Abstract
Background: Kaposi sarcoma associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) associated lymphomas, which often develop in human immunodeficiency virus (HIV) infected patients with advanced AIDS, present predominantly as primary effusion lymphoma (PEL) or, less frequently, as “solid” extracavitary based lymphomas, associated with serous effusions. These last lymphomas, also called “solid PEL”, have been reported before the development of an effusion lymphoma and after resolution of PEL. Interestingly, KSHV/HHV-8 associated lymphomas that present as solid or extracavitary based lesions in HIV seropositive patients without serous effusions have been reported recently.
Methods/Results: This paper provides evidence for the existence of a previously undescribed KSHV/HHV-8 associated lymphoma in HIV seronegative patients without serous effusions. These lymphomas exhibit a predilection for the lymph nodes and display anaplastic large cell morphology. These tumours were completely devoid of common cell type specific antigens, including epithelial and melanocytic cell markers. B and T cell associated antigens and other commonly used lymphoid markers were absent or weakly demonstrable in a fraction of the tumour cells. Conversely, immunohistochemical studies showed strong immunostaining with plasma cell reactive antibodies.
Conclusions: Analysis of viral infection and immunohistological studies are of primary importance to define this lymph node based KSHV/HHV-8 associated lymphoma with anaplastic large cell morphology and plasmablastic immunophenotype occurring in HIV seronegative patients without serous effusions.
Keywords: lymphoma, KSHV/HHV8 associated lymphomas, anaplastic large cell lymphoma, plasmablastic lymphoma, diffuse large B cell lymphoma
Kaposi sarcoma associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), was initially discovered in tissue biopsies of AIDS related Kaposi sarcoma,1 a tumour consistently infected by this virus. In addition to Kaposi’s sarcoma, KSHV/HHV-8 is also associated with three distinct lymphoproliferative diseases occurring most often in patient with human immunodeficiency virus (HIV) infection/AIDS,2 namely: primary effusion lymphoma (PEL),3 multicentric Castleman disease (MCD),4 and MCD associated plasmablastic lymphoma.5,6 A new KSHV/HHV-8 associated lymphoproliferative disease, termed “germinotropic lymphoproliferative disorder”, has recently been described in HIV seronegative patients.7
“Because of the morphological and immunophenotypical features of these large cell tumours, we propose that they should be classified as KSHV/HHV-8 associated lymphomas with anaplastic large cell morphology and plasmablastic immunophenotype”
Intriguingly, cases of KSHV/HHV-8 associated extracavitary lymphoma have been reported in HIV seropositive patients with serous effusions.2,8,9,10 KSHV/HHV-8 associated extracavitary lymphomas have been reported before the development of an effusion lymphoma8 and after the resolution of PEL.11 These cases, also called “solid PEL”, primarily involved extranodal tissue locations and had identical morphology, immunophenotype, KSHV/HHV-8 viral status, and immunoglobulin light chain gene rearrangements to their PEL counterparts. This group of KSHV/HHV-8 associated lymphomas in HIV seropositive patients with serous effusions might also include a recently reported case of syncronous presentation of a pleural cavity PEL and a tongue based KSHV/HHV-8 positive lymphoma. Both tumours displayed plasmablastic features.12
The spectrum of KSHV/HHV-8 associated lymphoproliferative diseases in the HIV setting has been expanded by the identification of cases of KSHV/HHV-8 associated extracavitary lymphomas without serous effusions.9,13–21 In general, these lymphomas are composed of a proliferation of immunoblastic-like cells, although in some cases the neoplastic cells show greater pleomorphism and anaplastic features. Among the KSHV/HHV-8 associated extracavitary lymphomas without serous effusions, a unique case simulating CD30 positive anaplastic large cell lymphoma in an HIV seropositive patient has recently been reported.22 It was a lymph node based lymphoma resembling CD30 positive ALCL both histologically and immunophenotypically.
KSHV/HHV-8 associated lymphoproliferative diseases have also been reported in HIV seronegative patients. These lymphomas were mainly PEL.23–28 However, cases of KSHV/HHV-8 associated extracavitary lymphoma have been reported in HIV seronegative patients with serous effusions.28,29
In our present report, we provide evidence for the existence of a previously undescribed KSHV/HHV-8 associated lymphoma in HIV seronegative patients without serous effusions. The reported lymphoma cases were extracavitary, with a predilection for the lymph nodes, and displayed anaplastic large cell morphology. Paraffin wax immunohistochemical studies showed that the tumour cells were strongly positive for the plasma cell reactive antibody MUM1/IRF4.30 B and T cell associated antigens and other commonly used lymphoid markers were absent or weakly demonstrable in a considerable proportion of tumour cells. Unequivocally, these tumours were completely devoid of common cell type specific antigens, including epithelial and melanocytic cell markers. Because of the morphological and immunophenotypical features of these large cell tumours, we propose that they should be classified as KSHV/HHV-8 associated lymphomas with anaplastic large cell morphology and plasmablastic immunophenotype. These lymphomas may correspond to a distinct entity within the heterogeneous group of diffuse large B cell lymphomas with plasmablastic differentiation.
MATERIALS AND METHODS
Case reports
The two HIV seronegative patients with KSH/HHV8 associated lymphomas were aged 52 and 75 years. Table 1 summarises their demographic and clinical features.
Table 1.
Demographic and clinical features of two HIV negative patients with KSHV/HHV-8 associated solid lymphomas
| Feature | Case 1 | Case 2 |
| Age/Sex | 75/M | 52/M |
| HHV-8 related disease | Negative | Negative |
| HHV-8 viraemia (cp/ml) | 231430 | 2850 |
| EBV viraemia (cp/ml) | Negative | 1074 |
| CD4 cell (count/μl) | 470 | 501 |
| PS (ECOG) | 3 | 1 |
| Stage (Ann Arbor) | IVB | IIIA |
| B symptoms | Severe weight loss | Absent |
| Site of involvement | LN, Waldeyer’s ring, soft tissue, spleen | LN, spleen |
| Bulky disease | Present | Present |
| Major laboratory abnormalities | ||
| Severe anaemia (⩽80 g/l) | Negative | Negative |
| Thrombocytopenia (⩽100×109/l) | Negative | Negative |
| Coomb’s test | Negative | Negative |
| Abnormal LDH serum concentration (⩾460 U/ml) | Present | Negative |
| Hypoalbuminaemia (⩽30 g/l) | Present | Negative |
| Treatment | CHOP | CEOP |
| Outcome (months) | Dead (2) | Alive (8) |
| Cause of death | NHL | – |
CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CEOP, cyclophosphamide, epidoxorubicin, vincristine, prednisone; EBV, Epstein-Barr virus; HHV-8, human herpesvirus type 8; HIV, human immunodeficiency virus; KSHV, Kaposi sarcoma associated herpesvirus; LDH, lactate dehydrogenase; LN, lymph node; NHL, non-Hodgkin lymphoma.
Poor general conditions (PS3) and severe weight loss were seen in one patient (case 1). Advanced stage disease (stages III–IV) occurred in both patients.
Tumour localisation included both generalised lymphadenopathy and spleen involvement. One patient (case 1) also showed extranodal localisation including gastrointestinal tract and Waldeyer involvement. Bone marrow involvement was absent in both patients. Neither of the patients developed lymphomatous effusions.
An abnormal serum lactate dehydrogenase serum concentration, hypoalbuminemia, thrombocytopenia, anaemia, and KSHV/HHV-8 viraemia were found in the patient with more advanced stage disease (case 1). Both patients received anthracycline based treatment. However, the clinical course of these patients was variable. Patient 1 had aggressive and rapidly fatal disease. He died after one course of chemotherapy for disease progression (survival, two months). At the time of this report, patient 2 is alive, and is still receiving chemotherapy, with good clinical response (survival, eight months).
Clinical evaluation
At diagnosis, both patients were evaluated for the extent of lymphoma by physical examination, chest radiography, computed tomography of the thorax and abdomen, bone marrow aspiration and biopsy, lumbar puncture for spinal fluid, blood chemistry, and CD4 cell count. The lymphoma stage was assigned according to the Ann Arbor staging system.31 Informed written consent was obtained from the patients, and tissue collection was approved by the institutional review board.
The two reported cases of KSHV/HHV-8 associated lymphoma occurring in HIV seronegative patients without serous effusions belonged to a series of KSHV/HHV-8 associated lymphoproliferative diseases referred to a single institution (Aviano Cancer Centre, Italy) where they were diagnosed and treated. Three other cases of KSHV/HHV-8 associated lymphoma developing in HIV seropositive patients without serous effusions belonged to the same institutional series. These three cases, together with case 1 reported here, were included in a previous study focusing on their relatedness to AIDS related PEL.16 Case 1, in which the lymphoma developed in an HIV seronegative patient, was included mainly for epidemiological purposes. Within the series of KSHV/HHV-8 associated lymphoproliferative diseases there were also 15 PELs (13 in HIV seropositive and two in HIV seronegative patients). PEL cases were classified on the basis of the unusual clinicopathological and virological (for example, KSHV/HHV-8 positivity) characteristics of the lymphoma. Six of 15 PEL cases were included in a previous study based on the gene profiling expression analysis of AIDS related lymphomas.32
Analysis of viral infection
The lymph node samples from both cases presented here were investigated for tumour infection by KSHV/HHV-8 and Epstein-Barr virus (EBV).
The presence of KSHV/HHV-8 was ascertained by immunohistochemistry using a rat monoclonal antibody against the KSHV/HHV-8 latency associated nuclear antigen encoded by viral open reading fragment (ORF) 73 (Advanced Biotechnologies, Columbia, Maryland, USA). The cases were also tested for viral interleukin 6 (vIL-6), a KSHV/HHV-8 cytokine homologue, using a rabbit polyclonal antibody (Advanced Biotechnologies). Bouin’s or formalin fixed, paraffin wax embedded tissue sections were pretreated in a microwave oven at 250 W for 30 minutes in EGTA solution (1mM, pH 8), and then immunostained on an automated immunostainer (Nexes, Ventana Medical Systems Inc, Tucson, Arizona, USA) according to a modified version of the company’s protocols. Negative controls, which were invariably negative, consisted of omission of the primary antibody and substitution with phosphate buffered saline. Positive controls for ORF73 consisted of KS biopsy samples, whereas positive controls for vIL-6 consisted of AIDS associated MCD biopsy samples.
The presence of KSHV/HHV-8 was confirmed by polymerase chain reaction (PCR) analysis of three KSHV/HHV-8 regions (KS233, ORFK9-3, and ORF72) using previously reported primer sequences.33 PCR analysis was performed on DNA isolated from formalin tissues using the Nucleospin tissue system (Macherey-Nagel GmbH and Co, Düren, Germany) according to the manufacturer’s protocol, with minor modifications. Each PCR reaction used approximately 0.2 µg of genomic DNA, 100 pmol of each primer, 2 U Taq polymerase, 100mM of each dNTP, 1.5mM Tris hydrochloride (pH 9.0), and 0.1% Triton X-100 in a final volume of 50 µl. PCR amplification was carried out at 95°C for six minutes (one cycle); 95°C for 15 seconds, 60°C for 15 seconds, and 72°C for 30 seconds (35 cycles); and 72°C for five minutes (one cycle). Amplification was performed with a GeneAmp PCR system 9600 (Perkin-Elmer, Norwalk, Connecticut, USA). PCR products were visualised on a 2% agarose gel containing ethidium bromide.
Infection by EBV was investigated by several approaches, including in situ hybridisation (ISH) and PCR. EBV encoded small non-coding RNA (EBER) ISH was performed on Bouin’s or formalin fixed tissue sections, as described previously.34,35 PCR analysis of EBV was performed with primers SL-1 and SL-3, representative of the EBV nuclear antigen 1 (EBNA-1) gene, as reported previously.36
In the EBV positive case, immunostaining for latent membrane protein 1 (LMP1) was performed with an LMP1 specific antibody (Dako A/S, Glostrup, Denmark) and the alkaline phosphatase antialkaline phosphatase method.37
KSHV/HHV-8 serology was performed by an immunoflorescence assay.38 KSHV/HHV-8 quantitative PCR was performed on DNA extracted from plasma using the QiAmp Kit (Qiagen Gmbh, Hilden, Germany). Real time (TaqMan) PCR was used for the detection of the HHV-8 67 bp capsid protein gene, as described previously in detail.38
EBV plasma DNA: a PCR assay based on LMP2A sequence amplification was performed as described previously.39
Molecular analysis of clonality
ISH was used to detect immunoglobulin light chain mRNA. The κ and λ light chain mRNA was detected in Bouin’s fixed, paraffin wax embedded sections using a commercial kit (Dako A/S) under conditions recommended by the supplier. PCR analysis of immunoglobulin heavy chain (IgVH) and light chain (IgVL) and T cell receptor γ chain gene rearrangements was carried out according to standard protocols as described previously.16
Immunohistological studies
Our study cases had been immunophenotyped as previously described40 on paraffin wax embedded lymph node tissues, which were fixed in Bouin’s solution or neutral buffered formalin. Plasma cell markers included MUM1/IRF4,30 Vs38c,41 and CD138.42 A monoclonal antibody (Serotec, Oxford, UK) was used to test the expression of cytoplasmic IgG (CIgG).
To investigate further the phenotype of KSHV/HHV-8 associated “solid” lymphomas, we tested the expression of an additional panel of proteins that are specifically expressed by PEL tumour cells, as previously documented by gene expression profiling analysis.32 Using this type of gene expression data, AIDS-PEL was assigned to a plasmablastic cell of origin. Those antigens for which antibodies suitable for paraffin wax embedded tissue sections were available were analysed by means of immunohistochemistry. The KSHV/HHV-8 associated lymphomas were tested for the expression of the following proteins: granzyme A (GRA), aquaporin-3 (AQP3), selectin P ligand (SELPLG), and vascular endothelial growth factor (VEGF). They were investigated using commercially available antibodies (GRA, AQP3, SELPLG, and VEGF; Santa Cruz Biotechnology, Santa Cruz, California, USA). They were tested on paraffin wax embedded sections after antigen retrieval (30 minutes in EGTA solution in a microwave oven at 250 W) and immunostained on an automated immunostainer (Nexes). Negative controls were performed as described above.
RESULTS
Demonstration of KSHV/HHV-8 by immunohistochemistry and PCR
Immunohistochemistry for the KSHV/HHV-8 ORF73 protein revealed that this antigen was present in the nuclei of almost all of the tumour cells of both cases (figs 1, 2). In contrast, staining for vIL-6 was cytoplasmic. vIL-6 was detected on most tumour cells of case 2 (fig 2), whereas it was restricted to a limited number of tumour cells in case 1 (not shown). On PCR analysis, bands compatible with KSHV/HHV-8 genomic sequences were detected in both cases (figs 1 and 2).
Figure 1.
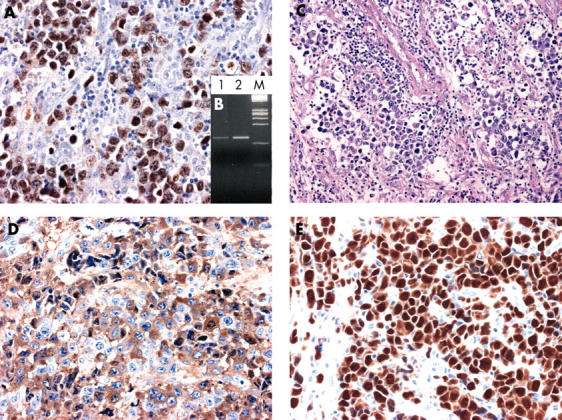
Kaposi sarcoma associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) associated “solid” lymphoma; case 1. (A) Immunohistochemistry for KSHV/HHV-8 open reading fragment 73 (ORF73) protein. The ORF73 protein is detected in the nucleus of almost all of the tumour cells (original magnification, ×40). (B) Polymerase chain reaction for KSHV/HHV-8 ORF72. A band compatible with the genomic sequence is detectable. Lane 1, DNA extracted from case 1; lane 2, DNA extracted from a positive control (CRO-AP/6 primary effusion lymphoma cell line); M, DNA molecular weight marker. (C) A lymph node infiltrated by large anaplastic cells exhibiting a sinusoid pattern of growth. Haematoxylin and eosin staining; original magnification, ×40. (D) Immunohistochemistry for cytoplasmic IgG showing that the tumour cells are positive for this antigen (original magnification, ×40). (E) Immunohistochemistry for MUM1/IRF4. All tumour cells show intense nuclear staining for MUM1/IRF4 (original magnification, ×40).
Figure 2.
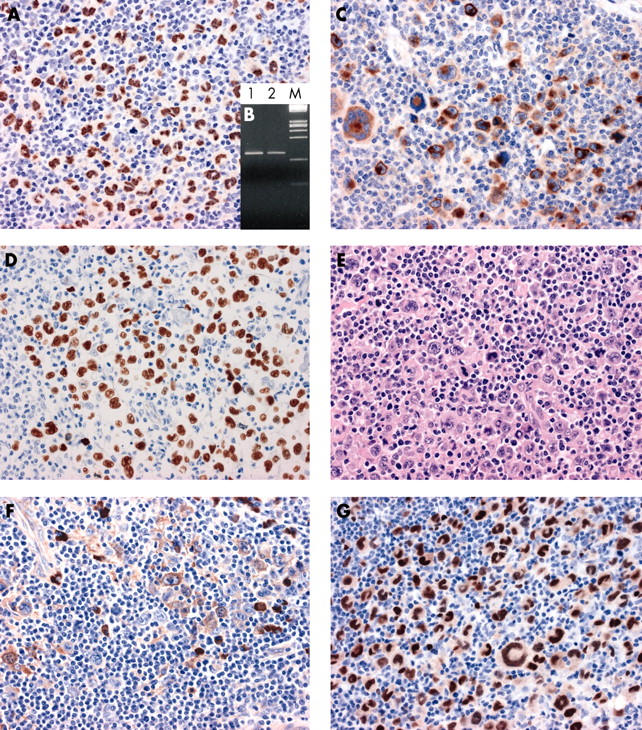
Kaposi sarcoma associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) associated “solid” lymphoma; case 2. (A) Immunohistochemistry for KSHV/HHV-8 open reading fragment 73 (ORF73) protein. The ORF73 protein is detected in the nucleus of almost all of the tumour cells (original magnification, ×40). (B) Polymerase chain reaction for KSHV/HHV-8 ORF72. A band compatible with the genomic sequence is detectable. Lane 1, DNA extracted from case 1; lane 2, DNA extracted from a positive control (CRO-AP/6 primary effusion lymphoma cell line); M, DNA molecular weight marker. (C) Immunohistochemistry for KSHV/HHV-8 viral interleukin 6 protein, with most tumour cells showing cytoplasmic staining for this antigen (original magnification, ×40). (D) Demonstration of Epstein-Barr virus (EBV) by EBV encoded small non-coding RNA in situ hybridisation (original magnification, ×40). (E) A lymph node infiltrated by large anaplastic cells. Several giant cells display multilobated nuclei. Haematoxylin and eosin staining; original magnification, ×40. (F) Immunohistochemistry for cytoplasmic IgG, with several tumour cells positive for this antigen (original magnification, ×40). (G) Immunohistochemistry for MUM1/IRF4. All tumour cells display intense nuclear staining for MUM1/IRF4 (original magnification, ×40).
Histopathological analysis of the KSHV/HHV-8 associated lymphomas
In both cases the tumours showed predominantly lymph node involvement. Microscopically, the lymph nodes were infiltrated by diffusely growing large neoplastic cells, which displayed anaplastic features. In some areas of the lymph nodes, the neoplastic cells showed a distinctive sinusoid pattern of growth. In these areas, groups of large anaplastic cells were located essentially within the sinuses (fig 1). The tumour cells were discohesive and had a moderate to abundant amount of cytoplasm. They showed striking variations in size and had more or less eccentrically placed, horse shoe-like nuclei. In case 2, multilobated nuclei and multinucleated giant cells were numerous (fig 2).
Demonstration of EBV by ISH, immunohistochemistry, and PCR
Using EBER ISH, only one case (case 2) was positive for EBV (fig 2). These results were confirmed by PCR analysis of the EBNA-1 region of EBV. EBV infection was not associated with LMP1 expression (table 2).
Table 2.
Virological and immunophenotypic characteristics of KSHV/HHV-8 associated solid lymphomas
| Case 1 | Case 2 | |
| KSHV (IHC) | ORF73+ | ORF73+ |
| vIL-6− | vIL-6+ | |
| KSHV (PCR) | KS330+ | KS330+ |
| ORF72+ | ORF72+ | |
| ORF9-3+ | ORF9-3− | |
| EBER (ISH) | − | + |
| EBNA-1 (PCR) | − | + |
| LMP1 (IHC) | − | − |
| CLA/CD45 | − | −/+ |
| EMA | + | −* |
| MUM1/IRF4 | + | + |
| CD138/syn-1 | + | −/+ |
| VS38c | −/+ | + |
| CD30 | −/+ | − |
| ALK (ALK1) | − | − |
| CD20 | − | − |
| CD40 | − | − |
| CD79a | − | − |
| CD3 | −/+ | − |
| CD5 | − | − |
| CD43 | −/+ | −/+ |
| CD45RO | − | − |
| CD10 | − | − |
| Ki-67/MIB1 | + | + |
| κ/λ chain | − | − |
| CIgG | + | + |
| CD15 | − | − |
| CD68 | − | − |
| S100 | − | − |
| Keratin (AE1/AE3) | − | − |
| Keratin (MNF116) | − | − |
| Granzyme A | −/+ | −* |
| AQP3 | −* | + |
| SELPLG | + | + |
| VEGF | + | + |
+, almost all neoplastic cells positive; +/−, most neoplastic cells positive; −/+, minority of neoplastic cells positive; −*, few neoplastic cells positive; −, all neoplastic cells negative; NE, not evaluable.
AQP3, aquaporin 3; CIgG, cytoplasmic IgG; CLA, common leucocyte antigen; EBER, Epstein-Barr virus encoded small non-coding RNA; EBNA-1, Epstein-Barr virus nuclear antigen 1; EMA, epithelial membrane antigen; HHV-8, human herpesvirus type 8; IHC, immunohistochemistry; ISH, in situ hybridisation; KSHV, Kaposi sarcoma associated herpesvirus; ORF, open reading fragment; PCR, polymerase chain reaction; SELPLG, selectin P ligand; syn-1, syndecan 1; VEGF, vascular endothelial growth factor.
Molecular analysis of clonality
Table 2 shows the results with regard to clonality. In both cases, ISH studies failed to detect monoclonality. Neither case yielded IgVH or IgVL rearrangement, although the cases were readily amplified with a control gene fragment of similar size. Analysis of T cell receptor γ rearrangement was negative (not shown).
Immunophenotypical analysis
Table 2 shows the results of the immunophenotypical analysis. Immunohistological analysis revealed that only case 2 was positive for CD45, whereas only case 1 displayed positivity for CD3 and CD30. The staining for all these markers was weak and restricted to a small proportion of tumour cells. CD3 was mainly localised in the cytoplasm. Conversely, both cases showed intense positive staining for the plasma cell associated markers CD138 and MUM1/IRF4 (table 2). In particular, all tumour cells were strongly positive for MUM1/IRF4 (figs 1, 2). Furthermore, several tumour cells were positive for CIgG (figs 1, 2). Finally, in both cases a subset of cells was positive for CD43.
To investigate the tumour phenotype further, we tested the expression of an additional panel of proteins that are specifically expressed by PEL tumour cells.32 Both cases displayed a profile that is clearly indistinguishable from that of PEL. In fact, both cases expressed GRA, AQP3, SELPLG, and VEGF (table 2).
DISCUSSION
KSHV/HHV-8 associated lymphomas, which often develop in HIV infected patients with advanced AIDS, present predominantly as PEL2,10 or, less frequently, as solid, extracavitary based lymphomas, associated with serous effusions.2,8,9,10 Interestingly, KSHV/HHV-8 associated lymphomas that present as solid or extracavitary based lesions in HIV seropositive patients without serous effusions have been reported recently.9,13–16,18–21 In our present report, we provide evidence of the existence of KSHV/HHV-8 associated solid, extracavitary based lymphomas in HIV seronegative patients without serous effusions. Characteristically, these lymphomas had a predilection for the lymph nodes and displayed anaplastic large cell morphology. Immunohistochemistry showed unequivocally that these tumours lacked epithelial and melanocytic cell markers. Based on the immunophenotypical results, there were difficulties in assigning a lymphoid lineage in these KSHV/HHV-8 associated lymphomas using common phenotypic markers (table 2). However, although molecular studies failed to detect monoclonality, the presence of CIgG in these cases suggested that these tumours were related to cells of the plasma cell series. The detection of strong immunostaining with plasma cell reactive antibodies confirmed that these lymphomas were related to cells of the B cell system and had undergone plasmacellular differentiation.
Consistent with these results, these KSHV/HHV-8 associated solid lymphomas expressed a series of proteins that are specifically expressed by PEL tumour cells, confirming the phenotypic relatedness of these lymphomas to PEL. With regard to these features, it is important to note that previous gene expression data have suggested that the tumour cells of PEL are related to a mature B cell shifting towards terminal plasma cell differentiation.32
“Although molecular studies failed to detect monoclonality, the presence of cytoplasmic IgG in these cases suggested that these tumours were related to cells of the plasma cell series”
Both patients presented with diffuse lymphadenopathy and spleen involvement. However, virological features and clinical outcome were significantly different between the two patients. Patient 1 had more advanced stage and rapidly fatal disease in addition to the presence of haematological abnormalities and a high KSHV/HHV-8 viral load compared with patient 2. These data suggest a correlation between the evolution of clinical and virological events. It is noteworthy that a high HHV-8 viral load correlates with clinical exacerbation of MCD in HIV infected patients.43,44
There are both similarities and important differences between KSHV associated germinotropic lymphoproliferative disorder and our present cases of lymphoma that harbour KSHV/HHV-8 in HIV seronegative patients. Germinotropic lymphoproliferative disorder occurs in immunocompetent HIV seronegative individuals without serous effusion, presents as localised lymphadenopathy, and shows a favourable response to treatment. This disorder is composed of plasmablasts with a pronounced tropism for the germinal centres of lymphoid follicles. In contrast, the lymphomas described here presented at an advanced stage of disease (stage III–IV), with generalised lymphadenopathy, and spleen involvement. One patient (case 1) also showed extranodal localisation. Microscopically, these lymphomas were characterised by large neoplastic cells, with anaplastic features, diffusely infiltrating the lymph nodes. A distinctive sinusoidal pattern of growth was also seen.
Take home messages.
We report a previously undescribed Kaposi sarcoma associated herpesvirus/human herpesvirus 8 associated lymphoma in human immunodeficiency virus seronegative patients without serous effusions
These lymphomas show a predilection for the lymph nodes and display anaplastic large cell morphology
They were completely devoid of common cell type specific antigens but showed strong immunostaining with plasma cell reactive antibodies
Thus, analysis of viral infection and immunohistological studies are of primary importance to define this tumour
Previous studies have shown that the biological and genetic features of KSHV/HHV-8 associated solid lymphomas closely mimic those of AIDS related PEL, suggesting that KSHV/HHV-8 associated solid lymphomas should be considered as a “tissue based or extracavitary variant of classic PEL”.16,17 Accordingly, the KSHV/HHV-8 associated lymph node based lymphomas in HIV seronegative patients described here may be part of the spectrum of HIV unrelated PEL, and may represent its solid lymphoma counterpart. These KSHV/HHV-8 associated solid lymphomas in HIV seronegative patients displaying a predilection for the lymph nodes and anaplastic large cell morphology are distinct clinical and pathological entities, although they share an identical biological base with classic PEL. However, because the large tumour cells showed an immunophenotypic profile of differentiated plasma cells with absent expression of B cell markers and strong reactivity for plasma cell associated antigens, such as CD38 and CD138, both the KSHV/HHV8 associated lymphomas may be classified within the heterogeneous group of diffuse large B cell lymphomas with plasmablastic differentiation.45
In conclusion, our study expands the spectrum of KSHV/HHV-8 associated lymphomas presenting primarily as solid or extracavitary based lesions, irrespective of HIV status. The awareness of the existence of this type of KSHV/HHV-8 associated lymphoma in HIV seronegative patients and the knowledge of its features should lead to the use of plasma cell reactive antibodies and antibodies to IgG chains when the common lymphoid markers are not demonstrable in an undifferentiated large cell tumour. In fact, in these cases, the negative immunostaining for CD20 and CD45 could erroneously exclude a lymphoma from the diagnostic alternatives.
It is evident that molecular diagnostic techniques, including analysis of viral infection and immunohistological studies, are of primary importance to define this lymph node based KSHV/HHV-8 associated lymphoma with anaplastic large cell morphology and plasmablastic immunophenotype occurring in HIV seronegative patients.
Acknowledgments
Supported in part by Istituto Superiore di Sanità, Programma Nazionale di Ricerca sull’AIDS – Progetto Patologia, Clinica e Terapia dell’AIDS, 2002–2003, Rome, Italy and by the Ministero della Sanita’, RF 2002, Rome, Italy.
Abbreviations
AQP3, aquaporin-3
CigG, cytoplasmic IgG
EBER, Epstein-Barr virus encoded small non-coding RNA
EBNA-1, Epstein-Barr virus nuclear antigen 1
EBV, Epstein-Barr virus
GRA, granzyme A
HHV-8, human herpesvirus 8
HIV, human immunodeficiency virus
ISH, in situ hybridisation
LMP, latent membrane protein
KSHV, Kaposi sarcoma associated herpesvirus
MCD, multicentric Castleman disease
ORF, open reading fragment
PEL, primary effusion lymphoma
SELPLG, selectin P ligand
VEGF, and vascular endothelial growth factor
vIL-6, viral interleukin 6
The patients gave their informed consent for these case reports to be published.
REFERENCES
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994;226:1865–9. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Knowles DM. The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin Cancer Biol 1999;9:165–74. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body cavity-based lymphomas. N Engl J Med 1995;332:1186–91. [DOI] [PubMed] [Google Scholar]
- 4.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995;86:1276–80. [PubMed] [Google Scholar]
- 5.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 2000;95:1406–12. [PubMed] [Google Scholar]
- 6.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002;99:2331–6. [DOI] [PubMed] [Google Scholar]
- 7.Du M-Q, Diss TC, Liu H, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 2002;100:3415–18. [DOI] [PubMed] [Google Scholar]
- 8.DePond W, Said JW, Tasaka T, et al. Kaposi’s sarcoma-associated lymphoma of the bowel: report of two cases in HIV-positive men with secondary effusion lymphomas. Am J Surg Pathol 1997;2:719–24. [DOI] [PubMed] [Google Scholar]
- 9.Katano H, Suda T, Morishita, et al. Human herpesvirus 8-associated solid lymphomas that occur in AIDS patients take anaplastic large cell morphology. Mod Pathol 2000;13:77–85. [DOI] [PubMed] [Google Scholar]
- 10.Knowles DM. Etiology and pathogenesis of AIDS-related non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am 2003;17:785–820. [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, Chang KL, Gaal K, et al. Primary effusion lymphoma with subsequent development of a small bowel mass in an HIV-seropositive patient: a case report and literature review. Am J Surg Pathol 2002;26:1363–7. [DOI] [PubMed] [Google Scholar]
- 12.Mate JL, Navarro JT, Ariza A, et al. Oral solid form of primary effusion lymphoma mimicking plasmablastic lymphoma. Hum Pathol 2004;35:632–5. [DOI] [PubMed] [Google Scholar]
- 13.Aboulafia DM. HHV-8-a and EBV-associated nonepidermotrophic large B-cell lymphoma presenting as a foot rash in a man with AIDS. AIDS Patient Care STDS 2002;16:139–45. [DOI] [PubMed] [Google Scholar]
- 14.Beaty MW, Kumar S, Sorbara L, et al. A biphenotypic HHV8-associated primary bowel lymphoma. Am J Surg Pathol 1999;23:992–4. [DOI] [PubMed] [Google Scholar]
- 15.Buske C, Hannig H, Hiddemann W, et al. Human herpesvirus-8 (HHV-8) DNA associated with anaplastic large cell lymphoma of the B-cell type in an HIV-1-positive patient. Int J Cancer 1997;73:303–4. [DOI] [PubMed] [Google Scholar]
- 16.Carbone A, Gloghini A, Vaccher E, et al. KSHV/HHV-8-positive solid lymphomas. A tissue-based variant of primary effusion lymphoma. J Mol Diagn 2005;7:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadburn A, Hyjek E, Mathew S, et al. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol 2004;28:1401–16. [DOI] [PubMed] [Google Scholar]
- 18.Costes V, Faumont N, Cesarman E, et al. Human herpesvirus-8-associated lymphoma of the bowel in human immunodeficiency virus-positive patients without history of primary effusion lymphoma. Hum Pathol 2002;33:846–9. [DOI] [PubMed] [Google Scholar]
- 19.Engels EA, Pittaluga S, Whitby D, et al. Immunoblastic lymphoma in persons with AIDS-associated Kaposi’s sarcoma: a role for Kaposi’s sarcoma-associated herpesvirus. Mod Pathol 2003;16:424–9. [DOI] [PubMed] [Google Scholar]
- 20.Morand P, Buisson M, Collandre H, et al. Human herpesvirus 8 and Epstein Barr-virus in a cutaneous B-cell lymphoma and a malignant cell line established from the blood of an AIDS patient. Leuk Lymphoma 1999;35:379–87. [DOI] [PubMed] [Google Scholar]
- 21.Navarro JT, Ribera JM, Junca J, et al. Anorectal lymphoma without effusion associated with human herpesvirus-8 and type 1 Epstein-Barr virus in an HIV-infected patient. Hum Pathol 2003;34:630. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q, Chang KL, Gaal KK, et al. KSHV/HHV8-associated lymphoma simulating anaplastic large cell lymphoma. Am J Surg Pathol 2004;28:693–7. [DOI] [PubMed] [Google Scholar]
- 23.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br J Haematol 1996;94:533–43. [DOI] [PubMed] [Google Scholar]
- 24.Cesarman E, Nador RG, Aozasa K, et al. Kaposi’s sarcoma-associated herpesvirus in non-AIDS-related lymphomas occurring in body cavities. Am J Pathol 1996;149:53–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Jones D, Ballestas ME, Kaye KM, et al. Primary-effusion lymphoma and Kaposi’s sarcoma in a cardiac-transplant recipient. N Engl J Med 1998;339:444–9. [DOI] [PubMed] [Google Scholar]
- 26.Said JW, Tasaka T, Takeuchi S, et al. Primary effusion lymphoma in women: report of two cases of Kaposi’s sarcoma herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative woman. Blood 1996;88:3124–8. [PubMed] [Google Scholar]
- 27.Strauchen JA, Hauser AD, Burstein DA, et al. Body cavity-based malignant lymphoma containing Kaposi’s sarcoma-associated herpesvirus in an HIV-negative man with previous Kaposi’s sarcoma. Ann Intern Med 1996;125:822–5. [DOI] [PubMed] [Google Scholar]
- 28.Teruya-Feldstein J, Zauber P, Setsuda JE, et al. Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman’s disease and primary effusion lymphoma. Lab Invest 1998;78:1637–42. [PubMed] [Google Scholar]
- 29.Ariad S, Benharroch D, Lupu L, et al. Early peripheral lymph node involvement of human herpesvirus 8-associated, body cavity-based lymphoma in a human immunodeficiency virus-negative patient. Arch Pathol Lab Med 2000;124:753–5. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen H, Hiscott J, Pitha PM. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev 1997;8:293–312. [DOI] [PubMed] [Google Scholar]
- 31.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on non-Hodgkin’s disease classification. Cancer Res 1971;31:1860–1. [PubMed] [Google Scholar]
- 32.Klein U, Gloghini A, Gaidano G, et al. Gene expression profile analysis of AIDS-related primary effusion lymphoma (PEL) suggests a plasmablastic derivation and identifies PEL-specific transcripts. Blood 2003;101:4115–21. [DOI] [PubMed] [Google Scholar]
- 33.Pan L, Milligan L, Michaeli J, et al. Polymerase chain reaction detection of Kaposi’s sarcoma-associated herpesvirus—optimized protocols and their application to myeloma. J Mol Diagn 2001;3:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbone A, Cilia AM, Gloghini A, et al. Establishment of HHV-8 positive and HHV-8 negative lymphoma cell lines from primary lymphomatous effusions. Int J Cancer 1997;73:562–9. [DOI] [PubMed] [Google Scholar]
- 35.Carbone A, Cilia AM, Gloghini A, et al. Establishment and characterization of EBV-positive and EBV-negative primary effusion lymphoma cell lines harbouring human herpesvirus type-8. Br J Haematol 1998;102:1081–9. [DOI] [PubMed] [Google Scholar]
- 36.Gaidano G, Pastore C, Gloghini A, et al. Distribution of human herpesvirus-8 sequences throughout the spectrum of AIDS-related neoplasia. AIDS 1996;10:941–9. [DOI] [PubMed] [Google Scholar]
- 37.Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal antialkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984;32:219–29. [DOI] [PubMed] [Google Scholar]
- 38.Tedeschi R, Dillner J, De Paoli P. Laboratory diagnosis of human herpes virus 8 infection in humans. Eur J Clin Microbiol Infect Dis 2002;21:831–44. [DOI] [PubMed] [Google Scholar]
- 39.Pratesi C, Bortolin MT, D’Andrea M, et al. Quantitative plasma/serum EBV DNA load by LMP2A determination in an Italian cohort of NPC patients. J Clin Virol 2003;28:155–64. [DOI] [PubMed] [Google Scholar]
- 40.Carbone A, Gloghini A, Larocca LM, et al. Expression profile of MUM1/IRF4, BCL-6, and CD138/syndecan-1 defines novel histogenetic subsets of human immunodeficiency virus-related lymphomas. Blood 2001;97:744–51. [DOI] [PubMed] [Google Scholar]
- 41.Turley H, Jones M, Erber W, et al. VS38: a new monoclonal antibody for detecting plasma cell differentiation in routine sections. J Clin Pathol 1994;47:418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wijdenes J, Vooijs WC, Clément C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol 1996;94:318–23. [DOI] [PubMed] [Google Scholar]
- 43.Grandadam M, Dupin N, Calvez V, et al. Exacerbations of clinical symptoms in human immunodeficiency virus type 1-infected patients with multicentric Castleman’s disease are associated with a high increase in Kaposi’s sarcoma herpesvirus DNA load in peripheral blood mononuclear cells. J Infect Dis 1997;75:1198–201. [DOI] [PubMed] [Google Scholar]
- 44.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 2000;96:2069–73. [PubMed] [Google Scholar]
- 45.Colomo L, Loong F, Rives S, et al. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol 2004;28:736–47. [DOI] [PubMed] [Google Scholar]


