Abstract
Background: It is still not clear whether native or platelet count adjusted platelet rich plasma (PRP) should be used for platelet aggregation measurements.
Aim: To evaluate the necessity of using adjusted PRP in platelet function testing.
Methods: Platelet aggregation with native PRP and adjusted PRP (platelet count: 250/nl, obtained by diluting native PRP with platelet poor plasma) was performed on the Behring Coagulation Timer (BCT®) using ADP, collagen, and arachidonic acid as agonists. Healthy subjects, patients on antiplatelet treatment, and patients with thrombocytosis (platelet counts in PRP > 1250/nl) were investigated.
Results: No significant differences in the maximum aggregation response were seen when using either native or adjusted PRP from healthy subjects and patients on antiplatelet treatment. Nevertheless, some patients taking aspirin or clopidogrel showed reduced inhibition of ADP and arachidonic acid induced aggregation in adjusted PRP but not in native PRP. The maximum velocity of healthy subjects and patients on antiplatelet treatment varied significantly as a result of the degree of dilution of the adjusted PRP. Surprisingly, the BCT provided good results when measuring platelet aggregation of native PRP from patients with thrombocytosis, whereas commonly used aggregometers could not analyse platelet aggregation of native PRP in these patients.
Conclusion: The time consuming process of PRP adjustment may not be necessary for platelet aggregation measurements. Moreover, using adjusted PRP for monitoring aspirin or clopidogrel treatment may falsify results. Therefore, it may be better to use native PRP for platelet aggregation measurements, even in patients with thrombocytosis.
Keywords: platelet aggregation, platelet rich plasma, antiplatelet treatment, thrombocytosis
Platelet aggregometry, developed in the 1960s, revolutionised the ability to identify and diagnose alterations in platelet function.1 The use of a panel of platelet agonists such as ADP or collagen triggers classic platelet responses, including shape change and primary and secondary aggregation.2–4 The recorded response depends mainly upon the functioning of the platelets.5,6 Optical aggregometers are widely used in clinical situations to diagnose platelet dysfunction, and work on the principle that the light transmission of platelet rich plasma (PRP) increases with the progress of platelet aggregation,.7–9 However, the wide variety of methods for platelet aggregation measurement hampers the standardisation of platelet aggregometry.8
“The use of a panel of platelet agonists such as ADP or collagen triggers classic platelet responses, including shape change and primary and secondary aggregation”
This paper focuses on the controversial issue of using PRP that has been adjusted to a certain platelet count for platelet aggregation measurements. In general, adjusted PRP is used to compare platelet aggregation results between different patients.10 Investigations of aspirin or clopidogrel resistance have been performed using platelet count adjusted PRP.11–13 However, this common practice does not reflect the individual native response to platelet agonists.14 Moreover, the adjustment of PRP using platelet poor plasma (PPP) may falsify results. PPP is obtained by vigorous centrifuging. Thus, physiological agonists are released from platelets activated by the high shear stress and adjusting PRP with PPP may affect platelet aggregation measurements. The aim of our study was to compare platelet aggregation investigations using either native or platelet count adjusted PRP. Furthermore, this is the first study to report platelet aggregation results of patients with thrombocytosis using native PRP.
METHODS
Subjects
Twenty five healthy subjects (13 women, 12 men) without a history of bleeding disorders were included in our study. Their median age was 36 years (range, 20–65). During the 10 days before the examination they had not taken acetylsalicylic acid (ASA), non-steroidal anti-inflammatory drugs, or other medications known to affect platelet function. In addition, we also studied 10 patients (three men, seven women; median age, 62 years) with arterial occlusive diseases treated with ASA 100 mg/day (seven of 10), clopidogrel 75 mg/day (one of 10), or a combination of clopidogrel and ASA (two of 10) as antiplatelet agents. Furthermore, we investigated platelet aggregation in six patients (four men, two women; median age, 46 years) with thrombocytosis. Their platelet count in PRP was above 1250 platelets/nl. Two of these patients were treated with ASA 100 mg/day.
Informed consent was obtained from all subjects and our study was approved by the local ethics committee.
Blood samples
Blood was drawn by clean venipuncture from an antecubital vein using a 21 gauge butterfly cannula system (Multifly®-Set; 21G × 1½ TW, 0.8 × 19 mm; Sarstedt, Nümbrecht, Germany). Blood was collected in the morning between 8.00 and 12.00 am into plastic syringes (Monovette®; Sarstedt). First, an EDTA blood sample was drawn for a full blood cell count, followed by two 3 ml tubes containing 0.106 mol/litre trisodium citrate for analysing platelet aggregation by optical aggregometry. We ensured that the samples were mixed adequately by gently inverting the tubes. To obtain comprehensible results tests were performed only if platelet counts of the EDTA samples were above 100/nl. PRP was obtained by centrifuging citrated whole blood at room temperature at 140 ×g for five minutes. The platelet count was measured on the Sysmex® KX-21, an automatic multiparameter blood cell counter. In the healthy subjects, the native PRP platelet count ranged between 348 and 711/nl, in the patients undergoing antiplatelet treatment it ranged between 320 and 717/nl, and in the patients with thrombocytosis it was between 1258 and 1535/nl. To produce PPP (platelet count < 10/nl), citrated whole blood was centrifuged more vigorously at 1500 ×g for 15 minutes. Adjusted PRP with a platelet count of 250/nl was obtained by diluting native PRP with the subject’s PPP.
Platelet aggregation
The platelet function test on the fully automated Behring Coagulation Timer (BCT®; Dade Behring, Düdingen, Switzerland) was used for investigations of platelet function. The BCT detects platelet aggregate formation in PRP by changes in light transmission (wavelength, 620 nm) at 37°C. Platelet aggregation agonists are introduced automatically into the PRP. We used the agonists ADP (AppliChem, Darmstadt, Germany), collagen (Moelab, Hilden, Germany), and arachidonic acid (Moelab) to stimulate aggregation. The final agonist concentration in PRP was 2 μmol/litre for ADP, 0.21 mg/ml for collagen, and 0.55 mg/ml for arachidonic acid. The BCT measures the maximum aggregation response, the maximum angle, and the maximum velocity of the aggregation curve. In addition, the difference between the beginning and the endpoint of the absorption curve can be obtained. On the BCT it is possible to measure two samples at the same time.
Statistical analysis
Agreement between native and platelet count adjusted platelet aggregation tests was assessed by SPSS Software package. The Mann-Whithney U test was used and p values < 0.05 were considered significant.
RESULTS
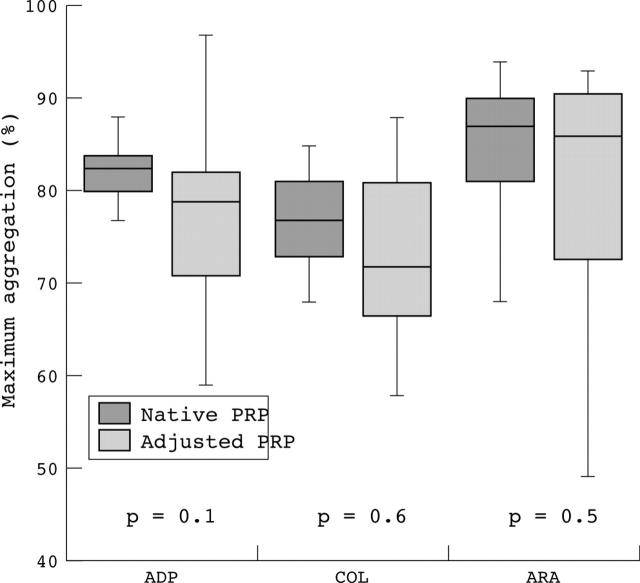
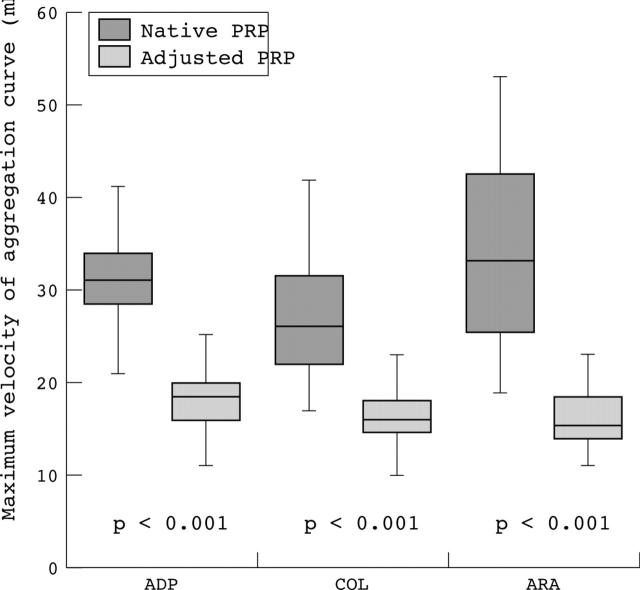
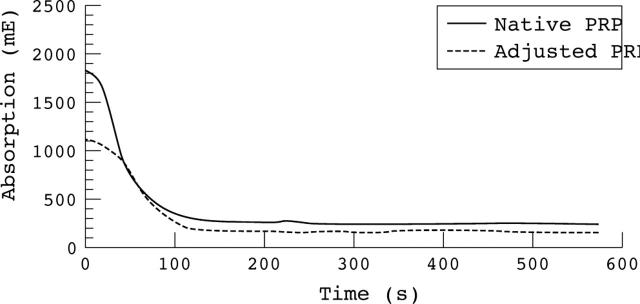
The results of the maximum aggregation response of the healthy subjects for different agonists did not differ significantly using either native or adjusted platelet rich plasma for platelet aggregation investigations. The variation in the results was much larger when using adjusted PRP (fig 1). In contrast, the results of the maximum velocity of aggregation curve showed a significant difference using native or adjusted PRP for platelet aggregation (fig 2). When using native PRP, the aggregation curve started at much higher values than when using platelet count adjusted PRP (fig 3).
Figure 1.
Maximum aggregation response using different aggregation agonists. Maximum aggregation response of healthy subjects in per cent on the Behring Coagulation Timer using ADP, collagen (COL), and arachidonic acid (ARA) as agonists. No significant difference was seen using native (dark boxes) or adjusted (light boxes) platelet rich plasma (PRP).
Figure 2.
Maximum velocity of the aggregation curve using different agonists. Maximum velocity of the aggregation curve of healthy subjects on the Behring Coagulation Timer using ADP, collagen (COL), and arachidonic acid (ARA) as agonists. A significant difference was seen using native (dark boxes) or adjusted (light boxes) platelet rich plasma (PRP).
Figure 3.
Aggregation curves obtained when using native and adjusted platelet rich plasma (PRP). The absolute extinction is decreased when using platelet count adjusted plasma for platelet aggregation measurements.
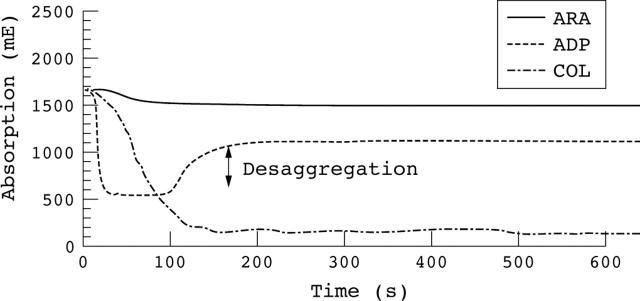
Figure 4 shows the typical aggregation curve of patients taking ASA as antiplatelet treatment.
Figure 4.
Typical aggregation curve obtained for patients taking acetylsalicylic acid (ASA). The ADP induced aggregation shows a desaggregation in the curve. The collagen (COL) induced aggregation is not inhibited, whereas the arachidonic acid (ARA) induced aggregation is inhibited by ASA.
Table 1 shows the aggregation curve data for the patients undergoing antiplatelet treatment.
Table 1.
Aggregation response at the end of the aggregation curve and maximum velocity of patients undergoing antiplatelet treatment using native and adjusted PRP
| Patient | Age (years) | Sex | Antiplatelet treatment | Platelets/nl in native and adjusted PRP | Aggregation response at end of aggregation (%)* | Max. velocity (mE/sec) | ||||
| ADP | COL | ARA | ADP | COL | ARA | |||||
| 1 | 56 | M | ASA | Native: 320 | 15 (des: 75) | 89 | 6 | 40 | 25 | 3 |
| Adjusted: 255 | 10 (des: 70) | 80 | 5 | 30 | 21 | 2 | ||||
| 2 | 58 | M | ASA | Native: 639 | 45 (des: 30) | 87 | 16 | 38 | 29 | 53 |
| Adjusted: 268 | 35 (des: 10) | 76 | 14 | 12 | 10 | 32 | ||||
| 3 | 66 | F | ASA | Native: 364 | 60 (des: 10) | 76 | 35 | 23 | 16 | 5 |
| Adjusted: 265 | 52 (des: 13) | 68 | 22 | 12 | 8 | 2 | ||||
| 4 | 80 | F | ASA | Native: 382 | 50 (des: 16) | 80 | 38 | 21 | 14 | 4 |
| Adjusted: 253 | 55 | 68 | 30 | 10 | 7 | 2 | ||||
| 5 | 41 | F | ASA | Native: 600 | 46 (des: 30) | 87 | 17 | 39 | 24 | 3 |
| Adjusted: 265 | 44 (des: 20) | 82 | 20 | 14 | 8 | 3 | ||||
| 6 | 64 | F | C | Native: 717 | 50 (des: 20) | 83 | 79 | 37 | 31 | 44 |
| Adjusted: 250 | 20 (des: 20) | 86 | 27 (des: 20) | 12 | 11 | 10 | ||||
| 7 | 46 | F | ASA | Native: 500 | 60 (des: 25) | 84 | 11 | 49 | 29 | 7 |
| Adjusted: 244 | 54 (des: 15) | 79 | 18 | 18 | 12 | 3 | ||||
| 8 | 78 | F | ASA+C | Native: 393 | 1 (des: 12) | 55 | 3 | 10 | 16 | 1 |
| Adjusted: 248 | 2 (des: 10) | 52 | 4 | 8 | 10 | 1 | ||||
| 9 | 68 | M | ASA | Native: 559 | 21 (des: 15) | 56 | 21 | 21 | 18 | 4 |
| Adjusted: 256 | 25 (des: 17) | 52 | 21 | 11 | 10 | 6 | ||||
| 10 | 66 | F | ASA+C | Native: 442 | 59 (des: 20) | 79 | 25 | 33 | 12 | 8 |
| Adjusted: 260 | 55 (des: 15) | 79 | 31 | 16 | 11 | 3 | ||||
*des, desaggregation in %.
ARA, arachidonic acid; ASA, acetylsalicylic acid; C, clopidogrel; COL, collagen; PRP, platelet rich plasma.
Patient 6 had the highest platelet count and showed no inhibition of the ADP induced aggregation although taking clopidogrel. This effect was seen only when platelet aggregation was measured in native PRP. Using adjusted PRP for measurements the ADP and arachidonic acid induced aggregation seemed to be inhibited by clopidogrel. In all patients, the maximum velocity was significantly reduced when using platelet count adjusted PRP for aggregation measurements with different agonists. This was caused by the reduced platelet count after adjustment of PRP.
Surprisingly, the results obtained when measuring platelet aggregation on the BCT with native PRP from patients with thrombocytosis were good (table 2).
Table 2.
Aggregation response, maximum velocity, and starting point of the aggregation curve for patients with thrombocytosis measured on the BCT using native PRP
| Patient | Age (years) | Sex | Antiplatelet treatment | Platelets/nl in native PRP | Aggregation response at end of aggregation (%) | Max. velocity (mE/sec) | Start of aggregation (mE) | ||||||
| ADP | COL | ARA | ADP | COL | ARA | ADP | COL | ARA | |||||
| 1 | 22 | F | ASA | 1535 | 83 | 75 | 82 | 55 | 53 | 64 | 3199 | 2846 | 2842 |
| 2 | 50 | M | – | 1471 | 90 | 82 | 91 | 71 | 57 | 78 | 3289 | 2900 | 2892 |
| 3 | 55 | M | – | 1487 | 81 | 79 | 80 | 47 | 46 | 62 | 3025 | 2720 | 2842 |
| 4 | 43 | M | ASA | 1258 | 31 (des: 50) | 81 | 1 | 56 | 36 | 2 | 3326 | 3079 | 2967 |
| 5 | 42 | F | – | 1490 | 4 (des: 29) | 81 | 88 | 25 | 54 | 65 | 2591 | 2400 | 2393 |
| 6 | 61 | M | – | 1534 | 78 | 75 | 83 | 50 | 49 | 70 | 3221 | 3052 | 3064 |
*des, desaggregation in %.
ARA, arachidonic acid; ASA, acetylsalicylic acid; C, clopidogrel; COL, collagen; PRP, platelet rich plasma.
As seen in table 2, the aggregation curve of patients with thrombocytosis started at much higher values (2300–3300 mE) than in patients with normal platelets counts (∼ 1500 mE). In most cases, the maximum velocity and angle of the aggregation curve were also increased compared with the normal range (normal range for maximum velocity, 15–45 mE/sec). Although patient 1 was taking ASA 100 mg/day, the arachidonic acid induced aggregation was not inhibited as it was in patient 4. This may indicate non-responsiveness to that dose of ASA for patient 1. In contrast, patient 5 showed desaggregation of ADP induced aggregation although taking no medication.
No aggregation response could be detected when the same samples from these patients were measured on an optical manual aggregometer (PAP-4; Moelab) using native PRP. The special software available on the BCT enables accurate and precise measurement of the platelet aggregation response. Therefore, platelet aggregation in patients with thrombocytosis whose platelet counts are > 1500/nl in native PRP can be analysed on the BCT.
DISCUSSION
Clearly defined procedures for blood sampling and preanalytical handling are necessary for all specialised coagulation laboratories, but these procedures may vary according to whether the laboratory is a research or a routine clinical laboratory.10 There are various methods to measure platelet aggregation,5 but international standardisation criteria have not yet been defined. Our study focused on the controversial method of using platelet count adjusted PRP to measure platelet aggregation. PRP adjustment is usually performed to standardise and compare platelet aggregation results between patients. However, using platelet count adjusted PRP may falsify the individual responses to platelet agonists.
“Platelet poor plasma is produced by vigorous centrifugation, and physiological agonists may be released by the platelets, which are activated by shear stress”
We found that platelet function can be compared between patients without adjusting PRP. There was no significant difference in the values of the maximum aggregation response. As expected, the maximum velocity of aggregation was reduced when using adjusted PRP, because adjustment reduces the number of platelets. The maximum aggregation response varied greatly in healthy subjects when using adjusted PRP. This may result from increased amounts of physiological agonists in adjusted PRP, which is diluted with PPP. PPP is produced by vigorous centrifugation, and physiological agonists may be released by the platelets, which are activated by shear stress. Therefore, it is more useful to measure platelet aggregation with native PRP because this platelet response reflects more accurately the in vivo situation.
The investigation of patients on antiplatelet treatment indicated that in some cases there are significant differences between platelet aggregation using native or adjusted PRP. No inhibition of ADP induced aggregation was seen in one patient taking clopidogrel when platelet aggregation was measured using native PRP. Using platelet count adjusted PRP from the same patient, ADP and arachidonic acid induced aggregation appeared to be inhibited by clopidogrel. Studies describing aspirin or clopidogrel responsiveness have been based on laboratory methods using adjusted PRP,11–13 but this may lead to false results. Therefore, native PRP can be used for platelet aggregation, and the time consuming process of platelet count adjustment is not needed for platelet function tests. The individual response to platelet agonists can be analysed using native PRP, even in patients with thrombocytosis.
The aggregation curve of patients with thrombocytosis started at much higher values than in patients with normal platelet counts, and the maximum velocity and angle of the aggregation curve were also increased in most cases compared with the normal range. Arachidonic acid induced aggregation was not inhibited in one patient who was taking acetylsalicylic acid 100 mg/day, perhaps because this patient was not responsive to this dose of ASA. In contrast, another patient showed desaggregation of ADP induced aggregation although taking no medication. As described in the article of Schafer,15 patients with secondary thrombocytosis may suffer from thrombotic, vascular, and also bleeding complications. Platelet function abnormalities responsible for a bleeding tendency can be diagnosed by platelet aggregation tests. The treatment of patients with thrombocytosis with ASA could also be monitored by platelet aggregation tests. We recommend that investigations should be performed using native PRP, because the individual response of these patients to different agonists is most accurately tested by using native PRP, as our results show. Patients with an increased number of platelets showed different responses to antiplatelet drugs. However, further clinical trials with larger numbers of patients are urgently needed to validate the clinical relevance of platelet aggregation measurement with native PRP.
Take home messages.
No significant differences in the maximum platelet aggregation response were seen when using either native or adjusted platelet rich plasma (PRP) from healthy subjects and patients on antiplatelet treatment
The time consuming process of PRP adjustment may not be necessary for platelet aggregation measurements, even in patients with thrombocytosis and when comparing patients
Using adjusted PRP for monitoring aspirin or clopidogrel treatment may falsify results, so that it may be better to use native PRP
Acknowledgments
We thank Dr HU Pfeiffer from the Red Cross Blood Donor Service Baden-Würtemberg/Hessen, Frankfurt, Germany for his friendly support and Professor HK Breddin for critically reading the manuscript.
Abbreviations
ASA, acetylsalicylic acid
BCT, Behring Coagulation Timer
PPP, platelet poor plasma
PRP, platelet rich plasma
REFERENCES
- 1.Yardumian DA, Mackie IJ, Machin SJ. Laboratory investigation of platelet function: a review of methodology. J Clin Pathol 1986;39:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.British Society for Haematology. Guidelines on platelet function testing. J Clin Pathol 1988;4:1322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost 2001;86:222–32. [PubMed] [Google Scholar]
- 4.Pongracz E. Measurement of platelet aggregation during antiplatelet therapy in ischemic stroke. Clin Hemorheol Microcirc 2004;30:237–42. [PubMed] [Google Scholar]
- 5.Moffat KA, Ledford-Kraemer MR, Nichols WL, et al. Variability in clinical laboratory practice in testing for disorders of platelet function. Thromb Haemost 2005;93:549–53. [DOI] [PubMed] [Google Scholar]
- 6.De Gaetano G, Donati MB, Garattini S. The effects of drugs on platelet function tests. Thromb Haemost 1976;36:281. [PubMed] [Google Scholar]
- 7.Breddin HK, Harder S. Usefulness of tests to measure platelet function. Vasa 2003;32:123–9. [DOI] [PubMed] [Google Scholar]
- 8.Walker ID. Blood collection and sample preparation: pre-analytical variation. In: Jespersen J, Bertina RM, Haverkate F, eds. Laboratory techniques in thrombosis, a manual. Second revised edition of ECAT assay procedure. Dortrecht, the Netherlands: Kluwer Academic Publisher 1999:21–8.
- 9.Breddin HK, Grun H, Krzywanek HJ, et al. On the measurement of platelet aggregation. Thromb Haemost 1976;35:669–91. [PubMed] [Google Scholar]
- 10.Hutton RA, Ludlam CA. ACP Broadsheet 122. Platelet function testing. J Clin Pathol 1989;42:858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gum PA, Kottke MK, Welsh PA, et al. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol 2003;41:961–5. [DOI] [PubMed] [Google Scholar]
- 12.Matetzsky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004;109:3171–5. [DOI] [PubMed] [Google Scholar]
- 13.Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischaemic stroke. Stroke 1994;25:2331–6. [DOI] [PubMed] [Google Scholar]
- 14.Budde U. Diagnosis of platelet function defects with platelet aggregometers. J Lab Med 2002;26:564–71. [Google Scholar]
- 15.Schafer A. Thrombocytosis. N Engl J Med 2004;350:1211–19. [DOI] [PubMed] [Google Scholar]