Abstract
Background: Apocrine carcinoma is rare and often occurs in the axilla. This is the second apocrine carcinoma arising in bilateral axillae with associated apocrine hyperplasia to be reported.
Aims/Methods: Because benign apocrine tumours may be precursors of cancer, this case was investigated immunohistochemically and histologically, and a literature (English and Japanese) review undertaken of cases with coexistent malignant and benign apocrine tumours in the axilla to elucidate the relation between apocrine carcinoma and benign apocrine tumours.
Results: Only four cases of axillary apocrine carcinoma with benign apocrine tumours were identified in the literature. In each case, benign apocrine hyperplasia was situated within and surrounding the adenocarcinomatous nests. Staining for epithelial membrane antigen revealed three patterns: (1) poorly differentiated tumour cells showing strong cytoplasmic staining; (2) combined luminal surface and cytoplasmic staining of glandular cells; and (3) a strongly positive lineal staining pattern at the luminal membrane surface, comprising one or two apocrine hyperplastic secretory cells. The basal lesions of apocrine hyperplasia were strongly positive for α smooth muscle actin, whereas the periphery of adenomatous lesions showed weaker positive staining, even though the periphery of adenocarcinomatous lesions was negative.
Conclusions: All five apocrine carcinomas with benign apocrine tumours occurred in elderly Japanese men who had bilateral benign apocrine tumours even if affected by unilateral axillary apocrine carcinoma. The immunohistochemical results support the notion that apocrine hyperplasia is a precursor of cancer and that apocrine carcinoma, adenoma, and hyperplasia may be successive steps in the linear progression to carcinoma.
Keywords: apocrine carcinoma, benign apocrine tumour, immunohistochemical study, epithelial membrane antigen
Apocrine carcinoma comprises a group of rare primary cutaneous adenocarcinomas, which show features of apocrine differentiation and most frequently arise in regions of high apocrine gland density—particularly in the axilla.1,2 Rarely, they also arise in the Moll’s glands of the eyelids. Apocrine neoplasms occasionally cause diagnostic problems clinically and pathologically, because it is difficult to make a pathological distinction between benign and malignant apocrine neoplasms.3,4 Most apocrine carcinomas exist for less than one year before diagnosis. In addition, slow growing lesions can be present as painless, solitary, or multiple, solid to cystic masses, ranging in size from 1 to over 5 cm. These lesions tend to vary in colour from red to purple, and show ulceration of the overlying skin. The tumours are initially locally invasive, and systemic dissemination is often associated with regional lymph node metastases. Wide, local excision is the standard treatment for such lesions, and although apocrine carcinoma responds poorly to chemotherapy, adjuvant radiotherapy may be used in cases with advanced local or regional lesions.
“Apocrine carcinoma comprises a group of rare primary cutaneous adenocarcinomas, which show features of apocrine differentiation”
In this report, we review the clinicopathological findings of published cases of apocrine carcinoma with benign apocrine tumours. In addition, we discuss the case of an 89 year old man suffering from apocrine carcinoma with aberrant apocrine hyperplasia independently occurring on the bilateral axillae.
CASE REPORT
In October 2000, an 89 year old man visited our hospital because of a painless tumour of the left axilla, which had slowly enlarged since he first noticed it six months earlier.
Physical examination revealed a slightly soft, dome shaped tumour consisting of two masses; one mass was thumb sized and the other was hen egg sized. It was adherent to the overlying skin with ulceration, and at that time no mass was present in the right axilla. A biopsy specimen revealed skin appendage carcinoma, and an enhanced computed tomography scan of the thorax and abdomen revealed no tumour mass except for the left axillary tumour. The patient underwent radical excision with left axillary lymph node resection in December 2000. Laboratory data at that time—including serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen—were within the normal ranges.
The patient showed no remarkable change in his left axilla, but he noted a thumb sized tumour without skin colour change in his right axilla in September 2001. This tumour was resected with local anaesthesia a few weeks later and revealed atypical apocrine hyperplasia. In November 2002, he noted a new, thumb sized tumour with multiple small nodules on his right axilla. This tumour and the nodules were radically resected in December 2002. In the 23 months since this operation, the patient has been well without local recurrence or metastasis.
MATERIAL AND METHODS
We examined the resected bilateral axillae tumours in December 2000 and December 2002 by haematoxylin and eosin, periodic acid Schiff (with and without diastase), and iron staining.
In addition, we examined them immunohistologically using antibodies to α smooth muscle actin (αSMA; Dako, Glostrup, Denmark), S-100 (Dako), epithelial membrane antigen (EMA; Dako), gross cystic disease fluid protein 15 (Signet Pathology Systems, Dedham, Massachusetts, USA), cytokeratin (AE1+AE3, Nichirei, Tokyo, Japan), progesterone receptor (Immunotek, Marseille, France), the oestrogen receptor (Immunotek), c-erbB-2 (human epidermal growth factor receptor type 2; HER-2; Roche, Indianapolis, Indiana, USA), Ki-67 (Dako), p53 (Dako), and carcinoembryonic antigens (Dako).
RESULTS
All the resected bilateral axillae tumours had similar histological and immunohistological characteristics. The tumours were located in the dermal to subcutaneous tissue. Tumour nests were gathered in their centres, and these nests exhibited papillary adenocarcinoma with varying degrees of differentiation (fig 1A). The tumour cells contained abundant eosinophilic cytoplasm and showed apocrine-like decapitation (fig 2A). In poorly differentiated adenocarcinomatous areas, the tumour cells formed strands or solid nests within a hyalinised fibrous stroma in the upper dermis (fig 2B). Cells within and surrounding the centre varied from solid coalescent nests (fig 1B) to gland forming regions, with several layers of lining epithelial cells, which indicated apocrine adenoma (fig 2C). Some lobular masses, whose luminal layers were composed of cuboidal or columnar secretory cells without atypicality and a dilated lumen containing eosinophilic secreted material with occasional calcification (fig 2D), were situated within and spreading around these adenocarcinomatous and adenomatous nests (fig 1C). The resected left axillary lymph node showed no metastatic cancer cells, and there was no evidence of ectopic breast tissue.
Figure 1.
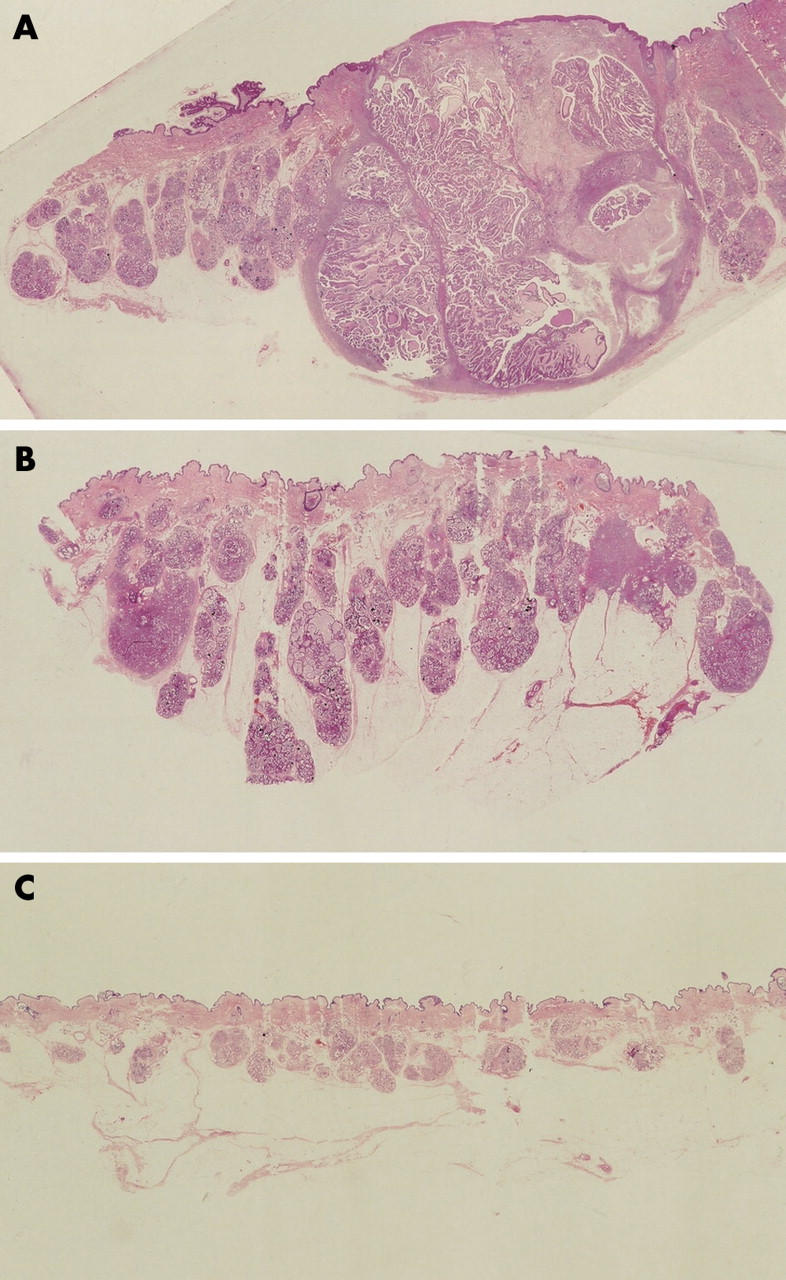
Gross view of the right axillary tumour resected in December 2002. (A, B) Tumour nests are gathered in the centres and show variation from solid coalescent nests within and surrounding the centre. Some lobular masses are within and spread around the centre.
Figure 2.
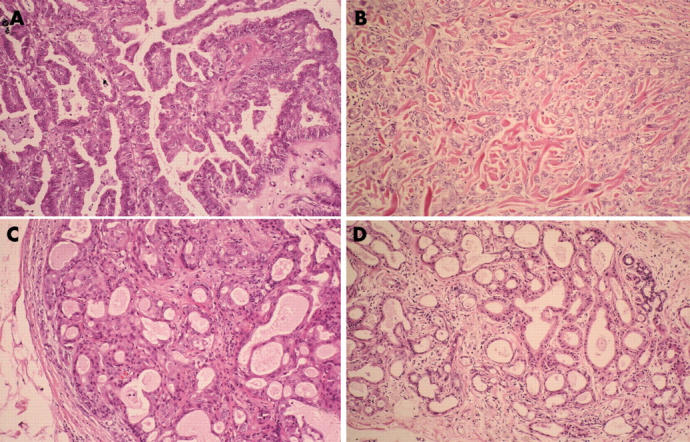
(A) The tumour cells of the centre exhibit papillary adenocarcinoma with varying degrees of differentiation, contain abundant eosinophilic cytoplasm, and show apocrine-like decapitation. (B) In poorly differentiated areas, the tumour cells form strands or solid nests within a hyalinised fibrous stroma in the upper dermis. (C) The cells within and surrounding the centre contain gland forming regions with several layers of lining epithelial cells, indicating the presence of apocrine adenoma. (D) The luminal layers of the lobular masses are composed of cuboidal or columnar secretory cells without atypicality and a dilated lumen, which contain eosinophilic secreted material with occasional calcification. Haematoxylin and eosin stain; original magnification, ×33.
The cytoplasm of these tumour cells contained periodic acid Schiff diastase resistant granules and no iron positive granules.
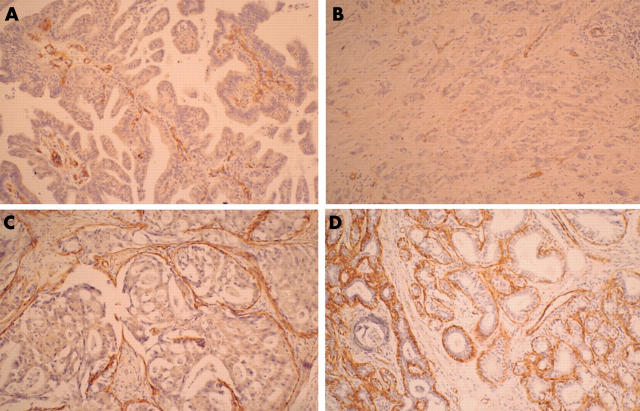
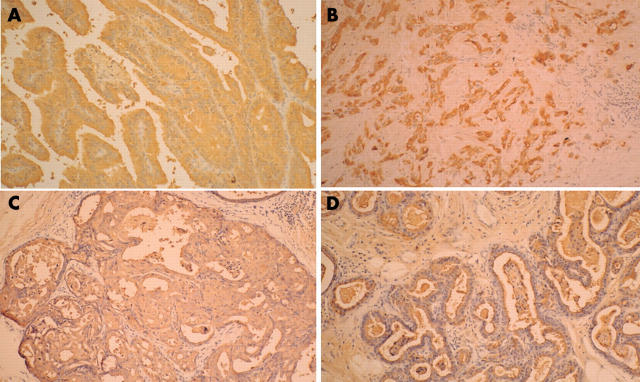
Table 1 outlines the results of the immunohistochemical studies. The basal lesions of apocrine hyperplasia were strongly positive for S-100 and αSMA (fig 3D), but the periphery of the adenomatous lesions stained weakly or moderately for these proteins (fig 3C), and both were negative in the adenocarcinomatous lesions (fig 3A, B). The findings for EMA and gross cystic disease fluid protein 15 were similar; the cytoplasm of the papillary and poorly differentiated adenocarcinoma cells was strongly positive (fig 4A, B). The luminal membrane surface, composed of one or two apocrine hyperplastic secretory cells, also showed strong positive staining (fig 4D), and the luminal surface and cytoplasm of the adenomatous glandular cells showed positive staining to the same degree (fig 4C). Tumour cells of the adenocarcinomatous and adenomatous lesions were strongly positive for cytokeratin, whereas the hyperplastic cells showed weak positive staining for cytokeratin. Adenocarcinomatous and adenomatous cells were weakly positive for the progesterone receptor and Ki-67. The nuclei of the adenocarcinomatous and adenomatous cells were moderately positive for p53, and negative for CEA, the oestrogen receptor, and HER-2.
Table 1.
Immunohistochemical profile of our case
| Marker | PDC | PAC | AA | AH |
| αSMA | − | − | +* | +++† |
| S-100 | − | − | +* | +++† |
| EMA | +++ | +++ | +++‡ | +++§ |
| GCDFP-15 | +++ | +++ | +++‡ | +++§ |
| Cytokeratin | +++ | +++ | +++ | + |
| PR | + | + | + | − |
| ER | − | − | − | − |
| HER-2 | − | − | − | − |
| Ki-67 | 12.5% | 6.4% | 3.8% | − |
| p53 | 46.4% | 63.6% | 54.4% | − |
| CEA | − | − | − | − |
Staining intensity: +++, strong; +, weak; −, negative staining.
*Positive only in peripheral lesions; †positive only in basal lesions; ‡positive only at the luminal surface and in glandular cells; §positive only at the luminal surface.
αSMA, α smooth muscle antigen; AA, apocrine adenoma; AH, apocrine hyperplasia; CEA, carcinoembryonic antigen; EMA, epithelial membrane antigen; ER, oestrogen receptor; GCDFP-15, gross cystic disease fluid protein 15; PAC, papillary apocrine carcinoma; PDA, poorly differentiated carcinoma; PR, progesterone receptor.
Figure 3.
(A, B) The adenocarcinomatous lesions are negative for α smooth muscle actin (αSMA), although (C) the peripheral areas of the adenomatous lesions are weakly or moderately positive. (D) The basal lesions of apocrine hyperplasia show strong positive staining for αSMA.
Figure 4.
(A, B) The cytoplasm of the papillary adenocarcinoma cells and the poorly differentiated tumour cells was strongly positive for epithelial membrane antigen (EMA). (C) The luminal surface and the cytoplasm of the glandular cells showed the same degree of positive staining for EMA. (D) The luminal membrane surface, composed of one or two secretory cells, also showed strong positive staining for EMA.
DISCUSSION
Because both tumours developed independently of one another and there was an absence of regional lymph node or systemic metastasis, we diagnosed this case as an independent occurrence of bilateral axillary apocrine adenocarcinoma. To our knowledge, only Yoshida et al have reported a case of apocrine adenocarcinoma arising in bilateral axillae.5 Their case occurred in an 88 year old Japanese man who complained of bilateral axillae masses, which consisted of different degrees of adenocarcinoma, foci of in situ carcinoma, and non-neoplastic but hyperplastic apocrine gland. Their histological findings were very similar to ours. Furthermore, a review of the literature revealed only three additional cases where there was coexistence of malignant and benign apocrine tumours (table 2).6–8 All of these patients were elderly Japanese men whose clinical features were similar to those of triple extramammary Paget’s disease. Twenty eight cases of triple extramammary Paget’s disease, which were thought to be related to carcinoma of the apocrine glands, have been reported in Japan,9 but there are no reports from other countries. All but one of the 28 patients with triple Paget’s disease was male. With this in mind, Koseki et al speculated that some systemic factors might be involved,9 and in particular that androgens might play a role in the pathological process involved in triple Paget’s disease. The only other case report of apocrine carcinoma arising in bilateral axillae5 revealed that multiple apocrine carcinoma has some clinical features in common with triple extramammary Paget’s disease.
Table 2.
Clinical data of patients with apocrine adenocarcinoma and benign apocrine tumours in the literature
| First author | Age | Sex | Histopathology |
| Yoshida4 | 88 | Male | Bilateral axillary apocrine adenoacarcinoma with in situ carcinoma and hyperplastic apocrine gland |
| Nishikawa5 | 69 | Male | Left axillary apocrine adenocarcinoma with bilateral hyperplastic apocrine gland |
| Isei6 | 82 | Male | Right axillary apocrine adenocarcinoma with bilateral apocrine adenoma |
| Amo7 | 62 | Male | Left axillary apocrine adenocarcinoma with bilateral hyperplastic apocrine adenoma |
| Present case | 89 | Male | Bilateral axillary apocrine adenoacarcinoma with apocrine adenoma and hyperplastic apocrine gland |
In both the previous cases and the present case of apocrine carcinoma with axillary apocrine hyperplasia, the tumours consisted of poorly differentiated adenocarcinoma to benign apocrine hyperplasia. Nishikawa and colleagues6 reported a case of apocrine adenocarcinoma of the left axilla associated with hamartomatous apocrine gland hyperplasia of both axillae, and suggested that hyperplastic apocrine glands with a hamartomatous appearance may be precancerous. Recently, Amo et al reported on apocrine carcinoma in the left axilla with tubular apocrine adenoma in the right axilla.8 Two of the five cases in their review (those of Yoshida et al and Isei et al) showed regional lymph node metastasis, and only one of the patients died; death was the result of severe respiratory insufficiency with cutaneous recurrence of the tumour 38 months after initial surgery. The other patients remained alive 22 to 46 months after initial surgery. This finding suggests that apocrine carcinoma with apocrine hyperplasia has a favourable prognosis.
Our present case, along with the cases of Isei and Amo, was immunohistologically examined using antibodies to EMA and αSMA, and the results of all three cases were the same. Yoshida et al also reported αSMA results identical to those seen here.5
“This is the first reported case of apocrine carcinoma with detailed results of immunohistochemistry using epithelial membrane antigen”
In breast cancer, carcinoma in situ and invasive breast cancer frequently coexist.10,11 The αSMA and cytokeratin results for four such cases of apocrine carcinoma with apocrine hyperplasia showed the same characteristic findings for epitheliosis, ductal carcinoma in situ, and clinging carcinoma in breast cancer.12,13 Most epithelial tumours—including squamous cell carcinoma, breast carcinoma, and large cell lung carcinoma—are positive for EMA.14 Antibodies against EMA also stain normal sweat glands and the apocrine naevus, which shows staining of the luminal membrane surface, although the epidermis is negative. Staining for EMA is consistently positive in both extramammary and mammary Paget’s disease.15 Some cases of apocrine adenocarcinoma were positive for EMA,5,16 but the results were not described in detail. During the neoplastic transformation of breast cancer cells, the morphological expression of EMA may change. Luna-Moré et al reported on the EMA staining patterns of 330 breast carcinomas, and these patterns could be classified into three groups: lineal, cytoplasmic, and negative.17 Furthermore, they suggested that EMA is a differentiation marker and histological prognostic factor. Recently, Misago and colleagues18 reported low grade sebaceous carcinoma associated with glandular structures. They pointed out that these glandular structures were positive for EMA at the luminal surface and in the cytoplasm of luminal cells, and showed a focal positive reaction against EMA only in well differentiated areas in sebaceous carcinomas. In our case, EMA revealed lineal staining in benign apocrine hyperplasia, cytoplasmic staining in poorly differentiated cancer cells, and combined staining of the luminal surface and cytoplasm of glandular nest cells. This is the first reported case of apocrine carcinoma with detailed results of immunohistochemistry using EMA. The αSMA, cytokeratin, and p53 results suggest that apocrine carcinoma, apocrine adenoma, and apocrine hyperplasia are successive steps in a linear progression model terminating in apocrine carcinoma, as in invasive breast cancer10,11 and low grade sebaceous carcinoma.18 Further investigations will be needed to determine whether the EMA results are identical in apocrine adenocarcinoma and breast cancer, as the αSMA and cytokeratin characteristics are.
Take home messages.
We report a second case of apocrine carcinoma with benign apocrine tumours in both axillae
Apocrine carcinoma, apocrine adenoma, and apocrine hyperplasia are successive steps in a linear progression model terminating in apocrine carcinoma
Acknowledgments
We thank Dr Ogawa, Dr Isei, and Dr Amo for providing us with specimens from their cases, and Mr Labrecque for editing the manuscript.
Abbreviations
αSMA, α smooth muscle actin
EMA, epithelial membrane antigen
REFERENCES
- 1.Murphy GF, Elder DE. Apocrine adenocarcinoma. In: Rosai J, Sobin LH, eds. Atlas of tumor pathology. Non-melanocytic tumors of the skin. Washington, DC: Armed Forces Institute of Pathology, 1990:119–20.
- 2.Chamberlain RS, Huber K, White JC, et al. Apocrine gland carcinoma of the axilla. Review of the literature and recommendations for treatment. Am J Clin Oncol 1999;22:131–5. [DOI] [PubMed] [Google Scholar]
- 3.Kage M, Nakamura Y, Ozumi K. A case report of equivocal neoplasm originating from an apocrine gland on the eyelid. Acta Pathol Jpn 1990;40:431–4. [DOI] [PubMed] [Google Scholar]
- 4.Shintaku M, Tsuta K, Yoshida H, et al. Apocrine adenocarcinoma of the eyelid with aggressive biological behavior: report of a case. Pathol Int 2002;52:169–73. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida A, Kodama Y, Hatanaka S, et al. Apocrine adenocarcinoma of the bilateral axillae. Acta Pathol Jpn 1991;41:927–32. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa Y, Tokusashi Y, Saito Y, et al. A case of apocrine adenocarcinoma associated with hamartomatous apocrine gland hyperplasia of both axillae. Am J Surg Pathol 1994;18:832–6. [DOI] [PubMed] [Google Scholar]
- 7.Isei T, Yasuda H, Haradaet A, et al. A case of axillary apocrine adenocarcinoma arising from apocrine adenoma. Skin Cancer 1995;10:156–9. [Google Scholar]
- 8.Amo Y, Kawano N. A case of ductal apocrine carcinoma in the left axilla with tubular apocrine adenoma in the right axilla. J Dermatol 2003;30:72–5. [PubMed] [Google Scholar]
- 9.Koseki S, Mitsuhashi Y, Yoshikawa K, et al. A case of triple extramammary Paget’s disease. J Dermatol 1997;24:535–8. [DOI] [PubMed] [Google Scholar]
- 10.Boecker W, Moll R, Dervan P, et al. Usual ductal hyperplasia of the breast is a committed stem (progenitor) cell lesion distinct from atypical ductal hyperplasia and ductal carcinoma in situ. J Pathol 2002;198:458–67. [DOI] [PubMed] [Google Scholar]
- 11.Going JJ. Stages on the way to breast cancer. J Pathol 2003;199:1–3. [DOI] [PubMed] [Google Scholar]
- 12.Raju U, Crissman JD, Zarbo RJ, et al. Epitheliosis of the breast. An immunohistochemical characterization and comparison to malignant intraductal proliferations of the breast. Am J Surg Pathol 1990;14:939–47. [DOI] [PubMed] [Google Scholar]
- 13.Böcker W, Bier B, Freytag G, et al. An immunohistochemical study of the breast using antibodies to basal and luminal keratins, alpha-smooth muscle actin, vimentin, collagen IV and laminin. Part II: epitheliosis and ductal carcinoma in situ. Virchows Arch A Pathol Anat Histopathol 1992;421:323–30. [DOI] [PubMed] [Google Scholar]
- 14.Elenitsas R, Belle PV, Elder D. Laboratory methods. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s histopathology of the skin. 8th ed. Philadelphia: Lippincott-Raven, 1997:57.
- 15.Jones RR, Spaull J, Gusterson B. The histogenesis of mammary and extramammary Paget’s disease. Histopathology 1989;14:409–16. [DOI] [PubMed] [Google Scholar]
- 16.Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. A clinicopathologic, immunocytochemical, and ultrastructural study. Cancer 1993;71:375–81. [DOI] [PubMed] [Google Scholar]
- 17.Luna-Moré S, Rius F, Weil B, et al. EMA: a differentiation antigen related to node metastatic capacity of breast carcinomas. Pathol Res Pract 2001;197:419–25. [DOI] [PubMed] [Google Scholar]
- 18.Misago N, Narisawa Y. Sebaceous carcinoma with apocrine differentiation. Am J Dermatopathol 2001;23:50–7. [DOI] [PubMed] [Google Scholar]