Abstract
Background: Deoxycytidine kinase (dCK) is responsible for the activation of several clinically important deoxynucleoside analogues used for the treatment of haematological and solid malignancies.
Aim: To measure dCK expression in tumour cells from different origins.
Method: A rabbit antihuman dCK antibody was used for the immunocytochemical detection of dCK expression in three leukaemic cell lines (HL60, U937, and CCRF-CEM) and 97 patient samples (paediatric acute myeloid leukaemia (AML) and lymphoid leukaemia (ALL), retinoblastoma, paediatric brain tumours, and adult non-small cell lung cancer (NSCLC)).
Results: CCRF-CEM, U937, and HL60 cells stained positively for dCK and the degree of expression correlated with dCK activity. dCK expression varied between tumour types and between individual patients within one tumour type. dCK was located predominantly in the cytoplasm. The staining intensity was scored as negative (0), low (1+), intermediate (2+), or high (3+). Expression of dCK was high in AML blasts. In contrast, brain tumour samples expressed low amounts of dCK. dCK staining ranged from low (1+) to high (3+) in ALL blasts, retinoblastoma, and NSCLC tissue samples. Staining was consistent (interobserver variability, 88%; κ = 0.83) and specific. Western blotting detected the dCK protein appropriately at 30 kDa, without additional bands.
Conclusions: Immunocytochemistry is an effective and reliable method for determining the expression of dCK in patient samples and requires little tumour material. This method enables large scale screening of dCK expression in tumour samples.
Keywords: deoxycytidine kinase, immunocytochemistry, childhood acute leukaemia, solid tumours, deoxynucleoside analogues
Deoxycytidine kinase (dCK) phosphorylates 2′-deoxycytidine to its monophosphate form, which is the rate limiting step in deoxynucleoside salvage.1–3 dCK is also required for activation of several clinically important deoxynucleoside analogues.4,5 These include the classic deoxycytidine analogues cytarabine (ara-C) and 5-aza-2′deoxycytidine, in addition to the purine analogues cladribine and fludarabine, which are widely used for the treatment of childhood and adult leukaemia. Several novel deoxynucleoside analogues with activity in solid tumours (for example, gemcitabine and troxacitabine) also require activation by dCK.6,7 Resistance to deoxynucleoside analogues in cell lines has been linked to dCK deficiency.8,9,10,11,12,13 Transfection and induction of dCK restored sensitivity to ara-C and gemcitabine.14 Furthermore, a strong correlation between the antitumour effect of gemcitabine and dCK activity has been observed.15
“Resistance to deoxynucleoside analogues in cell lines has been linked to deoxycytidine kinase deficiency”
The dCK gene is localised on chromosome 4.16–18 Human dCK functions as a homodimer of 60 kDa, consisting of two identical 30.5 kDa subunits.17 dCK is expressed in a constitutive manner throughout the cell cycle,2,19,20 and is expressed predominantly in lymphoid tissues, indicating cell type specific regulation.16,17,21,22 Moreover, dCK expression can be upregulated in certain malignancies.23 The molecular mechanism leading to tissue specific transcription of dCK has not yet been elucidated. Another level of control could involve regulation of the subcellular localisation of the enzyme. dCK has been described as a cytoplasmic protein, but when overexpressed in vitro it was localised mainly in the nucleus.19,24
We have established an immunocytochemical staining method for the detection of dCK, using a rabbit antihuman dCK antibody directed against the C-terminal end of the dCK protein. Immunocytochemical detection could be a valuable tool for pretreatment screening of dCK values in malignancies to be treated with deoxynucleoside analogues, because it requires only a small number of tumour cells, and cellular morphology is maintained, allowing studies on the subcellular localisation of dCK. In our present study, we describe the methodology of immunocytochemical staining for dCK in cell lines and patient samples from different tumour types, including childhood acute myeloid leukaemia (AML), childhood acute lymphoblastic leukaemia (ALL), and adult non-small cell lung cancer (NSCLC). Some paediatric retinoblastoma and brain tumour (peripheral neural ectodermal tumour and ependymoma) samples were included in our analyses for comparative purposes, because they are extremely resistant to ara-C in vitro, possibly as a result of a deficiency in dCK.25,26
MATERIALS AND METHODS
Cell lines
The leukaemic cell lines HL60, U937, and CCRF-CEM were cultured as described previously.27 Cytospin preparations were made with 50 µl cell suspensions (0.5 × 106 cells/ml in phosphate buffered saline (PBS) containing 5% human serum albumin). Cytospins were air dried on silica gel for at least 48 hours and subsequently stored at –20°C.
Leukaemic samples
Bone marrow or peripheral blood samples taken for routine diagnostic purposes were obtained with informed consent and institutional review board approval from 31 children with de novo AML and 29 children with de novo ALL. Mononuclear cells were separated by density gradient centrifugation (Lymphoprep; density 1.077 g/ml; Nycomed Pharma, Oslo, Norway). Where necessary, the proportion of malignant cells was enriched, as described previously,28 after which the percentage of leukaemic cells was determined morphologically (May-Grünwald-Giemsa staining; Merck, Darmstadt, Germany) and cytospins were prepared.
Solid tumours
Primary tumour material was collected with informed consent and institutional review board approval from 12 children diagnosed with a brain tumour (peripheral neural ectodermal tumour (n = 7) or ependymoma (n = 5)) and from 10 patients with retinoblastoma. Tumour samples were collected fresh and under sterile conditions in PBS containing 100 IU/ml penicillin, 100 µg/ml streptomycin, and 0.125 µg/ml amphotericin (Gibco BRL, Breda, The Netherlands). Cell suspensions were made as described previously.25,26 The viability of the cells was determined by trypan blue exclusion. The final percentage of malignant cells was assessed by May-Grünwald-Giemsa staining and cytospin preparations were made.25 Formalin fixed, paraffin wax embedded tumour samples from 15 patients with NSCLC were evaluated. Tissue sections were cut into 4 µm thick sections and mounted on poly-L-lysine coated slides. Slides were stored at room temperature.
Immunocytochemical and histochemical staining
Cryopreserved cytospins were thawed at room temperature (30 minutes). All incubations were performed at room temperature. Cells were fixed using 10% formaldehyde (20 minutes). Endogenous myeloperoxidase was inactivated with PBS/1% H2O2 for 10 minutes. Slides were washed (PBS, 3 × 10 minutes) and blocked (10% normal swine serum; 30 minutes; Sigma, St Louis, Missouri, USA). Slides were incubated with the primary antibody, a rabbit polyclonal anti-dCK antibody,24 diluted 1/200 in 1% bovine serum albumin/10% pooled human serum/PBS (two hours). After PBS wash steps, the secondary antibody, biotinylated swine antirabbit (1/300 dilution in PBS/1% bovine serum albumin/2% pooled human serum, 30 minutes; Dako, Glostrup, Denmark) was applied. Slides were rinsed (PBS) and incubated with horseradish peroxidase conjugated streptavidin (1/100 dilution in PBS/1% bovine serum albumin, 45 minutes; Dako). Peroxidase activity was determined using 1mM 3,3′diaminobenzidine (Sigma), 0.05M imidazole (Merck), and 0.036% H2O2 in 0.05M Tris buffer (pH 7.4) for 10 minutes. Cells were counterstained using Mayer’s haematoxylin solution (Merck) and embedded in malinol. CCRF-CEM cells were used as a positive control in each experiment. Negative controls were performed for each sample by omitting the primary antibody. The staining was examined by light microscopy. dCK protein expression was determined by scoring the intensity of the staining: negative (0), low (1+), intermediate (2+), or high (3+), by two observers (IH and AJFB) in independent sessions, without knowledge of each others results.
NSCLC tissue sections were dewaxed and rehydrated by incubation in 100% xylene (2 × 10 minutes), followed by incubation in decreasing ethanol concentrations. Sections were fixed and endogenous myeloperoxidase was blocked (methanol/0.3% H2O2; 30 minutes). Antigen retrieval was performed by heating the slides in a microwave for 15 minutes in 10mM citrate buffer solution (pH 6.0). Slides were cooled down at room temperature for at least 30 minutes and washed (PBS). Non-specific staining was blocked using normal swine serum (1/10 dilution; 10 minutes). The remainder of the protocol was performed as described above.
dCK activity assay
dCK activity was determined using 3H-chlorodeoxyadenosine as a substrate (final concentration 50µM; specific activity, 0.19 µCi/nmol) with 5mM MgATP as phosphate donor and 100mM NaF to prevent nucleotide breakdown, as described in detail previously.27 Enzyme activity was expressed as nmol 3H-chlorodeoxyadenosine monophosphate formed/hour/mg protein.
Immunoblotting
To determine the specificity of the antibody, immunoblotting was carried out on two leukaemic cell lines (U937 and HL60) and two AML patient samples. Western blotting was performed as described previously by electrophoresis on 12.5% sodium dodecyl sulfate polyacrylamide gels and transfer by electroblotting to nitrocellulose membranes.27 Purified dCK protein with a histidine tag was used as a positive control. Blocking was performed with blocking buffer (5% non-fat dry milk blocking grade in PBS/0.1% Tween 20 for one hour) and the blot was probed with rabbit antihuman dCK polyclonal antibody (1/5000 dilution; one hour at room temperature) and goat antirabbit secondary antibody conjugated to horseradish peroxidase (1/3000 dilution; one hour at room temperature (Santa Cruz Biotechnology, Santa Cruz, California, USA)). Peroxidase activity was visualised using the enhanced chemiluminescence (ECL) kit (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK).
Statistics
The κ statistic was used to assess agreement between observers.29 Otherwise the data analysis was merely descriptive.
RESULTS
Immunocytochemistry and histochemistry
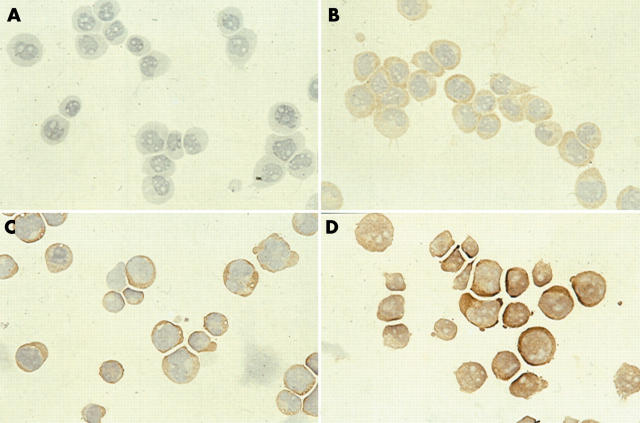
Titration of the primary antibody between 1/50 and 1/1000 resulted in optimal staining when a concentration of 1/200 was used. Optimal fixation of cytospins was with 10% formaldehyde. Other fixatives (such as methanol, 4% paraformaldehyde, or acetone) resulted in non-evaluable samples. Good cellular morphology was crucial because cytospins of poor quality resulted in non-evaluable stainings. Negative controls did not stain. HL60, CCRF-CEM, and U937 cells stained positive for dCK, but to different degrees (fig 1). Staining was strongest in the HL60 cells, which also showed nuclear staining, and lowest in U937. In CCRF-CEM and U937 cells dCK was located exclusively in the cytoplasm. Immunocytochemistry and western blotting data were in agreement with dCK activity (table 1). In clinical samples, CCRF-CEM served as a positive control and gave similar staining results in each experiment.
Figure 1.
Immunocytochemical staining of deoxycytidine kinase in the leukaemic cell lines. (A) U937 phosphate buffered saline negative control, (B) U937, (C) CCRF-CEM, and (D) HL60.
Table 1.
Deoxycytidine kinase (dCK) activity in leukaemic cell lines
| Cell line | dCK activity (nmol/hr/mg protein) |
| HL60 | 31.9 (4.9) |
| CCRF-CEM | 22.7 (2.4) |
| U937 | 14.1 (2.2) |
Values are the mean (SE) of three experiments.
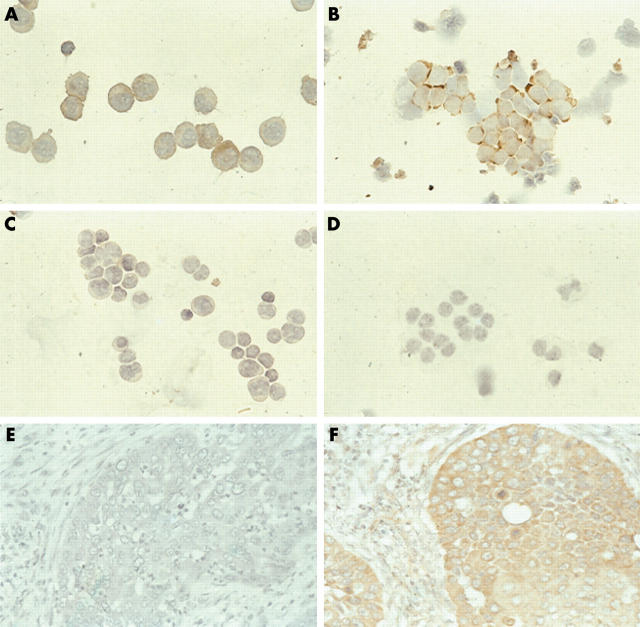
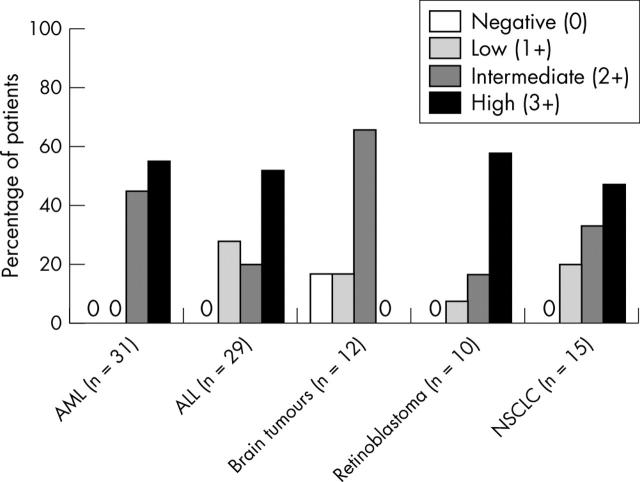
In all tumour samples, expression varied and a predominantly cytoplasmic staining was seen (figs 2, 3). Nuclear staining was also seen in four of 31 AML samples and in two of 29 ALL samples. Expression of dCK was either intermediate (2+) or strong (3+) in AML blasts, but in ALL, retinoblastoma, and NSCLC expression ranged from low (1+) to high (3+). Staining for dCK was weak (1+) or intermediate (2+) in brain tumours. dCK was not detectable in two brain tumour samples. The reproducibility of staining was tested by replicate staining of 10 patient samples and was found to be good (80%). The interobserver variability was low: in 72 of 82 cases the first observation was in agreement (κ = 0.83); disagreement only involved scores of 2+/3+ (intermediate/high). At re-examination by both observers agreement was reached, and this consensus was used in the final analysis.
Figure 2.
Immunocytochemical staining of deoxycytidine kinase (dCK) in patient samples from different tumour types. (A) An acute myeloid leukaemia sample and (B) a retinoblastoma sample with strong expression of dCK (3+). (C) An acute lymphoid leukaemia sample with weak staining (1+); (D) A brain tumour sample negative for dCK (0). Non-small cell lung cancer tissue stained for dCK: (E) phosphate buffered saline negative control and (F) a sample with high dCK expression (3+)
Figure 3.
Expression of the deoxycytidine kinase (dCK) protein in different tumour types. The acute myeloid leukaemia (AML) and retinoblastoma samples showed high expression of dCK (predominantly 3+). In contrast, the brain tumour samples showed low expression of dCK (0–2+) and in acute lymphoid leukaemia (ALL) samples dCK expression was intermediate (2+). NSCLC, non-small cell lung cancer.
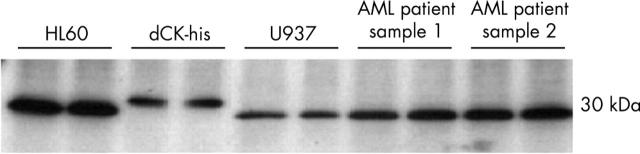
Western blotting
Western blotting was performed on two cell lines and two AML patient samples to evaluate the specificity of the antibody (fig 4). The band for dCK was located at 30 kDa, as expected, and no bands were seen at 60 kDa. The purified dCK-his protein was clearly visible. No additional bands were seen on the blots, indicating high specificity for dCK in both the leukaemic cell lines and patient samples.
Figure 4.
Western blot using the anti-deoxycytidine kinase (dCK) antibody (1/5000 dilution) on the leukaemic cell lines HL60 and U937, purified dCK protein (dCK-his), and two AML patient samples. Samples (20 µg protein) were loaded in duplicate and β actin was used for normalisation. The band for dCK was located at 30 kDa, as expected. The purified dCK protein band was located slightly higher because of the histidine tag. No additional bands were seen. The intensity of the band for the CCRF-CEM cells was intermediate, as described previously.27
DISCUSSION
We describe the immunocytochemical staining of dCK with a rabbit polyclonal antibody. This method is rapid, reproducible, and can be used to evaluate dCK expression in different malignancies. Although various antibodies against dCK have been described and used for western blotting, immunocytochemistry of patient samples has not been reported previously.19,23,24 The antibody was highly specific on both western blotting and immunocytochemistry. The specificity of this antibody was tested earlier and found to be blocked by the same recombinant dCK used as a control in fig 4.24 The interobserver variability was good. In patient samples, it was possible to discriminate between negative, low, intermediate, and high expression of dCK. There were clear differences between tumour types. In most samples, dCK was located exclusively in the cytoplasm, which is in agreement with previous studies that also describe dCK as a cytoplasmic enzyme.2,18 However, a study by Johansson et al reported that dCK can be found in the nucleus and that the N-terminal region (amino acids, 6–23) of the dCK protein contains a nuclear import signal.19 Hatzis et al showed that dCK is predominantly located in the cytoplasm in several cell types, but when overexpressed it was mainly located in the nucleus.24 Our study shows that nuclear localisation can be seen in several AML and ALL samples with high dCK expression. Nuclear staining was never seen in samples with a low or intermediate degree of dCK expression. Retinoblastomas showed positive staining, although they are highly resistant to ara-C in vitro.25,30 The presence of a mutation in the retinoblastoma tumour suppressor gene, characteristic for this disease, possibly encodes for general drug resistance. Brain tumour samples, which are also very resistant to ara-C in vitro,26 showed weak dCK staining. Therefore, dCK expression might play a role in ara-C resistance in this tumour type.
“Brain tumour samples, which are very resistant to cytarabine in vitro, showed weak deoxycytidine kinase staining”
The patients with AML included in our study were all treated with ara-C containing protocols. Unfortunately, we were not able to assess the relation between dCK protein expression and in vivo response to treatment because of the heterogeneity of the treatment received. Patients were treated according to different treatment regimens and several different dosing schedules. However, we are currently assessing the relation between dCK protein expression and in vitro ara-C sensitivity. In addition, we are using immunocytochemistry to screen a large group of samples from patients with NSCLC, who have been treated with gemcitabine, for pretreatment dCK expression and its relation to in vivo response.
Our study shows that immunocytochemistry is an effective method to determine the degree of expression of dCK in patient samples, and only requires a small amount of tumour material. This will enable the evaluation of dCK expression as a predictor of initial response to treatment and final outcome.
Take home messages.
We measured the immunohistochemical expression of deoxycytidine kinase (dCK), which is responsible for the activation of several clinically important deoxynucleoside analogues used for the treatment of haematological and solid malignancies, in several cell lines and tumour types
Immunocytochemistry was found to be an effective and reliable method for determining the expression of dCK in patient samples and required little tumour material
The use of this method will enable the evaluation of dCK expression as a predictor of initial response to treatment and final outcome
Acknowledgments
The authors thank all the research technicians in the paediatric oncology research laboratory at the VU University Medical Centre, Amsterdam, all medical doctors who helped in providing tumour samples, and Professor P van der Valk (department of pathology) for his advice. The study was performed in the setting of the international BFM study group, especially the AML committee (chaired by GJL Kaspers).
Abbreviations
ALL, acute lymphoid leukaemia
AML, acute myeloid leukaemia
ara-C, cytarabine
dCK, deoxycytidine kinase
NSCLC, non-small cell lung cancer
PBS, phosphate buffered saline
REFERENCES
- 1.Bohman C, Eriksson S. Deoxycytidine kinase from human leukemic spleen: preparation and characteristics of homogeneous enzyme. Biochemistry 1988;27:4258–65. [DOI] [PubMed] [Google Scholar]
- 2.Arner ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther 1995;67:155–86. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson S, Munch-Petersen B, Johansson K, et al. Structure and function of cellular deoxyribonucleoside kinases. Cell Mol Life Sci 2002;59:1327–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Clercq E. Antiviral drugs: current state of the art. J Clin Virol 2001;22:73–89. [DOI] [PubMed] [Google Scholar]
- 5.Peters GJ, Van der Wilt CL, Van Moorsel CJ, et al. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol Ther 2000;87:227–53. [DOI] [PubMed] [Google Scholar]
- 6.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol 2002;3:415–24. [DOI] [PubMed] [Google Scholar]
- 7.Galmarini CM, Mackey JR, Dumontet C. Nucleoside analogues: mechanisms of drug resistance and reversal strategies. Leukemia 2001;15:875–90. [DOI] [PubMed] [Google Scholar]
- 8.Verhoef V, Sarup J, Fridland A. Identification of the mechanism of activation of 9-beta-D-arabinofuranosyladenine in human lymphoid cells using mutants deficient in nucleoside kinases. Cancer Res 1981;41:4478–83. [PubMed] [Google Scholar]
- 9.Bhalla K, Nayak R, Grant S. Isolation and characterization of a deoxycytidine kinase-deficient human promyelocytic leukemic cell line highly resistant to 1-beta-D-arabinofuranosylcytosine. Cancer Res 1984;44:5029–37. [PubMed] [Google Scholar]
- 10.Stegmann AP, Honders MW, Kester MG, et al. Role of deoxycytidine kinase in an in vitro model for AraC- and DAC-resistance: substrate–enzyme interactions with deoxycytidine, 1-beta-D-arabinofuranosylcytosine and 5-aza-2′-deoxycytidine. Leukemia 1993;7:1005–11. [PubMed] [Google Scholar]
- 11.Ruiz van Haperen V, Veerman G, Eriksson S, et al. Development and molecular characterization of a 2′,2′-difluorodeoxycytidine-resistant variant of the human ovarian carcinoma cell line A2780. Cancer Res 1994;54:4138–43. [PubMed] [Google Scholar]
- 12.Hapke DM, Stegmann AP, Mitchell BS. Retroviral transfer of deoxycytidine kinase into tumor cell lines enhances nucleoside toxicity. Cancer Res 1996;56:2343–7. [PubMed] [Google Scholar]
- 13.Dumontet C, Fabianowska-Majewska K, Mantincic D, et al. Common resistance mechanisms to deoxynucleoside analogues in variants of the human erythroleukaemic line K562. Br J Haematol 1999;106:78–85. [DOI] [PubMed] [Google Scholar]
- 14.Stegmann AP, Honders WH, Willemze R, et al. Transfection of wild-type deoxycytidine kinase (dck) cDNA into an AraC- and DAC-resistant rat leukemic cell line of clonal origin fully restores drug sensitivity. Blood 1995;85:1188–94. [PubMed] [Google Scholar]
- 15.Kroep JR, Loves WJ, Van der Wilt CL, et al. Pretreatment deoxycytidine kinase levels predict in vivo gemcitabine sensitivity. Mol Cancer Ther 2002;1:371–6. [PubMed] [Google Scholar]
- 16.Chottiner EG, Shewach DS, Datta NS, et al. Cloning and expression of human deoxycytidine kinase cDNA. Proc Natl Acad Sci U S A 1991;88:1531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JJ, Walker S, Chen E, et al. Genomic structure and chromosomal localization of the human deoxycytidine kinase gene. Proc Natl Acad Sci U S A 1993;90:431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell BS, Song JJ, Johnson EE, et al. Regulation of human deoxycytidine kinase expression. Adv Enzyme Regul 1993;33:61–8. [DOI] [PubMed] [Google Scholar]
- 19.Johansson M, Brismar S, Karlsson A. Human deoxycytidine kinase is located in the cell nucleus. Proc Natl Acad Sci U S A 1997;94:11941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson M, Karlsson A. Cloning of the cDNA and chromosome localization of the gene for human thymidine kinase 2. J Biol Chem 1997;272:8454–8. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson A, Johansson M, Eriksson S. 2 cloning and expression of mouse deoxycytidine kinase. Pure recombinant mouse and human enzymes show differences in substrate specificity. J Biol Chem 1994;269:24374–8. [PubMed] [Google Scholar]
- 22.Chen EH, Johnson EE, Vetter SM, et al. Characterization of the deoxycytidine kinase promoter in human lymphoblast cell lines. J Clin Invest 1995;95:1660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spasokoukotskaja T, Arner ES, Brosjo O, et al. Expression of deoxycytidine kinase and phosphorylation of 2-chlorodeoxyadenosine in human normal and tumour cells and tissues. Eur J Cancer 1995;31A:202–8. [DOI] [PubMed] [Google Scholar]
- 24.Hatzis P, Al Madhoon AS, Jullig M, et al. The intracellular localization of deoxycytidine kinase. J Biol Chem 1998;273:30239–43. [DOI] [PubMed] [Google Scholar]
- 25.Schouten-van Meeteren AY, Van der Valk P, Van der Linden HC, et al. Histopathologic features of retinoblastoma and its relation with in vitro drug resistance measured by means of the MTT assay. Cancer 2001;92:2933–40. [PubMed] [Google Scholar]
- 26.Schouten van Meeteren AY, Van der Valk P, Van der Linden HC, et al. Features of proliferation and in vitro drug resistance in central primitive neuro-ectodermal tumours. Neuropathol Appl Neurobiol 2002;28:200–9. [DOI] [PubMed] [Google Scholar]
- 27.Van der Wilt CL, Kroep JR, Loves WJ, et al. Expression of deoxycytidine kinase in leukaemic cells compared with solid tumour cell lines, liver metastases and normal liver. Eur J Cancer 2003;39:691–7. [DOI] [PubMed] [Google Scholar]
- 28.Kaspers GJ, Zwaan CM, Pieters R, et al. Cellular drug resistance in childhood acute myeloid leukemia. A mini-review with emphasis on cell culture assays. Adv Exp Med Biol 1999;457:415–21. [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–74. [PubMed] [Google Scholar]
- 30.Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res 1987;78:858–68. [PubMed] [Google Scholar]