Abstract
Background: Aminopeptidase N (CD13) is expressed in normal and neoplastic liver tissue, where it exhibits a characteristic canalicular pattern (CD13can), similar to that seen for CD10 and when antibodies crossreact with biliary glycoprotein I (p-CEA).
Aim: To compare the putative diagnostic use of CD13can in differentiating between hepatocellular (HCC) and non-hepatocellular carcinomas metastatic to the liver (non-HCC).
Methods: A retrospective study comparing 53 HCC specimens with 32 non-HCC specimens. Immunostaining was performed with HepPar1 and antibodies directed against CD10, CD13, p-CEA, and α fetoprotein (AFP).
Results: In the HCC group, a canalicular staining pattern was found for CD13, p-CEA, and CD10 in 51, 43, and 33 specimens, respectively. HepPar1 was positive in 29 and AFP in 17 HCC specimens. In the non-HCC group, canalicular immunostaining for CD10 and p-CEA was confined to non-neoplastic liver tissue. One poorly differentiated cholangiocarcinoma showed apical expression of CD13, resembling to some extent CD13can. Sensitivity and specificity were 96.2% and 97.0%, respectively, for CD13can, 81.1% and 100% for p-CEAcan, 62.3% and 100%, for CD10can, 54.7% and 99.9% for HepPar1, and 32.1% and 100% for AFP.
Conclusions: These results show that CD13can is more sensitive in differentiating between HCC and non-HCC than CD10can, p-CEAcan, HepPar1, and AFP.
Keywords: liver, hepatocellular carcinoma, aminopeptidase N, CD13
Primary and metastatic malignant tumours of the liver may demonstrate a wide variety of histological patterns and the surgical pathologist is often challenged with biopsy specimens that yield only a small fraction of the liver mass lesion. Hepatocellular carcinomas (HCCs) and cholangiocarcinomas or metastatic tumours can often be differentiated using routine light microscopy, but it may be difficult to make this distinction, and special stains are needed. Special stains of diagnostic value in differentiating between HCC and non-HCC neoplasms include immunostaining with polyclonal antibodies crossreacting with biliary glycoprotein 1 (p-CEA),1,2,3,4,5,6,7,8,9,10,11,12,13 immunostaining with antibodies directed against α fetoprotein (AFP),1–3,6,7 CD10,9–14 and HepPar1,6,10–16 and detection of albumin mRNA by in situ hybridisation.1,7,17–20 Immunostaining for p-CEA and CD10 shows a characteristic canalicular pattern, with a sensitivity ranging from 50% to 90% and a specificity of almost 100%. Other antibodies and antigens have been tested, but these have proved to be less useful or have not yet been confirmed, including α1 antitrypsin,21 monoclonal antibodies directed against carcinoembryonic antigen,3,6,8 various cytokeratins,1,2,6,20,22 epithelial membrane antigen,8 erythropoiesis associated antigen,21 factor XIII,2 and p28GANK.14
“CD13 might be useful as an additional marker in differentiating between hepatocellular carcinoma and non-hepatocellular neoplasms”
It was shown recently that aminopeptidase N (CD13) is expressed in both normal and neoplastic liver tissue, where it exhibits a canalicular distribution pattern (CD13can) similar to that seen for p-CEA and CD109,23; thus, CD13 might be useful as an additional marker in differentiating between HCC and non-hepatocellular neoplasms. The aim of our retrospective study was to investigate the sensitivity, specificity, and spatial distribution of CD13 in HCC and non-HCC compared with immunostaining for p-CEAcan, CD10can, HepPar1, and AFP.
MATERIALS AND METHODS
Case selection
Fifty three HCC specimens, comprising 50 liver biopsies and three resection specimens, were retrieved from the archive of the department of pathology, Otto-von-Guericke-University, Germany. All cases were reviewed before study inclusion. In addition, most cases had been discussed during weekly clinicopathological conferences. Cases with ambiguous clinical or histological diagnostic features were not included in our study. The diagnosis of HCC was based on cytological, histological, and clinical criteria. Several clinical criteria supported the diagnosis of HCC, including evidence of a chronic diffuse liver disease with either liver fibrosis or cirrhosis, raised serum AFP values, and absence of an extrahepatic primary tumour. The specimens were obtained from 50 patients with an average age of 70.1 years (range, 49–95), 40 of whom were male and 10 were female (male to female ratio, 4 : 1). The HCCs were categorised into well (G1), moderately (G2), or poorly (G3) differentiated types, corresponding to Edmondson’s grades I/II, III, or IV, respectively.24,25 As a control group (non-HCC), we selected 32 biopsy specimens of liver metastases from 32 patients; the primary site of the malignant tumour was confirmed clinically and/or histologically (table 1). The average age of the patients in the non-HCC group was 68.2 years (range, 40–88) with 20 male and 12 female patients (male to female ratio, 1.6 : 1).
Table 1.
Non-hepatocellular carcinomas
| No. | Age (years) | Sex | Diagnosis | Primary |
| 1 | 75 | M | Small cell carcinoma | Lung |
| 2 | 81 | M | Small cell carcinoma | Lung |
| 3 | 47 | M | Small cell carcinoma | Lung |
| 4 | 75 | F | Poorly differentiated non-small cell cancer | Lung |
| 5 | 72 | M | Poorly differentiated non-small cell cancer | Lung |
| 6 | 76 | M | Poorly differentiated non-small cell cancer | Lung |
| 7 | 46 | F | Poorly differentiated invasive ductal carcinoma | Breast |
| 8 | 82 | F | Poorly differentiated invasive ductal carcinoma | Breast |
| 9 | 64 | F | Undifferentiated carcinoma NOS | Breast |
| 10 | 65 | F | Moderately differentiated invasive ductal carcinoma | Breast |
| 11 | 79 | F | Undifferentiated carcinoma NOS | Breast |
| 12 | 61 | M | Undifferentiated carcinoma NOS | Oesophagus |
| 13 | 43 | M | Undifferentiated carcinoma NOS | Oesophagus |
| 14 | 82 | M | Poorly differentiated neuroendocrine carcinoma | Stomach |
| 15 | 68 | M | Poorly differentiated adenocarcinoma, intestinal type | Stomach |
| 16 | 88 | M | Undifferentiated carcinoma NOS | Stomach |
| 17 | 79 | F | Poorly differentiated adenocarcinoma | Colon |
| 18 | 71 | M | Poorly differentiated cholangiocarcinoma | Liver |
| 19 | 64 | M | Poorly differentiated cholangiocarcinoma | Liver |
| 20 | 65 | M | Poorly differentiated adenocarcinoma NOS | Extrahepatic bile ducts |
| 21 | 71 | M | Poorly differentiated neuroendocrine carcinoma | Pancreas |
| 22 | 57 | M | Poorly differentiated neuroendocrine carcinoma | Pancreas |
| 23 | 82 | M | Undifferentiated carcinoma NOS | Pancreas |
| 24 | 63 | M | Undifferentiated carcinoma NOS | Pancreas |
| 25 | 73 | M | Poorly differentiated ductal adenocarcinoma | Pancreas |
| 26 | 58 | M | Poorly differentiated ductal adenocarcinoma | Pancreas |
| 27 | 75 | F | Poorly differentiated serous papillary carcinoma | Ovary |
| 28 | 40 | F | Poorly differentiated squamous cell carcinoma | Cervix |
| 29 | 63 | F | Poorly differentiated squamous cell carcinoma | Cervix |
| 30 | 71 | M | Well differentiated neuroendocrine carcinoma | CUP |
| 31 | 66 | F | Poorly differentiated neuroendocrine carcinoma | CUP |
| 32 | 70 | F | Small cell (neuroendocrine) carcinoma | CUP |
CUP, carcinoma of unknown primary; NOS, not otherwise specified.
Specimen processing
All biopsy and resection specimens were fixed in 10% buffered formalin and embedded in paraffin wax. Dewaxed serial sections were stained with haematoxylin and eosin, periodic acid Schiff with and without diastase pretreatment, and reticulin stain. Serial sections were cut at 3 µm and placed on Superfrost Plus glass slides.
Materials
Immunostaining was performed with monoclonal antibodies directed against CD10 (clone 56C6), CD13 (clone 38C12; both Novocastra Laboratories, distributed by Medac GmbH, Wedel, Germany), and HepPar1 (Dako, Glostrup, Denmark), and with polyclonal antibodies directed against AFP (Dako), and carcinoembryonic antigen (which crossreacts with biliary glycoprotein 1; p-CEA; Quartett, Berlin, Germany).
Immunohistochemistry
For immunostaining, sections were dewaxed in xylene and rehydrated in an alcohol series. Endogenous biotin was blocked using the endogenous biotin blocking kit from Ventana (Strasbourg, France). Immunostaining with p-CEA required blockade of endogenous peroxidase with 3% H2O2 for 15 minutes at room temperature (RT) before the addition of p-CEA for one hour at 37°C (1/500 dilution) in a moist chamber. This was followed by incubation with biotinylated secondary antibody and the streptavidin–peroxidase complex, each for 15 minutes at RT. Between steps, the sections were washed in Tris buffered saline. Immunostaining for CD10 and HepPar1 required pretreatment with 1mM EDTA (pH 8.0, 20 minutes, 450 W microwave oven), and for CD13 required pretreatment with 10mM sodium citrate (pH 6.0, 3 × 10 minutes, 600 W microwave oven). Sections were incubated with anti-CD10 (1/25 dilution) and anti-CD13 (1/50 dilution) for one hour at 37°C in a moist chamber, followed by incubation with biotinylated antimouse IgG/antirabbit IgG (1/200 dilution; Vector Laboratories; distributed by Camon, Wiesbaden, Germany) and ABC alkaline phosphatase reagent, each for 30 minutes at RT. Immunoreactions were visualised with the avidin–biotin complex method, applying a Vectastain ABC alkaline phosphatase kit (distributed by Camon) or an Ultratech horseradish peroxidase streptavidin–biotin universal detection system (Immunotech, Marseilles, France). Fast red and 3,3-diaminobenzidinetetrahydrochloride (DAB), respectively, served as chromogens.
Immunostaining with HepPar1 (1/50 dilution) was performed using the Ventana enhanced alkaline phosphatase red detection kit and the Ventana Nexus immunostainer. The primary antibody was incubated for 30 minutes at 37°C. The biotinylated secondary antibody and the alkaline phosphatase–streptavidin conjugate were applied according to the manufacturer’s instructions. Fast red served as chromogen.
Immunostaining with anti-AFP (1/100 dilution) was performed using the Ventana Basic DAB detection kit and the Ventana Nexus immunostainer. Endogenous peroxidase was blocked for four minutes at 37°C, according to the manufacturer’s instructions. The primary antibody was incubated for 26 minutes at 37°C. The biotinylated secondary antibody, the avidin–horseradish peroxidase conjugate, and the basic DAB solution were applied according to the manufacturer’s instructions. The reaction was enhanced with copper sulfate solution (four minutes at 37°C).
All specimens were counterstained with haematoxylin. Primary antibodies were omitted for negative controls and tissue specimens recommended by the manufacturers were used as positive controls.
RESULTS
Hepatocellular carcinomas
CD13 was detected in 51 of the 53 HCC specimens. Two CD13 immunostaining patterns were observed: cytoplasmic and cell membrane. Cell membrane staining was further divided into canalicular (delineating bile canaliculi) and non-canalicular patterns. A canalicular pattern was found in 51 specimens—all 14 well differentiated, 34 of 35 moderately differentiated, and three of four poorly differentiated HCCs (fig 1). Nine HCC specimens showed a cytoplasmic staining pattern and six non-canalicular staining of the cell membrane. Cytoplasmic staining of less than 10% of the tumour cells was found in six biopsies, staining of 10–50% of the tumour cells in three biopsies, and staining of greater than 50% of the tumour cells was not seen. Simultaneous cytoplasmic and cell membrane staining was found in nine of the 53 biopsy specimens. Non-neoplastic liver tissue showed a canalicular staining pattern and apical membranous staining of bile ducts (fig 2).
Figure 1.
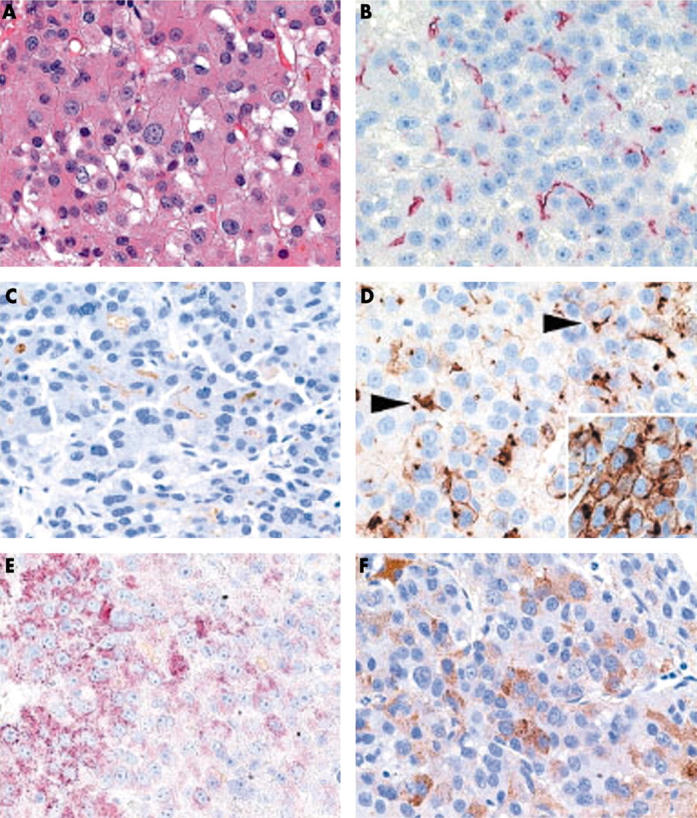
(A) A moderately differentiated hepatocellular carcinoma showing canalicular expression of (B) CD13 (aminopeptidase N), (C) p-CEA (antibody that crossreacts with biliary glycoprotein I), and (D, arrowheads) CD10, and cytoplasmic staining with (E) HepPar1 and (F) anti-AFP (α fetoprotein). (D, insert) Additional strong membranous expression was seen for CD10. (A) Haematoxylin and eosin; (B) anti-CD13, (C) anti-p-CEA, (D) anti-CD10, (E) HepPar1, and (F) anti-AFP, all with haematoxylin counterstain; original magnifications, ×400.
Figure 2.
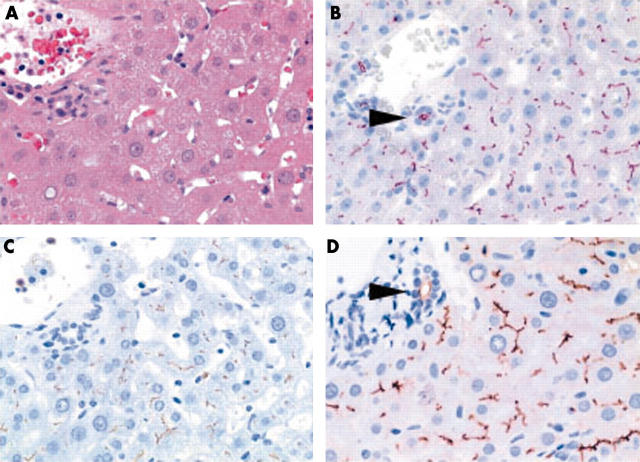
(A) Non-neoplastic liver tissue showing canalicular immunostaining with (B) anti-CD13 (aminopeptidase N), (C) anti-p-CEA (antibody that crossreacts with biliary glycoprotein I), and (D) anti-CD10. Bile ducts express CD10 and CD13 at the apical membrane (B and D; arrowheads). (A) Haematoxylin and eosin; (B) anti-CD13, (C) anti-p-CEA, and (D) anti-CD10, all with haematoxylin counterstain; original magnifications, ×400.
Immunostaining with p-CEA was found in 43 of 53 HCC specimens. The staining pattern was similar to that seen for CD13—cytoplasmic, canalicular, and non-canalicular (fig 1). A canalicular pattern was found in 43 specimens, a non-canalicular pattern in 13 specimens, and cytoplasmic staining was found in 13 specimens. Overall, the extent of the canalicular staining pattern was decreased in poorly differentiated HCCs; 10 of 14 well differentiated HCCs, 31 of 35 moderately differentiated HCCs, and two of four poorly differentiated HCCs showed a canalicular pattern. Non-neoplastic liver parenchyma showed canalicular immunostaining (fig 2).
CD10 was detected in 42 of the 53 HCC specimens. The staining pattern was similar to that seen for CD13 and p-CEA—cytoplasmic, canalicular, and non-canalicular (fig 1). A canalicular pattern was found in 33 specimens and the prevalence of canalicular staining correlated with the histological grade: 12 of 14 G1 HCCs, 19 of 35 G2 HCCs, and two of four G3 HCCs showed canalicular staining. Thirty HCC specimens had a cytoplasmic staining pattern and eight had non-canalicular staining of the cell membrane. Cytoplasmic staining of less than 10% of the tumour cells was found in 14 biopsies, staining of 10–50% in eight biopsies, and staining of greater than 50% in eight biopsies. Simultaneous cytoplasmic and cell membrane staining was found in 21 of the 53 biopsy specimens. There was no significant difference in the overall expression of CD10 between well and moderately differentiated HCCs. Non-neoplastic liver tissue showed a canalicular staining pattern in the parenchyma and apical membranous staining of bile ducts (fig 2).
Immunostaining with HepPar1 was found in 29 of 53 HCC specimens; HepPar1 stained the cytoplasm only (fig 1). The prevalence of HepPar1 immunostaining correlated inversely with the histological grade of the HCCs; HepPar1-staining was found in 10 of 14 well differentiated, 19 of 35 moderately differentiated, and none of the four poorly differentiated HCCs.
Immunostaining for AFP was found in 17 of 53 HCC specimens; AFP was found both in the cytoplasm and occasionally at the cell membrane (fig 1). The prevalence of AFP correlated with the histological grade of the HCCs; AFP was found in two of 14 G1, 12 of 35 G2, and three of four G3 HCCs.
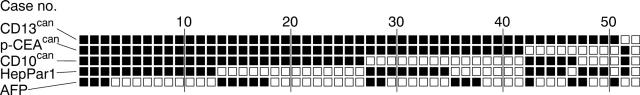
Figure 3 summarises the distribution pattern of all five immunohistochemical markers (CD13, p-CEA, and CD10—canalicular pattern only; HepPar1 and AFP—cytoplasm and/or cell membrane). Only two cases showed no CD13can immunostaining: one specimen stained for none of the immunohistochemical markers studied, although follow up biopsies clearly showed the HCC nature of the specimen. The second specimen showed canalicular immunostaining for CD10 and p-CEA, but lacked CD13 immunoreactivity. Interestingly, the bile canaliculi of HCCs were more often immunoreactive for CD13 than for p-CEA or CD10 (fig 3).
Figure 3.
Immunohistochemical expression profile of 53 hepatocellular carcinomas (HCCs) for CD13 (aminopeptidase N), p-CEA (antibody that crossreacts with biliary glycoprotein I), CD10, HepPar1, and AFP (α fetoprotein). Each column represents an individual biopsy sample from an HCC. Black squares denote positive canalicular immunostaining for CD13 (CD13can), p-CEA (p-CEAcan), and CD10 (CD10can), and cytoplasmic or membranous immunostaining with HepPar1 and for AFP, respectively; open squares denote no immunoreactivity in the tumour cells.
Non-hepatocellular carcinomas
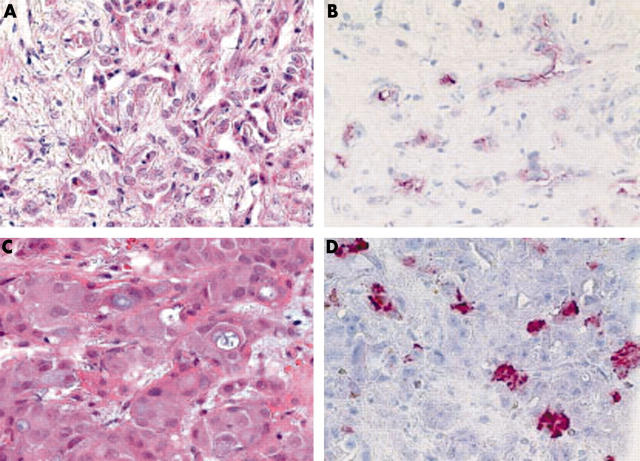
Among the non-HCCs, CD13 was present in the cytoplasm of one specimen—a poorly differentiated cholangiocarcinoma. Apical membranous staining was found in two cases—a metastasis of a poorly differentiated serous papillary carcinoma of the ovary and a poorly differentiated cholangiocarcinoma (fig 4). In the latter, CD13 immunostaining resembled canalicular staining of HCC. However, a desmoplastic stroma was present and all other HCC markers were negative (CD10can, p-CEAcan, HepPar1, and AFP).
Figure 4.
(A) A cholangiocarcinoma shows apical membranous expression of (B) CD13, resembling to some extent canalicular expression of hepatocytes. (D) Cytoplasmic HepPar1 immunostaining was found in another (C) cholangiocarcinoma. (A, C) Haematoxylin and eosin; (B) anti-CD13, (D) HepPar1, both with haematoxylin counterstain; original magnifications, ×400.
p-CEA staining was positive in 17 of the 32 specimens: staining was cytoplasmic only in 10, membranous only in one, and both cytoplasmic and membranous in six specimens. CD10 was present in the cytoplasm and at the cell membrane. Six of the 32 biopsy specimens showed cytoplasmic staining and four showed both cytoplasmic and cell membrane staining. In seven biopsy specimens, less than 10% of the tumour cells were immunoreactive, and more than 50% were immunoreactive in three. A canalicular staining pattern for CD10 and p-CEA was not detected in the non-HCC specimens.
HepPar1 immunostaining was found in only one specimen obtained from a poorly differentiated cholangiocarcinoma (fig 4). AFP staining was negative in all of the non-HCCs.
The sensitivity and specificity were calculated as 96.2% and 97.0%, respectively, for CD13can, 81.1% and 100% for p-CEAcan, 62.3% and 100%, for CD10can, 54.7% and 99.9% for HepPar1, and 32.1% and 100% for AFP.
Table 2 summarises the immunostaining results for HCC and non-HCC.
Table 2.
Immunostaining of hepatocellular (HCC) and non-hepatocellular (non-HCC) carcinomas
| HCC | Non-HCC | ||||
| Total n/n (%) | G1 n/n (%) | G2 n/n (%) | G3 n/n (%) | n/n (%) | |
| CD13 | |||||
| Cytoplasm | 9/53 (17.0) | 0/14 | 8/35 (22.9) | 1/4 (25.0) | 1/32 (3.1) |
| Cell membrane (non-canalicular) | 6/53 (11.3) | 3/14 (21.4) | 2/35 (5.7) | 1/4 (25.0) | 1/32 (3.1) |
| Cell membrane (canalicular) | 51/53 (96.2) | 14/14 | 34/35 (97.1) | 3/4 (75.0) | 1/32 (3.1) |
| p-CEA | |||||
| Cytoplasm | 13/53 (24.5) | 2/14 (14.3) | 9/35 (25.7) | 2/4 (50.0) | 16/32 (50.0) |
| Cell membrane (non-canalicular) | 14/53 (26.4) | 4/14 (28.6) | 8/35 (22.9) | 2/4 (50.0) | 7/32 (21.9) |
| Cell membrane (canalicular) | 43/53 (81.1) | 10/14 (71.4) | 31/35 (88.6) | 2/4 (50.0) | 0/32 |
| CD10 | |||||
| Cytoplasm | 30/53 (56.6) | 10/14 (71.4) | 18/35 (51.4) | 2/4 (50.0) | 10/32 (31.3) |
| Cell membrane (non-canalicular) | 8/53 (15.1) | 4/14 (28.6) | 3/35 (8.6) | 1/4 (25.0) | 4/32 (12.5) |
| Cell membrane (canalicular) | 33/53 (62.3) | 12/14 (85.7) | 19/35 (54.3) | 2/4 (50.0) | 0/32 |
| HepPar1 | 29/53 (54.7) | 10/14 (71.4) | 19/35 (54.3) | 0/4 | 1/32 (3.1) |
| AFP | 17/53 (32.1) | 2/14 (14.3) | 12/35 (34.3) | 3/4 (75.0) | 0/32 |
AFP, α fetoprotein; CD13, aminopeptidase N, p-CEA; antibody that crossreacts with biliary glycoprotein I.
DISCUSSION
Aminopeptidase N (CD13, APN) is a zinc dependent, cell membrane metallopeptidase, which has been shown to participate in the postsecretory processing of neuropeptides and peptide hormones. It is widely distributed and has been found in various cell types of organs and tissues, including benign and malignant tumours. The expression and putative pathophysiological role of CD13 has been studied in a variety of malignant tumours.26,27 The expression of CD13 has been linked to tumour cell proliferation, degradation of extracellular matrix, and metastatic behaviour.27–32 Almost all of these biological effects were attributed to the ectopeptidase activity.
Recently, it was shown that CD13 is also expressed by HCCs.23 Interestingly, CD13 mRNA showed no significant differences between non-tumorous liver and HCC, whereas CD13 protein values were slightly increased.23 CD13 may have a pathophysiological effect on hepatocarcinogenesis by cleaving regulatory peptides and peptide hormones. However, CD13 may have another role in HCC, which is unique to the liver—that is, the formation of bile canaliculi and production and secretion of bile acids. Previously, we found CD13 positive bile canaliculi in the fetal liver, focal nodular hyperplasia, non-tumorous liver, and HCC.23 This reflects its ubiquitous expression and its close association with the formation and function of bile canaliculi.33 CD13 positive bile canaliculi have been detected as early as 16 to 18 weeks of gestation,23 underscoring the role of this molecule in morphogenesis. Expression of CD13 is maintained at a constant level during liver regeneration.34 Thus, expression of CD13 seems to be required in various proliferation and differentiation states of the liver, which makes it an attractive diagnostic marker for surgical pathology.
“CD13 may have a role in hepatocellular carcinoma that is unique to the liver—that is, the formation of bile canaliculi and production and secretion of bile acids”
Because CD13 shows a specific canalicular staining pattern,23 similar to that seen for p-CEAcan and CD10can, our present study aimed to investigate the putative use of CD13can in differentiating HCCs from non-HCCs.
In our current series, both HCCs and non-HCCs expressed CD13 in the cytoplasm, at the cell membrane, or both, and the detection of CD13 itself was of no use in differentiating HCCs from non-HCCs. However, only non-neoplastic liver tissue and HCC showed a characteristic canalicular staining pattern, similar to that seen for p-CEA and CD10; this pattern was considered to be specific for HCCs and yielded a sensitivity of 96.2% and a specificity of 97.0% in our series. To evaluate further the diagnostic use of CD13can in differentiating HCC from non-HCC, we compared the sensitivity and specificity of CD13can with p-CEAcan, CD10can, HepPar1, and AFP—markers that have proved their diagnostic usefulness in surgical pathology.1–3,6,7,9,19–21 In our series, the sensitivity of CD13can was greater than that of p-CEAcan, CD10can, HepPar1, and AFP. Previous reports have described a canalicular staining pattern for p-CEA in 24–90% of cases1–4,6,7 and for CD10 in 28–86% of cases1–4,6,7; our figures were 81.1% and 62.3%, respectively. Thus, our values for canalicular immunostaining of anti-p-CEA and anti-CD10 are within the range of previous observations. Table 3 summarises the results of more recent studies investigating the diagnostic use of CD10can and p-CEAcan. Table 3 shows that the sensitivity of CD10can is much lower than that of p-CEAcan. However, as shown in our present study, the difference between p-CEAcan and CD13can is less pronounced, although unlike p-CEA and CD10, CD13 stained the cytoplasm of tumour cells less often, facilitating the recognition of even low numbers of bile canaliculi, which we consider to be an advantage of CD13, particularly in poorly differentiated HCCs.
Table 3.
Summary of recent studies investigating the sensitivity and specificity of canalicular immunostaining for CD10 and p-CEA
| First author | CD10can | p-CEAcan | ||
| HCC n/n (%) | Non-HCC n/n (%) | HCC n/n (%) | Non-HCC n/n (%) | |
| Borscheri9 | 43/63 (68.3%) | 0/25 (0%) | 60/63 (95.2%) | 0/25 (0%) |
| Chu11 | 50/96 (52.1%) | ND | 73/96 (76.0%) | ND |
| Lau13 | 17/42 (40.5%) | 0/65 (0%) | 29/42 (69.0%) | 0/56 (0%) |
| Lee15 | 21/75 (28.0%) | 0/399 (0%) | ND | ND |
| Lin35 | 19/22 (86.4%) | 0/23 (0%) | ND | ND |
| Morrison12 | 13/25 (52.0%) | 0/75 (0%) | 24/25 (96.0%) | 2/75 (2.7%) |
| Saad10 | 23/30 (76.7%) | 0/30 (0%) | 24/30 (80.0%) | 0/30 (0%) |
| Xiao36 | 9/15 (60.0%) | 0/19 (0%) | 15/15 (100%) | 0/19 (0%) |
| Our present study | 33/53 (62.3%) | 0/33 (0%) | 43/53 (81.1%) | 0/33 (0%) |
| Summary | 228/421 (54.2%) | 0/669 (0%) | 268/324 (82.7%) | 2/238 (0.8%) |
HCC, hepatocellular carcinoma; ND, not determined; p-CEA, antibody that crossreacts with biliary glycoprotein I.
CD13 was also expressed in bile ducts and in a cholangiocarcinoma, here resembling canalicular immunostaining. However, cholangiocarcinomas have an abundant desmoplastic stroma that aids in differentiating them from HCC in most cases.
HepPar1 is a monoclonal antibody that recognises a mitochondrial antigen of hepatocytes.6,37 In the past few years, several studies have investigated the sensitivity and specificity of HepPar1,10–16,38 and these were shown to range from 75% to 100% and from 66% to 100%, respectively. In our series, we found HepPar1 staining in only 29 of the 53 (54.7%) HCCs. This rather low sensitivity may be a sampling error, because most of our specimens were biopsies: HepPar1 staining is not homogeneous and only eight specimens showed HepPar1 staining in more than 50% of the tumour cells, and in most of our cases HepPar1 stained less than 50% of the tumour cells. Furthermore, in contrast to CD13, p-CEA, and CD10, immunostaining with HepPar1 does not show a hepatocyte specific staining pattern. Between 44% and 47% of gastric cancers react with HepPar1,16,38 and differentiating poorly differentiated gastric cancer from HCC using HepPar1 immunostaining only can be difficult at times. HepPar1 also occasionally stains cholangiocarcinomas (our present study)11,15,38, and pancreatic, colon, lung, adrenal, neuroendocrine, ovarian, and endocervical cancers.11,15,38 Thus, although HepPar1 frequently reacts with HCCs, it should be used cautiously and in conjunction with a panel of other antibodies, as recently stated by Fan et al.38
Take home messages.
Canalicular staining for CD13 (CD13can) is a highly specific marker of hepatocyte differentiation, with a sensitivity greater than that of p-CEAcan, CD10can, HepPar1, and α fetoprotein
Although CD13can does not differentiate between benign and malignant lesions, it is clearly useful for differentiating hepatocellular carcinoma (HCC) from non-HCC lesions
Further studies are needed to determine whether CD13 could replace p-CEA and CD10 in the diagnostic hepatopathology of HCCs and liver metastases
In our series, the sensitivity of CD13can, p-CEAcan, CD10can, and HepPar1 was superior to that of AFP; only 16 of the 53 HCCs expressed AFP. Previous studies have shown that between 17% and 62% of HCCs show immunostaining for AFP,1–3,6,7 and AFP immunostaining in our HCC specimens was within this range.
Sensitivity and specificity are influenced by many variables, with sampling being the most important. Biopsy specimens often provide only a small fraction of the tumour, so that a lack of immunostaining may simply be the result of inadequate sampling (see above). All five markers tested here are subject to sampling errors. Thus, to reduce sampling errors, using a battery of different markers has become common practice in cases where the histological diagnosis is not readily apparent from routine histochemical stains (for example, haematoxylin and eosin, periodic acid Schiff, and reticulin stain). By comparing the staining patterns of CD13, p-CEA, CD10can, HepPar1, and AFP, we were able to show that only one specimen was negative for all five markers. A combination of CD13can (as the most sensitive marker for the presence of bile canaliculi), AFP (as a sensitive marker for poorly differentiated HCCs), and HepPar1 staining was diagnostic in 98.1% of our HCCs, whereas CD13can and AFP together were diagnostic in 96.2% of cases. Future studies are needed to determine whether CD13 has the potential to replace p-CEA and CD10 in the diagnostic hepatopathology of HCCs and liver metastases.
In summary, canalicular staining for CD13 is a highly specific marker of hepatocyte differentiation, with a sensitivity greater than that of p-CEAcan and CD10can. Although CD13can does not differentiate between benign and malignant lesions, it is clearly of use in differentiating HCC from non-HCC.
Abbreviations
AFP, α fetoprotein
can, canalicular pattern
CD13, aminopeptidase N
DAB, 3,3-diaminobenzidinetetrahydrochloride
HCC, hepatocellular carcinoma
p-CEA, antibody that crossreacts with biliary glycoprotein I
RT, room temperature
REFERENCES
- 1.D’Errico A, Baccarini P, Fiorentino M, et al. Histogenesis of primary liver carcinomas: strengths and weaknesses of cytokeratin profile and albumin mRNA detection. Hum Pathol 1996;27:599–604. [DOI] [PubMed] [Google Scholar]
- 2.Hurlimann J, Gardiol D. Immunohistochemistry in the differential diagnosis of liver carcinomas. Am J Surg Pathol 1991;15:280–8. [DOI] [PubMed] [Google Scholar]
- 3.Ma CK, Zarbo RJ, Frierson HF Jr, et al. Comparative immunohistochemical study of primary and metastatic carcinomas of the liver. Am J Clin Pathol 1993;99:551–7. [DOI] [PubMed] [Google Scholar]
- 4.Koelma IA, Nap M, Huitema S, et al. Hepatocellular carcinoma, adenoma, and focal nodular hyperplasia. Comparative histopathologic study with immunohistochemical parameters. Arch Pathol Lab Med 1986;110:1035–40. [PubMed] [Google Scholar]
- 5.Wong MA, Yazdi HM. Hepatocellular carcinoma versus carcinoma metastatic to the liver. Value of stains for carcinoembryonic antigen and naphthylamidase in fine needle aspiration biopsy material. Acta Cytol 1990;34:192–6. [PubMed] [Google Scholar]
- 6.Minervini MI, Demetris AJ, Lee RG, et al. Utilization of hepatocyte-specific antibody in the immunocytochemical evaluation of liver tumors. Mod Pathol 1997;10:686–92. [PubMed] [Google Scholar]
- 7.Oliveira AM, Erickson LA, Burgart LJ, et al. Differentiation of primary and metastatic clear cell tumors in the liver by in situ hybridization for albumin messenger RNA. Am J Surg Pathol 2000;24:177–82. [DOI] [PubMed] [Google Scholar]
- 8.Christensen WN, Boitnott JK, Kuhajda FP. Immunoperoxidase staining as a diagnostic aid for hepatocellular carcinoma. Mod Pathol 1989;2:8–12. [PubMed] [Google Scholar]
- 9.Borscheri N, Roessner A, Röcken C. Canalicular immunostaining of neprilysin (CD10) as a diagnostic marker for hepatocellular carcinomas. Am J Surg Pathol 2001;25:1297–303. [DOI] [PubMed] [Google Scholar]
- 10.Saad RS, Luckasevic TM, Noga CM, et al. Diagnostic value of HepPar1, pCEA, CD10, and CD34 expression in separating hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration cytology. Diagn Cytopathol 2004;30:1–6. [DOI] [PubMed] [Google Scholar]
- 11.Chu PG, Ishizawa S, Wu E, et al. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol 2002;26:978–88. [DOI] [PubMed] [Google Scholar]
- 12.Morrison C, Marsh W Jr. Frankel WL. A comparison of CD10 to pCEA, MOC-31, and hepatocyte for the distinction of malignant tumors in the liver. Mod Pathol 2002;15:1279–87. [DOI] [PubMed] [Google Scholar]
- 13.Lau SK, Prakash S, Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175–81. [DOI] [PubMed] [Google Scholar]
- 14.Fu X, Tan L, Liu S, et al. A novel diagnostic marker, p28(GANK), distinguishes hepatocellular carcinoma from potential mimics. J Cancer Res Clin Oncol 2004;130:514–20. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Kim WH, Kang GH. Hepatocyte expressions in hepatocellular carcinomas, gastrointestinal neoplasms, and non-neoplastic gastrointestinal mucosa: its role as a diagnostic marker. J Korean Med Sci 2003;18:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui MT, Saboorian MH, Gokaslan ST, et al. Diagnostic utility of the HepPar1 antibody to differentiate hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration samples. Cancer 2002;96:49–52. [PubMed] [Google Scholar]
- 17.Murray GI, Paterson PJ, Ewen SW, et al. In situ hybridisation of albumin mRNA in normal liver and hepatocellular carcinoma with a digoxigenin labelled oligonucleotide probe. J Clin Pathol 1992;45:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Nalesnik MA, Carr BI. In situ hybridization of albumin mRNA in normal liver and liver tumors: identification of hepatocellular origin. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;64:361–5. [DOI] [PubMed] [Google Scholar]
- 19.Krishna M, Lloyd RV, Batts KP. Detection of albumin messenger RNA in hepatic and extrahepatic neoplasms. A marker of hepatocellular differentiation. Am J Surg Pathol 1997;21:147–52. [DOI] [PubMed] [Google Scholar]
- 20.Papotti M, Pacchioni D, Negro F, et al. Albumin gene expression in liver tumors: diagnostic interest in fine needle aspiration biopsies. Mod Pathol 1994;7:271–5. [PubMed] [Google Scholar]
- 21.Ganjei P, Nadji M, Albores-Saavedra J, et al. Histologic markers in primary and metastatic tumors of the liver. Cancer 1988;62:1994–8. [DOI] [PubMed] [Google Scholar]
- 22.Van Eyken P, Desmet VJ. Cytokeratins and the liver. Liver 1993;13:113–22. [DOI] [PubMed] [Google Scholar]
- 23.Röcken C, Carl-McGrath S, Gräntzdörffer I, et al. Ectopeptidases are differentially expressed in hepatocellular carcinomas. Int J Oncol 2004;24:487–95. [PubMed] [Google Scholar]
- 24.International Working Party. Terminology of nodular hepatocellular lesions. Hepatology 1995;22:983–93. [DOI] [PubMed] [Google Scholar]
- 25.MacSween RNM, Burt AD, Portmann BC, et al. Pathology of the liver. Edinburgh: Churchill Livingstone, 2002.
- 26.Mechtersheimer G, Moller P. Expression of aminopeptidase N (CD13) in mesenchymal tumors. Am J Pathol 1990;137:1215–22. [PMC free article] [PubMed] [Google Scholar]
- 27.Tokuhara T, Adachi M, Hashida H, et al. Neutral endopeptidase/CD10 and aminopeptidase N/CD13 gene expression as a prognostic factor in non-small cell lung cancer. Jpn J Thorac Cardiovasc Surg 2001;49:489–96. [DOI] [PubMed] [Google Scholar]
- 28.Saiki I, Fujii H, Yoneda J, et al. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer 1993;54:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menrad A, Speicher D, Wacker J, et al. Biochemical and functional characterization of aminopeptidase N expressed by human melanoma cells. Cancer Res 1993;53:1450–5. [PubMed] [Google Scholar]
- 30.Kido A, Krueger S, Haeckel C, et al. Possible contribution of aminopeptidase N (APN/CD13) to invasive potential enhanced by interleukin-6 and soluble interleukin-6 receptor in human osteosarcoma cell lines. Clin Exp Metastasis 1999;17:857–63. [DOI] [PubMed] [Google Scholar]
- 31.Hashida H, Takabayashi A, Kanai M, et al. Aminopeptidase N is involved in cell motility and angiogenesis: its clinical significance in human colon cancer. Gastroenterology 2002;122:376–86. [DOI] [PubMed] [Google Scholar]
- 32.Ishii K, Usui S, Sugimura Y, et al. Inhibition of aminopeptidase N (AP-N) and urokinase-type plasminogen activator (uPA) by zinc suppresses the invasion activity in human urological cancer cells. Biol Pharm Bull 2001;24:226–30. [DOI] [PubMed] [Google Scholar]
- 33.Lian WN, Tsai JW, Yu PM, et al. Targeting of aminopeptidase N to bile canaliculi correlates with secretory activities of the developing canalicular domain. Hepatology 1999;30:748–60. [DOI] [PubMed] [Google Scholar]
- 34.Bartles JR, Zhang LQ, Verheyen EM, et al. Decreases in the relative concentrations of specific hepatocyte plasma membrane proteins during liver regeneration: down-regulation or dilution? Dev Biol 1991;143:258–70. [DOI] [PubMed] [Google Scholar]
- 35.Lin F, Abdallah H, Meschter S. Diagnostic utility of CD10 in differentiating hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration biopsy (FNAB) of the liver. Diagn Cytopathol 2004;30:92–7. [DOI] [PubMed] [Google Scholar]
- 36.Xiao SY, Wang HL, Hart J, et al. cDNA arrays and immunohistochemistry identification of CD10/CALLA expression in hepatocellular carcinoma. Am J Pathol 2001;159:1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong AS, Sormunen RT, Tsui WM, et al. Hep Par 1 and selected antibodies in the immunohistological distinction of hepatocellular carcinoma from cholangiocarcinoma, combined tumours and metastatic carcinoma. Histopathology 1998;33:318–24. [DOI] [PubMed] [Google Scholar]
- 38.Fan Z, van de Rijn M, Montgomery K, et al. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol 2003;16:137–44. [DOI] [PubMed] [Google Scholar]