5.2 and CK7 and were negative for CK20. This is also consistent with the immunoprofile of breast carcinoma of usual types.12 In contrast, Merkel cell carcinomas are positive for CK20 and negative for CK7, whereas SCNCs of the lung are negative for both markers.13 This may be useful in differentiating between these tumours.
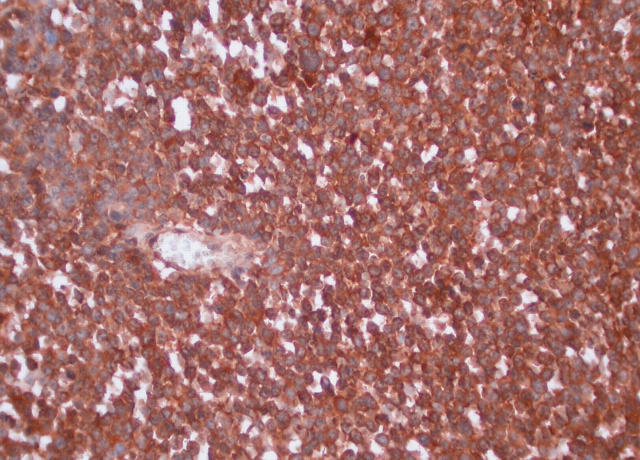
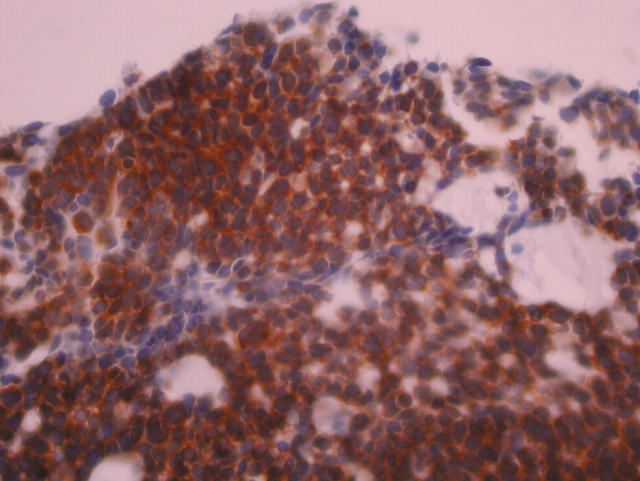
The expression of neuroendocrine markers by SCNC is inconsistent.9,14,15 This is not surprising in view of the fact that these tumours are poorly differentiated. Some have argued that the diagnosis of these tumours rests on routine haematoxylin and eosin morphology,14,15 and negative expression of neuroendocrine markers should not be used as an exclusion criterion. However, positive neuroendocrine markers will give strong support to the diagnosis, and this should be carefully searched for. All our cases showed diffuse positivity for neurone specific enolase (fig 2) and PGP9.5, weak to moderate staining for chromogranin (fig 3), and two were positive focally for synaptophysin, which as pointed out above supports the diagnosis.
Figure 2.
Section showing positive staining for neurone specific enolase in the invasive component.
Figure 3.
Section showing positive staining for chromogranin in the invasive component.
Positive expression of oestrogen and progesterone receptors in SCNC of the lung and a few other sites has been reported. Thus, their expression in SCNC is not definite proof of mammary origin. Oestrogen and progesterone receptors were expressed in 67% and 56% of cases reported by Shin et al. All three of our cases were negative for oestrogen and progesterone receptors and HER2.
In summary, SCNC is a distinct type of primary breast tumour. The prognosis may not be as poor as previously portrayed, especially for early stage disease.
Acknowledgments
The authors wish to thank Dr M Keane, Mr J Nee, and Dr N Faheem for providing clinical information on these cases, and Ms F Devlin for the high quality laboratory technical support.
Abbreviations
CK, cytokeratin
SCNC, small cell neuroendocrine carcinoma
WHO, World Health Organisation
REFERENCES
- 1.Samli B, Celik S, Evrensel T, et al. Primary neuroendocrine small cell carcinoma of the breast. Arch Pathol Lab Med 2000;124:296–8. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim NB, Briggs JC, Corbishley CM. Extrapulmonary oat cell carcinoma. Cancer 1984;54:1645–61. [DOI] [PubMed] [Google Scholar]
- 3.Richardson RL, Weiland LH. Undifferentiated small cell carcinomas in extrapulmonary sites. Semin Oncol 1982;9:484–96. [PubMed] [Google Scholar]
- 4.Shin SJ, DeLellis RA, Ying L, et al. Small cell carcinoma of the breast: a clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol 2000;24:1231–8. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki T, Shimazaki H, Aida S, et al. Primary small cell (oat cell) carcinoma of the breast: report of a case and review of the literature. Pathol Int 2000;50:914–18. [DOI] [PubMed] [Google Scholar]
- 6.Sapino A, Righi L, Cassoni P, et al. Expression of the neuroendocrine phenotype in carcinomas of the breast. Semin Diagn Pathol 2000;17:127–37. [PubMed] [Google Scholar]
- 7.Salmo EN, Connolly CE. Primary small cell carcinomas of the breast: report of a case and review of the literature. Histopathology 2001;38:277–8. [DOI] [PubMed] [Google Scholar]
- 8.Jundt G, Schulz A, Heitz PU, et al. Small cell neuroendocrine (oat cell) carcinoma of the male breast. Immunocytochemical and ultrastructural investigations. Virchows Arch A Pathol Anat Histopathol 1984;404:213–21. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:966–78. [DOI] [PubMed] [Google Scholar]
- 10.Hasleton PS. New WHO classification of lung tumours. Recent Advances in Histopathology 2001;19:115–29. [Google Scholar]
- 11.Miremadi A, Pinder SE, Lee AH, et al. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology 2002;40:215–22. [DOI] [PubMed] [Google Scholar]
- 12.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology 2002;40:403–39. [DOI] [PubMed] [Google Scholar]
- 13.Chan JK, Suster S, Wenig BM, et al. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol 1997;21:226–34. [DOI] [PubMed] [Google Scholar]
- 14.Guinee DG Jr, Fishback NF, Koss MN, et al. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol 1994;102:406–14. [DOI] [PubMed] [Google Scholar]
- 15.Suster S, Moran CA. Neuroendocrine carcinoma. In: Modern surgical pathology, Vol. 1. USA: Elsevier Science, :350–5.