Abstract
Background: Sentinel node (SN) status is the most important prognostic indicator in patients with cutaneous melanoma without clinically evident metastatic spread, but the procedure is associated with considerable morbidity. The LYVE-1 lymphatic marker offers the possibility of studying lymphangiogenesis and tumour metastasis within the primary excision.
Aims: To establish whether lymphatic vessel numbers/distribution within the primary tumour correlated with SN status. To assess whether tumour cells were easily demonstrable within lymphatics and could be used as a surrogate for SN status.
Methods: Double immunostaining for LYVE-1 and S100 in cutaneous biopsies from 18 SN+ patients with no lymphatic/vascular involvement on routine histology and 18 SN− patients matched for tumour thickness and ulceration.
Results: Lymphatic vessels were detected in all cases. Vessels within the tumour mass were suggestive of active lymphangiogenesis; those outside were mainly mature vessels with well defined walls. Tumour cells within lymphatics were detected in one of 18 SN− and five of 18 SN+ patients. Lymphatics containing tumour cells were all outside the tumour mass in well formed vessels, suggesting melanoma cell invasion into preformed lymphatics. There was no significant difference in lymphatic counts between SN+ and SN− patients. Although peritumorous lymphatic counts were higher in ulcerated than non-ulcerated melanomas, they did not vary with Breslow thickness.
Conclusion: LYVE-1 staining can reliably demonstrate lymphatic vessel distribution, but lymphatic counts cannot predict melanoma metastatic potential and cannot substitute for SN biopsy. LYVE-1 immunostaining can detect melanoma cells within lymphatics, but is unreliable in predicting melanoma metastasis, failing to detect metastatic spread in more than two thirds of patients with regional node metastasis.
Keywords: LYVE-1, malignant melanoma, lymphatic vessel, sentinel node biopsy, sentinel node status
The sentinel node status is the single most important prognostic factor in patients with cutaneous malignant melanoma,1–5 and is an integral part of the new American Joint Committee on Cancers (AJCC) staging system.6 The technique was initially described by Morton et al in 1992,7 and has since been modified by incorporating lymphoscintigraphy with intradermal injection of vital blue dye. This combined technique has greater success in identifying the sentinel node, approaching rates of up to 100%.8,9,10,11,12,13,14
“Until recently, there has been no marker specific for lymphatic vessels to enable their distinction from blood vessels”
When combined with conventional histology and immunostaining, the sentinel node technique is highly sensitive, with a false negative rate of only 2%. This figure is derived from 11 studies with 93 true nodal relapses out of 4750 sentinel node negative subjects.14a The sensitivity can be further enhanced by combining conventional histology and immunostaining with reverse transcription polymerase chain reaction.5,15–18 However, this is not normally used to guide management in individual patients because of a high rate of false positivity. In addition to providing crucial and accurate staging, sentinel lymph node biopsy (SLNB) is a minimally invasive method of identifying a group of patients who are harbouring occult nodal metastases and may benefit from therapeutic lymph node dissection and/or adjuvant treatment. A recent meta-analysis has confirmed an unequivocal benefit for interferon α in extending relapse free survival, and a marginal benefit for high dose interferon in prolonging overall survival.19 In addition, a retrospective study by Morton and colleagues has shown a 10 year survival benefit of 69% for sentinel node positive patients who underwent therapeutic lymph node dissection after SLNB compared with 37% for patients who underwent lymph node dissection at nodal relapse following initial surgery, which involved a wide excision only.20
In malignant melanoma, metastasis occurs via blood and lymphatic vessels. Angiogenesis has been studied extensively in the past with conflicting results. Therefore, its role in tumour spread remains controversial.21–23 Until recently, there has been no marker specific for lymphatic vessels to enable their distinction from blood vessels. Thus, although haemangiogenesis has been well investigated, little was known regarding tumour lymphangiogenesis. Recently, the identification of LYVE-1 (lymphatic vessel endothelial hyaluronan receptor 1), a lymph specific receptor for hyaluronan and a homologue of CD44, has provided a useful tool for research into tumour lymphangiogenesis.24 Hyaluronan is an extracellular matrix glycosaminoglycan present in the tissue matrix and body fluid of all vertebrates.25 LYVE-1 binds to hyaluronan on the luminal surface of lymphatic vessels and is completely absent from blood vessels. This has been demonstrated by double immunofluorescence staining with antibodies to LYVE-1 and to the vascular endothelial markers CD34 and von Willebrand factor.24 The only other sites of LYVE-1 expression are sinusoidal endothelial cells within the spleen, placental syncytiotrophoblasts,24 liver sinusoidal endothelium, and alveolar lining epithelium.26
A previous retrospective study reported increased lymphatic density in cutaneous biopsies of patients with clinically evident nodal metastases within one year of a diagnosis of primary cutaneous melanoma. The study also suggested that lymphatic density might be used as a novel prognostic indicator for the risk of lymph node metastasis in cutaneous melanoma.27 We were interested to know whether there is a difference in lymphangiogenesis when comparing SLNB positive and negative patients. In particular, we wished to establish whether LYVE-1 staining could substitute for sentinel node status and therefore avoid an invasive method of staging patients with melanoma.
MATERIALS AND METHODS
Patient selection
Subjects were identified retrospectively from a database of patients with malignant melanoma who had undergone SNLB preceded by preoperative lymphoscintigraphy between 1997 and 2001. Further material was available for the primary melanoma on 18 SLNB positive patients and these were matched with 18 SLNB negative patients. In none of these cases had lymphatic invasion been reported in the initial histological examination of a haematoxylin and eosin (H+E) section from the primary tumour. The two groups were matched for Breslow thickness and presence of ulceration, the two criteria that have independent prognostic value in the AJCC staging system (table 1). Serial sections from the original block were then cut and stained with H+E and double stained with S100 and LYVE-1 (see below).
Table 1.
Characteristics of the patients studied
| Characteristic | Sentinel node positive (n = 18) | Sentinel node negative (n = 18) |
| Stage T2a (Breslow 1.01–2 mm; non-ulcerated) | 4 | 5 |
| Median age at diagnosis (range) | 49 (35–59) | 48 (32–67) |
| Male/ Female | 1/3 | 3/2 |
| Stage T2b (Breslow 1.01–2 mm; ulcerated) | 2 | 2 |
| Median age at diagnosis (range) | 49 (36–61) | 37 (35–38) |
| Male/ Female | 1/1 | 1/1 |
| Stage T3a (Breslow 2.01–4 mm; non-ulcerated) | 4 | 5 |
| Median age at diagnosis (range) | 55 (35–82) | 52 (31–67) |
| Male/Female | 1/3 | 2/3 |
| Stage T3b (Breslow 2.01–4 mm; ulcerated) | 6 | 5 |
| Median age at diagnosis (range) | 56 (40–81) | 54 (30–60) |
| Male/Female | 6/0 | 4/1 |
| Stage T4a (Breslow >4 mm; non-ulcerated) | 2 | 3 |
| Median age at diagnosis (range) | 39 (28–50) | 60 (55–66) |
| Male/Female | 1/1 | 2/1 |
Histological staining
Paraffin wax embedded sections (4 μm thick) were cut and dried overnight at 37°C on to poly-L-lysine coated slides (superfrosted plus slides; VWR International, Poole, Dorset, UK). A routine H+E stain was performed on all sections studied. Immunocytochemistry involved double labelling using antibodies against polyclonal anti-S100 protein (supplied by Dakocytomation, Ely, Cambridgeshire, UK) and lymphatic endothelium (LYVE-1: gift from D Jackson, Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, UK).
The histological sections were dewaxed in xylene for four minutes and brought to water through graded alcohols. Slides were then trypsinised for 10 minutes in 100 ml distilled water containing 0.1 g trypsin in 0.01M calcium chloride, pH 7.0, at 37°C. The Envision double labelling system (Dakocytomation) was used for double labelling. After trypsinisation the slides were transferred to running tap water for 10 minutes and then incubated in a peroxide block for five minutes. They were then rinsed in water and transferred to Tris buffered saline (TBS). The first primary antibody was applied (anti-S100 protein; Dakocytomation) at a dilution of 1/2000. Sections were incubated for 30 minutes in a moist incubation chamber, followed by rinsing in TBS. A secondary linking polymer antibody bound to horseradish peroxidase was then applied for 30 minutes. TBS was used to rinse the slides and liquid DAB plus was added to develop the final reaction products for 10 minutes.
After completion of the primary staining sequence, slides were subjected to a second antigen retrieval step involving placing them in 500 ml of 0.01M sodium citrate solution, pH 6.0, and microwaving for 10 minutes at 700 W. After rinsing in running tap water, a double stain block solution was applied for three minutes (Dakocytomation), followed by a rinse in TBS. LYVE-1 was applied (1/500 dilution) to the tissue sections for 30 minutes in a moist incubation chamber and the slides were again rinsed in TBS. A secondary linking polymer antibody bound to alkaline phosphatase was added for 30 minutes, followed by another rinse in TBS. The sections were then treated with a substrate-chromagen solution (fast red) for 15 minutes to develop the final reaction products. Slides were rinsed in TBS for a final time and counterstained in Mayer’s haematoxylin for 30 seconds, after which they were mounted in Faramount aqueous mounting medium (Dakocytomation).
Histological review
The diagnosis of malignant melanoma, the Breslow thickness, and the presence or absence of ulceration were reviewed in all cases by two experienced dermatopathologists (RRJ and AR).
Estimation of lymph vessels using Chalkley point counting
The double immunocytochemically stained slides were assessed for lymphatic vessels using the guidelines for Chalkley point counting.28 The S100/ LYVE-1 stained sections were first scanned at low power to locate lymphatic hotspots, which were defined as a focus of LYVE-1 positive stained lymph vessels. Individual hotspots were then examined at ×40 magnification, using a Chalkley point array graticule mounted on an eyepiece. Each hotspot was given a score dependent on the number of points on the eyepiece coinciding with a LYVE-1 stained lymphatic vessel endothelium. An average score was calculated for each section based on the three highest hotspot scores counted.
We performed Chalkley counts of lymphatic proliferation in both peritumorous and tumorous regions for each melanoma slide. Counts were performed blind by two of the authors (DS and AR) and the average taken for each hotspot. “Peritumorous” was defined as the area confined within one microscopic field of the tumour border at ×40 magnification. “Tumorous” was defined as the region within the tumour mass itself, inclusive of stromal tissue and invagination of non-neoplastic tissue into the tumour. The tumorous lymphatic count (T-LC) included both intertumorous—that is, lymphatic vessels in the spaces between theques of tumour cells—and intratumorous vessels, which were compressed by or arising within a tumour nodule. Separate counts were performed for peritumorous lymphatics (PT-LC) and T-LC. In addition, intratumorous lymphatics (IT-LC) were counted separately and compared with the three highest hotspot scores among peritumorous and inter-peritumorous lymphatics combined (IPT-LC). In addition to the Chalkley scores, the presence of tumour cells within lymphatic vessels was also noted.
Statistical analysis
Comparisons between groups of continuous data were carried using either the Mann-Whitney or Kruskal-Wallis test, whereas for categorical data Fisher’s exact test was used. Group comparisons of paired data were made using the Wilcoxon signed ranks test. All quoted p values are two sided.
RESULTS
LYVE-1 positive lymph vessels seen in both peritumorous and tumorous regions of melanoma
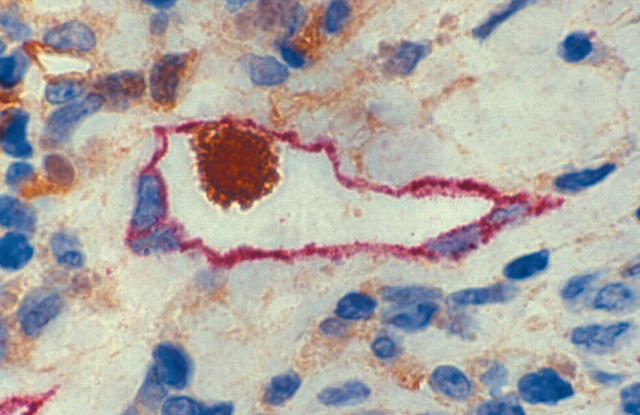
We sequentially assessed melanomas that had been stained with H+E and double labelled with immunocytochemistry for LYVE-1/S100. Lymphatic vessels were seen in all the melanoma slides studied, and were found to be distributed non-homogeneously as pockets of lymph vessel collections, which we termed hotspots. In general, we found mature lymphatic vessels with well defined endothelial cells and lumina devoid of blood cells in the peritumorous and intertumorous regions of the melanomas (fig 1). In contrast, intratumorous lymphatics had a fibrillary morphology without well defined lumina (fig 2). In addition to staining the lymphatic vessels, LYVE-1 also stained individual cells within the dermis, the origin of which is uncertain, although they may be precursor cells for new lymphatic vessels (fig 3).
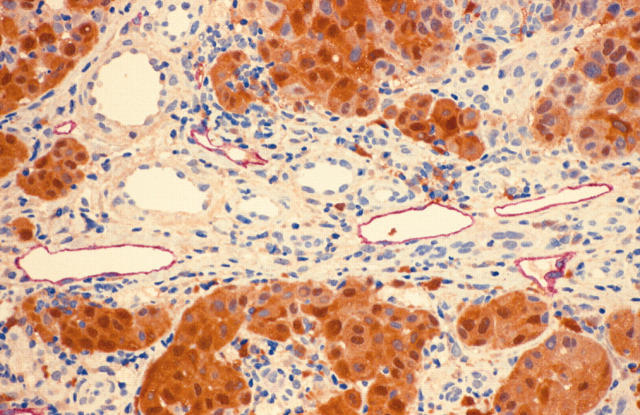
Figure 1.
Section of mature intertumorous lymphatic vessels stained with LYVE-1/S100 demonstrating well defined endothelial cells and a well formed lumen, devoid of blood cells. Note the four adjacent unstained blood vessels. (LYVE-1 stains red; S100 stains brown; original magnification, ×40).
Figure 2.
Section of an immature LYVE-1 positive lymphatic vessel within the tumour mass displaying a typical fibrillary pattern (LYVE-1/S100 stained section; LYVE-1 stains red; S100 stains brown; original magnification, ×100).
Figure 3.
Section showing individual dermal cells staining positively with LYVE-1. Note the mature LYVE-1 positive peritumorous lymphatic vessel and the adjacent unstained blood vessel. An S100 positive nerve bundle is present at the lower right pole (LYVE-1/S100 stained section; LYVE-1 stains red; S100 stains brown; original magnification, ×80).
Comparison of PT-LC with T-LC
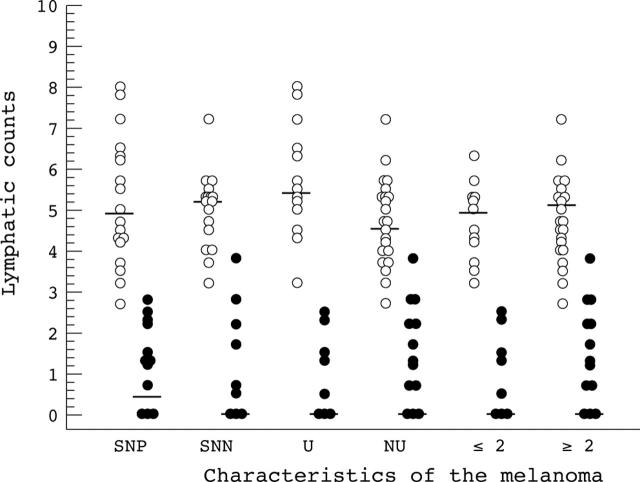
Tumorous lymphatics were defined as lymphatic vessels arising within the tumour mass, inclusive of stromal tissue invaginating between the tumour theques. The peritumorous region is classed as the area just outside the tumour margin, and in our study, within one microscopic field of the tumour border at ×40 magnification. Using this definition, we compared lymphatic vessel counts within the peritumorous and tumorous regions (fig 4).
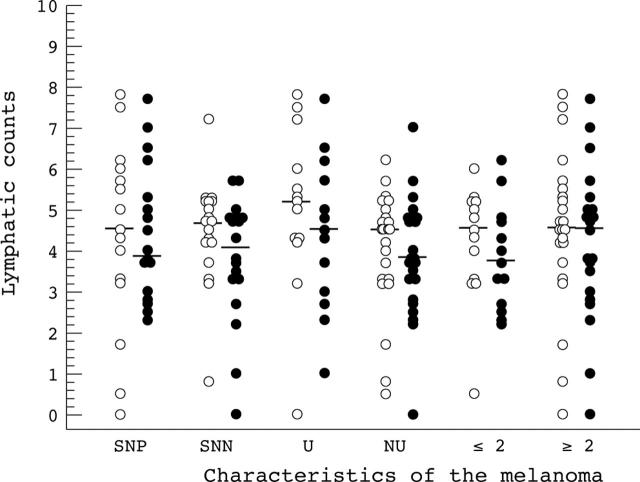
Figure 4.
Comparison of peritumorous lymphatic counts (open circles) and tumorous lymphatic counts (closed circles). SNP, sentinel node positive; SNN, sentinel node negative; U, ulcerated; NU, non-ulcerated; ⩽2/>2, Breslow thickness (mm).
In the SLNB positive group (18 patients), the PT-LCs had a median value of 4.5, with a range of 0.0–7.8, and the T-LCs had a median value of 3.9, with a range of 2.3–7.7. In the SLNB negative group (18 patients), the PT-LCs had a median value of 4.7, with a range of 0.8–7.2, and the T-LCs had a median value of 4.1, with a range of 0.0–5.7. PT-LC and T-LC were not significantly different between SLNB positive and SLNB negative patients (p = 0.82 and p = 0.57, respectively). No difference was found when PT-LC was compared with T-LC in SLNB positive and SLNB negative patients (p = 0.57 and p = 0.09, respectively).
The presence of ulceration in the primary tumour was used to divide patients into two groups—13 ulcerated melanomas with a median T-LC of 4.5 (range, 1.0–7.7) and 23 non-ulcerated tumours with a median T-LC of 3.8 (range, 0.0–7.0). Their difference was not significant (p = 0.36). A bigger difference was seen in the PT-LCs, with a median of 5.2 (range, 0.0–7.8) in the ulcerated tumours and median of 4.5 (range, 0.5–6.2) in the non-ulcerated melanomas. Their difference fell just short of significance (p = 0.08).
To investigate whether lymphatic vessel counts altered with tumour thickness, we divided the patients into those with thin tumours (Breslow, ⩽ 2 mm) and those with thick tumours (Breslow, > 2 mm). No significant difference was seen between thick and thin tumours in the PT-LCs (thin tumours: median, 4.5; range, 0.5–6.0; thick tumours: median, 4.5; range, 0.0–7.8; p = 0.58) or the T-LCs (thin tumours: median, 3.7; range, 2.2–6.2; thick tumours: median, 4.5; range, 0.0–7.7; p = 0.32).
Comparison of IPT-LC and IT-LC
It is possible that lymphatics arising within the tumour nodules are more important in determining lymphatic spread than intertumorous or peritumorous lymphatics, particularly because the intratumorous lymphatics showed a fibrillary morphology without well defined walls. This appearance suggests that lymphangiogenesis is occurring within the tumour mass itself. Therefore, we performed IT-TCs separately and compared these with IPT-LC (fig 5).
Figure 5.
Comparison of intertumorous and peritumorous lymphatic counts combined (open circles) with intratumorous lymphatic counts (closed circles). SNP, sentinel node positive; SNN, sentinel node negative; U, ulcerated; NU, non-ulcerated; ⩽ 2/>2, Breslow thickness (mm).
This revealed a significant increase in IPT-LC compared with IT-LC within SLNB positive patients (IPT-LC: median, 4.9; range, 2.7–8.0; IT-LC: median, 0.4; range, 0.0–2.8; p < 0.001) and a similar relation in SLNB negative patients (IPT-LC: median, 5.2; range, 3.2–7.2; IT-LC: median, 0.0; range, 0.0–3.8; p < 0.001). However, no difference was found when either IPT-LC or IT-LC was compared between SLNB positive and negative patients (p = 0.82 and p = 0.37, respectively).
We then compared lymphatic counts in ulcerated versus non-ulcerated melanomas. Again, there was no significant difference within the IT-LC group (ulcerated: median, 0.0; range, 0.0–2.5; non-ulcerated: median, 0.0; range, 0.0–3.8; p = 0.69), but there was a significant difference for the IPT-LC group, with a higher score among ulcerated tumours (ulcerated: median, 5.5; range, 3.2–8.0; non-ulcerated: median, 4.7; range, 2.7–7.2; p = 0.029).
The assessment of lymphatic counts as a function of Breslow thickness found no significant difference in either IPT-LC or IT-LC between thick and thin tumours (thin tumour IPT-LC: median, 5.0; range, 3.2–6.3; thick tumour IPT-LC: median, 5.2; range, 2.7–8.0; p = 0.28; thin tumour IT-LC: median, 0.0; range, 0.0–3.8; thick tumour IT-LC: median, 0.0; range, 0.0–2.8; p = 0.80).
Tumour emboli within lymphatic vessels
Double labelling with S100 and LYVE-1 allowed us to identify melanoma cells within lymphatic vessels. This can be difficult to visualise in H+E stained sections even with multiple sections (fig 6). None of the melanomas in our series had been reported as showing lymphatic invasion histologically. In the SLNB positive group we identified three patients with clumps of melanoma cells within a lymphatic, and examination of the serial H+E stained section revealed an identifiable deposit in one case only. In sections from four other patients there was a single S100 positive cell within a lymphatic vessel, and in two the cytology was that of a melanoma cell. In the two other cases there was doubt as to whether the S100 positive cells represented melanoma or an S100 positive antigen presenting cell. Further sections with staining for CD1a and MART-1 failed to resolve this issue because the cells in question were not present in the deeper sections.
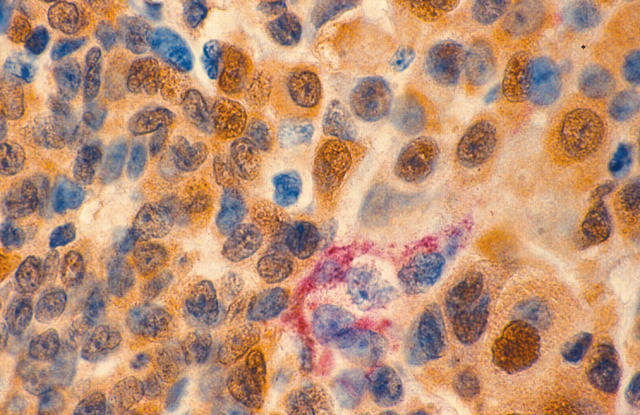
Figure 6.
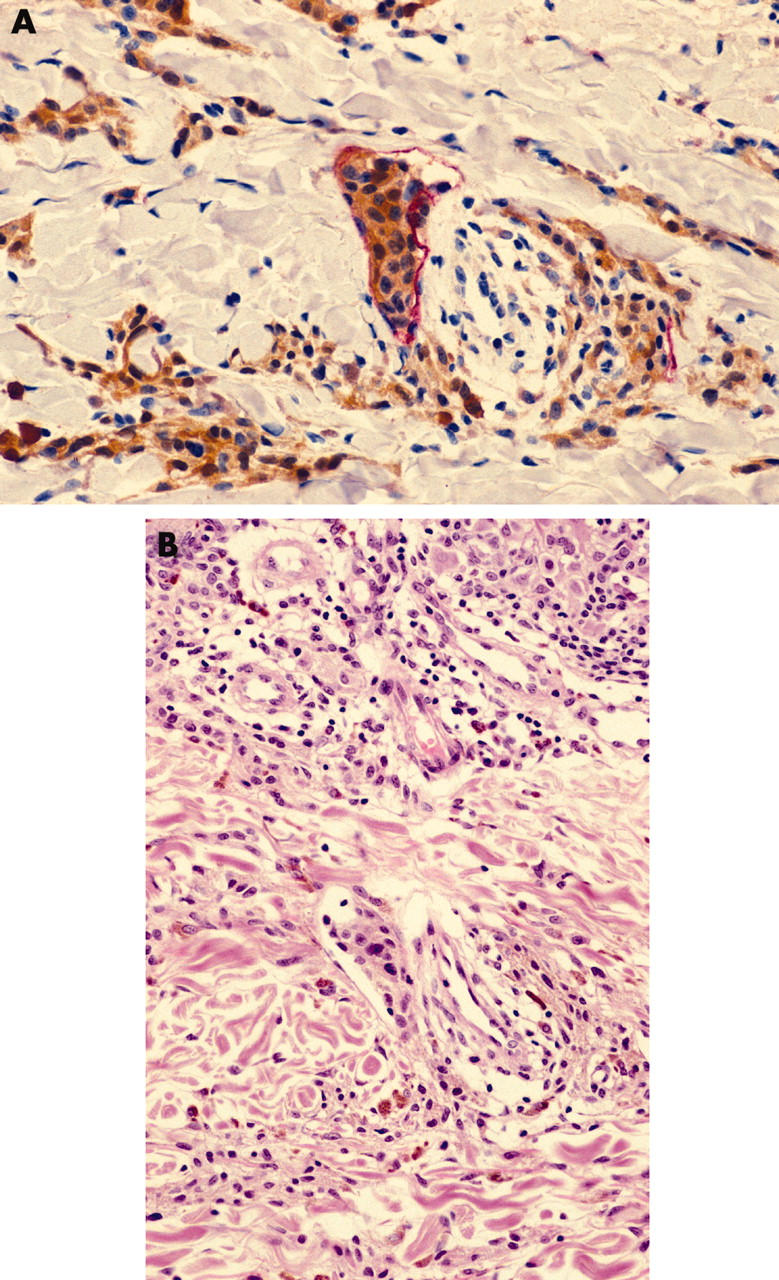
(A) LYVE-1/S100 stained section showing a cluster of melanoma cells within a LYVE-1 positive peritumorous lymphatic vessel (LYVE-1 stains red; S100 stains brown; original magnification, ×60). (B) A tumour embolus is present but less apparent in a serial section stained with haematoxylin and eosin (original magnification, ×60).
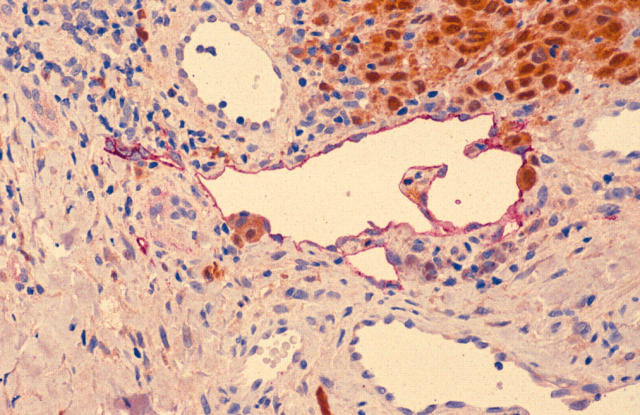
Within the SLNB negative group we identified a single melanoma cell within a lymphatic in one case only (fig 7), although interestingly this same case showed a group of melanoma cells lying immediately outside of a lymphatic within the deep reticular dermis and separate from the tumour itself. This was interpreted as an early in transit metastasis. The difference between the SLNB positive and negative groups is significant if all seven SLNB positive cases are included (p = 0.041), but not if only the five definite cases are included (p = 0.18).
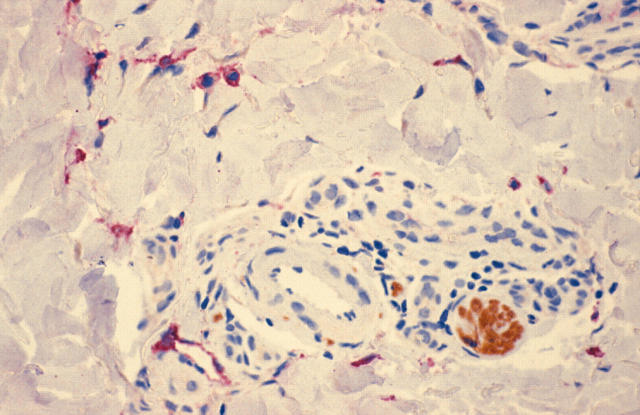
Figure 7.
LYVE-1/S100 stained section showing a single melanoma cell within a LYVE-1 positive lymphatic vessel (LYVE-1 stains red; S100 stains brown; original magnification, ×100).
In addition to finding tumour emboli within the lymphatic vessels we frequently saw melanoma cells impinging on lymph vessel walls in sentinel node positive and negative cases (fig 8).
Figure 8.
LYVE-1/S100 stained section showing a group of melanoma cells impinging on the wall of a peritumorous lymphatic vessel (LYVE-1 stains red; S100 stains brown; original magnification, ×60).
DISCUSSION
Selective lymphadenectomy was developed to identify patients who might benefit from a therapeutic lymph node dissection.7 It is now recognised as the most important prognostic factor in patients with melanoma, and sentinel node status has been incorporated into the new AJCC staging system for melanoma.29 However, the procedure is associated with postoperative complications including wound infections, lymphoedema, and nerve injury, and a less invasive method for staging patients with melanoma might avoid these problems. The discovery of LYVE-1 as a specific marker for lymphatic vasculature provides a possible non-invasive method for predicting disease outcome.
Staining with LYVE-1 enabled us to visualise malignant melanoma cells within lymphatic vessels, which were not apparent on routine H+E staining. In the node negative group, one patient (one of 18) showed melanoma cells within a lymph vessel compared with five (of 18) in the node positive group. The difference between the two groups was not significant (p = 0.18). S100 stained cells were also found in two additional node positive patients, although two independent histological opinions were unable to ascertain that these were definitely melanoma cells. Further stains failed to resolve this issue, and these two patients were therefore excluded. Interestingly, if they had been included the results would have reached significance, with a p value of 0.041. Even so, less than half and probably less than one third of SLNB positive patients showed evidence of lymphatic invasion histologically. Therefore, double immunostaining with LYVE-1 and S100 cannot be used as a substitute for the sentinel node status of a patient. Future studies may benefit from using a more specific melanoma marker such as MART-1, rather than S100, to avoid problems over interpretation of the cytology.
LYVE-1 also provides information on tumour lymphangiogenesis. Dadras et al found a significant difference in lymphatic density between patients with and without metastases, and suggested that the peritumorous lymphatic density may be used as a prognostic indicator of lymph node metastasis in patients with melanoma.27 We wished to investigate whether lymphangiogenesis could substitute for SLNB and therefore avoid the problems associated with this procedure. Our study, however, failed to demonstrate a significant difference in lymphatic counts between SLNB positive and negative individuals when applied to peritumorous, tumorous, and intratumorous lymphatics.
“Our study of the involvement of lymphatics in melanomas suggests that tumour cells gain access to the regional lymph nodes by invading preformed lymphatic vessels”
There are several reasons for the discordant findings between the two studies. First, the patients with metastasis delineated by Dadras had relapsed clinically, with nodal disease within a year of diagnosis, and this group must have had a very high metastatic potential. In contrast, our metastatic patients were identified by their sentinel node status—such patients have micrometastatic disease, but it is not possible to predict how quickly they would have relapsed had the nodal lesion been left undissected. Second, the Dadras group undertook a more complicated method of assessing lymphangiogenesis, which involved a computer assisted system for counting lymphatics per unit area (lymphatic density).27 We wished to investigate whether lymphangiogenesis could substitute for sentinel node status using a methodology that is routinely applicable to LYVE-1 stained sections. Lymphatic counts correlate closely with lymphatic density and have been used previously in studies using human breast cancer.30 In the event, not only did we find no significant difference between the two groups, but also the average counts for the peritumorous and tumorous lymphatics in the SLNB negative group were slightly higher than in the SLNB positive group. This is of interest because a previous study of LYVE-1 in melanoma showed that increased lymphatic density is associated with a less favourable outcome,31 the exact opposite of the conclusion by Dadras et al.27
Ulceration is another independent prognostic factor in the new AJCC staging system and could clearly affect lymphangiogenesis.29 In our study we found a significant difference (p = 0.029) between ulcerated and non-ulcerated melanomas when comparing the three hotspots with the highest lymphatic counts for the peritumorous and intertumorous areas combined versus the intratumorous areas. Vascular endothelial growth factor C has been thought to play an important role in tumour lymphangiogenesis, based on in vitro studies using melanoma cell lines and in vivo studies involving xenotransplantation of the melanoma cell lines into nude mice.31 In contrast, only low level and heterogeneous expression of vascular endothelial growth factor C has been found when studying human melanomas, and the levels of expression were not found to correlate with the metastatic potential of the primary tumour.27 Similar observations have also been made in metastatic human squamous cell carcinomas of the head and neck.32 It may be that vascular endothelial growth factor C is not the major lymphangiogenic factor in human malignant melanomas, and that other novel lymphatic factors, yet to be discovered, are more important. One could hypothesise from our results that whatever drives the process of lymphangiogenesis in malignant melanomas may be related to the process of ulceration in these tumours. Dadras et al did not undertake a direct comparison of ulcerated and non-ulcerated tumours, but did control for ulceration in their patient selection.27
Another difference between the two studies was in the number of cases showing intratumorous lymphatics. These arise between tumour cells and usually show an immature morphology with indistinct lumina and vessel walls suggestive of active lymphangiogenesis. In the Dadras study, cases showing any intratumorous lymphatics were seen more frequently among the metastatic group (78% v 37%), but no lymphatic densities were reported.27 In our study, we found no significant difference in the intratumorous lymphatic counts between the SLNB positive and negative groups, and no difference between the groups in the number of cases showing any intratumorous lymphatics. Instead, the number of patients demonstrating IT-LC was slightly higher in the node negative (12 of 18 patients) than the node positive group (nine of 18 patients). Lymphatic vessels in the peritumorous and intertumorous areas were found to display a mature morphology, with well defined lumina, suggesting that they might be preformed vessels and not the result of tumour lymphangiogenesis.
Take home messages.
Staining with the lymphatic marker LYVE-1 can reliably demonstrate lymphatic vessel distribution in excision biopsies, but lymphatic counts could not predict the metastatic potential of malignant melanoma and cannot substitute for sentinel lymph node biopsy
LYVE-1/S100 double immunostaining can detect melanoma cells within lymphatics but is unreliable in predicting melanoma metastasis, failing to detect metastatic spread in more than two thirds of patients with regional lymph node metastasis
In our series, we also compared tumours with a Breslow thickness of 2 mm or less with those of Breslow thickness greater than 2 mm. We found no significant difference in lymphatic counts. If lymphangiogenesis were an important predictor of metastatic potential then one would expect it to correlate with Breslow thickness.
There has been much debate as to whether tumours metastasise to regional lymph nodes by invading pre-existing lymph vessels or via lymphangiogenesis within the tumour mass. Although both our patient populations demonstrated intratumorous lymphatic vessels with morphology suggestive of new vessel formation we cannot be certain that these intratumorous lymphatics represent the conduit for tumour metastasis. There is a lack of intratumorous lymphangiogenesis in human breast cancers, yet this does not impede tumour metastasis to regional lymph nodes, and tumour emboli have been demonstrated in lymph vessels at the periphery of these tumours.30 It is possible that the intratumorous lymphatics in melanomas have a role other than channelling tumour cells. Certainly, other studies have alluded to a possible physiological role for tumour associated lymphatics, necessary for the functioning of the tumour as opposed to tumour dissemination.33 In our study, the observation of melanoma cells within lymphatic vessels was restricted to mature vessels located within the peritumorous area. This observation is consistent with the hypothesis that melanoma cells invade pre-existing lymphatic vessels rather than intratumorous lymphangiogenesis, occurring with consequent channelling of tumour cells.
In summary, LYVE-1 is valuable in the study of lymphangiogenesis. In our relatively small series of patients, the lymphatic counts failed to predict micrometastatic disease in the draining lymph node. The results suggest that lymphatic counts are not particularly useful in predicting the metastatic potential of malignant melanomas and therefore cannot be used as a substitute for SLNB. Nonetheless, there was a significant difference for ulceration, so that lymphangiogenesis may be related to the higher risk of metastatic disease seen with ulcerated tumours. Double immunostaining with LYVE-1 and S100 demonstrated the presence of tumour cells within lymphatics, with a higher percentage being seen in node positive patients. However, this fell short of significance, and because most node positive patients did not show this feature, it is less reliable than the sentinel node status of an individual patient. Our study of the involvement of lymphatics in melanomas suggests that tumour cells gain access to the regional lymph nodes by invading preformed lymphatic vessels.
Acknowledgments
Thanks to D Jackson, Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, UK for kindly supplying us with LYVE-1.
Abbreviations
AJCC, American Joint Committee on Cancers
H+E, haematoxylin and eosin
IPT-LC, inter-peritumorous positive peritumorous lymphatic counts
IT-LC, intratumorous lymphatic count
LYVE-1, lymphatic vessel endothelial hyaluronan receptor 1
PT-LC, peritumorous lymphatic count
SLNB, sentinel node biopsy
TBS, Tris buffered saline
T-LC, tumorous lymphatic count
REFERENCES
- 1.Gerschenwald J, Thompson W, Mansfield P, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel node status in 612 stage I and II melanoma patients. J Clin Oncol 1999;3:976–83. [DOI] [PubMed] [Google Scholar]
- 2.Wagner J, Corbett L, Myung Park H, et al. Sentinel lymph node biopsy for melanoma. Experience with 234 consecutive procedures. Plast Reconst Surg 2000;105:1956–66. [DOI] [PubMed] [Google Scholar]
- 3.Landi G, Polverelli M, Moscatelli G, et al. Sentinel lymph node biopsy in patients with primary cutaneous melanoma; study of 455 cases. J Eur Acad Dermatol Venereol 2000;14:35–45. [DOI] [PubMed] [Google Scholar]
- 4.Shivers S, Xang X, Li W, et al. Molecular staging of malignant melanoma. JAMA 1998;280:1410–15. [DOI] [PubMed] [Google Scholar]
- 5.Blaheta H-J, Ellwanger U, Schittek B, et al. Examination of regional lymph nodes by sentinel node biopsy and molecular analysis provides new staging facilities in primary cutaneous melanoma. J Invest Dermatol 2000;14:637–42. [DOI] [PubMed] [Google Scholar]
- 6.Balch C, Buzaid A, Soong S-J, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635–48. [DOI] [PubMed] [Google Scholar]
- 7.Morton DL, Wen D-R, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg 1992;127:392–9. [DOI] [PubMed] [Google Scholar]
- 8.Albertini JJ, Cruse CW, Rapaport D, et al. Intraoperative radiolymphoscintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg 1994;223:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershenwald JE, Tseng C-h, Thompson W, et al. Improved sentinel lymph node localisation in primary melanoma patients with the use of radiolabelled colloid. Surgery 1998;124:203–10. [PubMed] [Google Scholar]
- 10.Reitgen D, Cruse CW, Berman C, et al. The orderly progression of melanoma nodal metastases. Ann Surg 1994;220:759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson JF, McCarthy WH, Bosch CMJ, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res 1995;5:255–60. [DOI] [PubMed] [Google Scholar]
- 12.Gershenwald JE, Colome MI, Thompson W, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998;16:2253–60. [DOI] [PubMed] [Google Scholar]
- 13.Krag DN, Meijer SJ, Weaver DL, et al. Minimal-access surgery for staging of melanoma. Arch Surg 1995;130:654–8. [DOI] [PubMed] [Google Scholar]
- 14.Uren RF, Howman-Giles R, Thompson JF, et al. Lymphoscintigraphy to identify sentinel lymph nodes in patients with melanoma. Melanoma Res 1994;4:395–9. [DOI] [PubMed] [Google Scholar]
- 14a.Powell AM, Calonje E, Acland K, et al. Pattern of recurrence in patients with malignant melanoma and negative sentinel node biopsies. Br J Dermatol 2004;151 (suppl 68) :82–3. [Google Scholar]
- 15.Li W, Stall A, Shivers SC, et al. Clinical relevance of molecular staging for melanoma: comparison of RT-PCR and immunohistochemistry staining in sentinel lymph nodes of patients with melanoma. Ann Surg 2000;231:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivers SC, Wang X, Li W, et al. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA 1998;280:1410–15. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Heller R, Vanvoorhis N, et al. Detection of submicroscopic lymph node metastases with polymerase chain reaction in patients with malignant melanoma. Ann Surg 1994;220:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostick PJ, Morton DL, Turner RR, et al. Prognostic significance of occult metastases detected by sentinel lymphadenectomy and reverse transcriptase-polymerase chain reaction in early-stage melanoma patients. J Clin Oncol 1999;17:3238–44. [DOI] [PubMed] [Google Scholar]
- 19.Wheatley K, Ives N, Hancock B, et al. Does adjuvant interferon-alpha for high-risk melanoma provide worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev 2003;29:241–52. [DOI] [PubMed] [Google Scholar]
- 20.Morton DL, Hoon DSB, Cochran AJ, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg 2003;238:538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava A, Laidler P, Davies RP, et al. The prognostic significance of tumour vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma. A quantitative histologic study. Am J Pathol 1988;133:419–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Straume O, Salvesen HB, Akslen LA. Angiogenesis is prognostically important in vertical growth phase melanomas. Int J Oncol 1999;15:595–9. [DOI] [PubMed] [Google Scholar]
- 23.Busam KJ, Berwick M, Blessing K, et al. Tumour vascularity is not a prognostic factor for malignant melanoma of the skin. Am J Pathol 1995;147:1049–56. [PMC free article] [PubMed] [Google Scholar]
- 24.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999;144:789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992;6:2397–404. [PubMed] [Google Scholar]
- 26.Carreira CM, Nasser SM, Tomaso E, et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res 2001;61:8079–84. [PubMed] [Google Scholar]
- 27.Dadras SS, Paul T, Bertoncini J, et al. Tumour lymphangiogenesis. A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 2003;162:1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalkley H. Method for the quantitative morphological analysis of tissues. J Natl Cancer Inst 1943;4:47–53. [Google Scholar]
- 29.Balch C, Buzaid A, Soong S-J, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635–48. [DOI] [PubMed] [Google Scholar]
- 30.Williams CSM, Leek RD, Robson AM, et al. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol 2003;200:195–206. [DOI] [PubMed] [Google Scholar]
- 31.Straume O, Jackson DG, Akslen LA. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 2003;9:250–6. [PubMed] [Google Scholar]
- 32.Beasley NJ, Prevo R, Banerji S, et al. Intratumoural lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 2002;62:1315–20. [PubMed] [Google Scholar]
- 33.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumours. Cancer Metastasis Rev 1990;9:253–66. [DOI] [PubMed] [Google Scholar]