Abstract
A child owning pet rats developed an eruptive fever with blisters, polyarthritis, and spectacular desquamation of the hands. Streptobacillus moniliformis was identified after culture of the child’s blister fluid and was detected in rat samples by molecular methods. Such detection in the pet of a human victim of rat bite fever has not been reported previously.
Keywords: rat bite fever, Streptobacillus moniliformis, pet rat, hand desquamation, molecular diagnosis
A 7 year old boy presented with fever, rash on the extremities, and intense bilateral arthralgia in the knees, ankles, elbows, and wrists. Physical examination showed maculopapular morbilliform exanthema on the palms and soles, associated with several blisters 3–8 mm in diameter, containing a whitish fluid, on the face and elbows. None of the painful joints was mobilisable. The right knee and both ankles were swollen.
Laboratory investigations showed an intense inflammatory syndrome (C reactive protein, 300 mg/litre; erythrocyte sedimentation rate, 60 mm/hour), normal leucocyte count, and normal cerebrospinal fluid examination. Samples of cerebrospinal fluid, blood, and blister fluid were cultured.
Possible diagnoses included rat bite fever (RBF). One month before onset the patient had acquired two pet rats. His parents reported frequent physical contact between the boy and his pets, but they were not aware that he had been bitten.
Pending the laboratory results, empirical intravenous combination treatment was started with erythromycin (30 mg/kg/day) and amoxicillin (100 mg/kg/day).
Serological tests were negative for Mycoplasma pneumoniae, Francisella tularensis, Leptospira spp, and Rickettsia spp. Polymerase chain reaction (PCR) analyses were negative for Rickettsia spp and Leptospira interrogans.
Blood and cerebrospinal fluid cultures remained sterile. However, blister fluid samples yielded smooth, shiny, grey colonies 1–2 mm in diameter on horse blood agar plates incubated in aerobic conditions, and on Columbia blood agar incubated in anaerobic conditions, after 48 hours at 36°C. Microscopic examination showed pleomorphic Gram negative rods. Tests for oxidase, catalase, nitrate reductase, and urease were negative. Because growth was slow, despite serum enrichment, biochemical identification was not possible and antibiotic susceptibility testing was also unsuccessful.
The isolate was finally identified using a broad range PCR technique based on amplification and sequencing of bacterial 16S ribosomal DNA.1 The amplified DNA of the isolate showed 99% similarity with the Genbank Streptobacillus moniliformis sequence Z35305 (type strain ATCC16467).
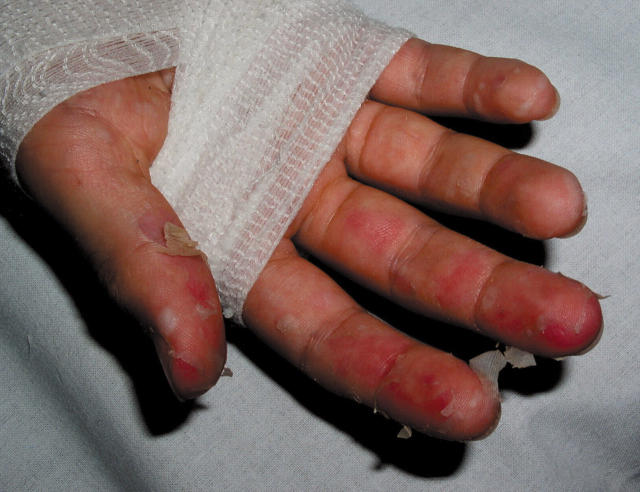
After five days of antibiotic treatment, the patient’s general status improved. At this time he developed spectacular bilateral desquamation of the fingers and toes (fig 1). The echocardiogram was normal, arguing against atypical Kawasaki disease. Erythromycin and amoxicillin were discontinued after seven and 15 days, respectively, without symptom recurrence. The patient was seen 15 days after discharge, and his physical examination was normal. He admitted at this time that he used to eat his pets’ faeces.
Figure 1.
Desquamation of the fingers in a patient with rat bite fever, resembling that seen in the subacute phase of Kawasaki disease.
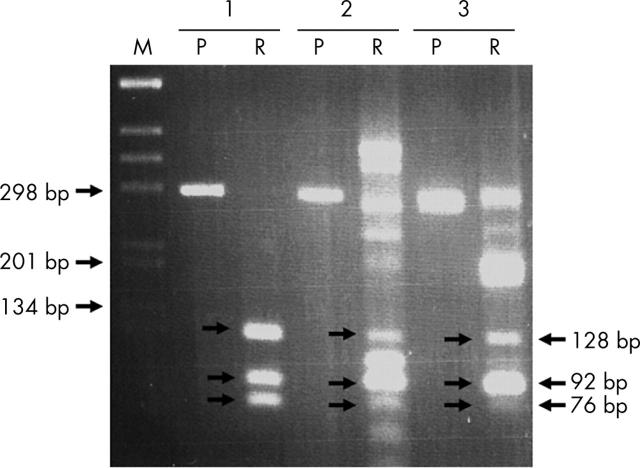
One of the apparently healthy pet rats was euthanised. Necropsy showed no macroscopic lesions. Fragments of oropharyngeal and tracheal mucosa were cultured. Various commensal microorganisms (mainly Pasteurella sp.) were isolated, together with small Gram negative bacilli with similar morphology to those isolated from the child’s blisters. Despite multiple subcultures, pure colonies of the putative Streptobacillus spp could not be obtained. We used molecular tools to detect S moniliformis. DNA was extracted from mixed culture on an agar plate and from the rat’s tracheal biopsy. The broad range PCR technique could not be used because of the polymicrobial DNA extracts; therefore, a specific PCR targeting S moniliformis using primers described by Boot and colleagues2 was performed. The 296 bp DNA fragment was amplified and restricted using BfaI, as described previously.2 This yielded supernumerary fragments but a S moniliformis signature (128, 92, and 76 bp fragments) was detected in the culture and biopsy DNA when compared with the isolated strain (fig 2).
Figure 2.
Agarose gel electrophoresis of undigested polymerase chain reaction fragment (P) or after digestion with BfaI (R). DNA extracts of the pure culture from the patient (lane 1), from the mixed culture of rat pharyngeal samples (lane 2), and from the rat tracheal biopsy (lane 3). M, molecular size marker.
DISCUSSION
RBF is a rare zoonosis caused by S moniliformis, a small fastidious pleomorphic Gram negative bacillus.3,4 It has been reported that S moniliformis is present in the nasopharyngeal flora of 50–100% of healthy wild living and laboratory rats,3–5 but few recent data are available. Asymptomatic carriage appears to be frequent.2 RBF is classically transmitted by rat bites, but human cases caused by scratches or mouth to mouth contact have also been described3,6; the mortality rate in untreated cases is about 10–13%.3–8 Ingestion of rat stools has not previously been linked to RBF, but S moniliformis is also responsible for Haverhill fever, a rare epidemic disease caused by ingestion of food or water contaminated by rat faeces.3–6,8
“Streptobacillus moniliformis is difficult to isolate and to identify with classic bacteriological techniques”
Our patient’s syndrome matched the classic description of RBF.3–7,9,10 In contrast, intense desquamation of the extremities has rarely been reported.9 This presentation in a young child may lead to an erroneous diagnosis of Kawasaki disease and delay effective antibiotic treatment. The desquamation could have been caused by immune or toxic factors.
Streptobacillus moniliformis is difficult to isolate and to identify with classic bacteriological techniques, because this fastidious bacterium requires enriched media and is inhibited by sodium polyanetholsulfonate, an additive present in most commercial blood culture bottles.3–5,8,9 Recently, molecular methods have been used to identify S moniliformis directly in human samples,6,8 in addition to cultures5 and organ biopsies of experimentally infected mice and rats.2 Although S moniliformis could not be isolated here in well individualised colonies by culture from pet specimens, its presence was strongly suggested both by the Gram colouration of cultures and molecular biology data (PCR restriction fragment length polymorphism) on DNA extracts from the cultures and rat biopsy. To our knowledge, the detection of S moniliformis by molecular methods in the pet of a human suffering from RBF has not been reported previously.
The difficulty of isolating S moniliformis, together with the polymorphic clinical manifestations of RBF,10 and the fact that RBF is not a notifiable disease may explain the lack of data on the incidence of this zoonosis.7,8,10 More cases of RBF have been reported in the past decade,5,7,10 but it is difficult to know whether this is because of the increasing number of unusual pets or improved microbiological methods and vigilance in reporting. Molecular studies of S moniliformis carriage among pet rodents would be of interest.
Take home messages.
We report the case of a child who owned pet rats and who developed an eruptive fever with blisters, polyarthritis, and spectacular desquamation of the hands
Streptobacillus moniliformis was identified after culture of the child’s blister fluid and was detected in rat samples by molecular methods
This is the first report of such a detection by molecular methods in the pet of a human victim of rat bite fever
Abbreviations
PCR, polymerase chain reaction
RBF, rat bite fever
REFERENCES
- 1.Gauduchon V, Chalabreysse L, Etienne J, et al. Molecular diagnosis of infective endocarditis: PCR amplification and direct sequencing on valve tissue. J Clin Microbiol 2003;41:763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boot R, Oosterhuis A, Thuis HC. PCR for the detection of Streptobacillus moniliformis. Lab Anim 2002;36:200–8. [DOI] [PubMed] [Google Scholar]
- 3.Denizot MJ, Hansen W, Bollet C. Streptobacillus moniliformis. In: Freney J, Renaud F, Hansen W, et al, eds. Précis de bactériologie clinique. Paris, France: ESKA, 2000:1495–8.
- 4.Hagelskjaer L, Sorensen I, Randers E. Streptobacillus moniliformis infection: 2 cases and a literature review. Scand J Infect Dis 1998;30:309–11. [DOI] [PubMed] [Google Scholar]
- 5.Wallet F, Savage C, Loiez C, et al. Molecular diagnosis of arthritis due to Streptobacillus moniliformis. Diagn Microbiol Infect Dis 2003;47:623–4. [DOI] [PubMed] [Google Scholar]
- 6.Berger C, Altwegg M, Meyer A, et al. Broad range polymerase chain reaction for diagnosis of rat-bite fever caused by Streptobacillus moniliformis. Pediatr Infect Dis J 2001;20:1181–2. [DOI] [PubMed] [Google Scholar]
- 7.Ojukwu IC, Christy C. Rat-bite fever in children: case report and review. Scand J Infect Dis 2002;34:474–7. [DOI] [PubMed] [Google Scholar]
- 8.Stehle P, Dubuis O, So A, et al. Rat bite fever without fever. Ann Rheum Dis 2003;62:894–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tattersall RS, Bourne JT. Systemic vasculitis following an unreported rat bite. Ann Rheum Dis 2003;62:605–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves MH, Janda JM. Rat-bite fever (Streptobacillus moniliformis): a potential emerging disease. Int J Infect Dis 2001;5:151–4. [DOI] [PubMed] [Google Scholar]