Abstract
Aims: Tumour angiogenesis is essential for carcinogenesis and facilitates the process of tumour development and metastasis. Vascular endothelial growth factor (VEGF) is a well characterised angiogenetic factor and is known to play a crucial role in new vessel development. To gain further insight into the effects of microvessel density and VEGF expression in colon cancer, their relation with tumour proliferation, ploidy status, and p53 expression was investigated in colon cancer.
Methods: Tissue samples of 50 archived colon cancers were analysed by immunohistochemistry for VEGF, p53, and the endothelial cell marker, von Willebrand factor (VWF), using specific antibodies. The same samples were re-cut for flow cytometric studies to obtain S phase fraction (SPF) and ploidy status.
Results: A positive significant correlation was found between SPF and angiogenesis. The median microvessel count in high SPF tumours was significantly higher than in low SPF ones. No association was found between VEGF expression and SPF. A positive correlation was found between ploidy status and p53 expression and microvessel count. Furthermore, a positive correlation was established between DNA ploidy, VEGF expression, and microvessel count.
Conclusion: This study provides evidence that in colon cancer, tumour growth may be stimulated by vascular supply, and the lack of a correlation between tumour cell proliferation and VEGF expression indicates that these two parameters may be regulated by separate mechanisms. Furthermore, the positive correlation between microvessel density, VEGF expression, and ploidy status provides more evidence that genetic alterations are involved in tumour angiogenesis.
Keywords: angiogenesis, colon cancer, S phase, ploidy, microvessel count
Angiogenesis, the development and formation of new blood vessels, is important in a variety of physiological and pathological processes, such as growth and differentiation, ovulation, wound healing, and neoplasia.1 Furthermore, increased vascular density has been shown to correlate with a higher incidence of metastasis and a worse prognosis in cancer.2,3
In 1971, Folkman proposed a new view of the role of blood vessels in tumour growth in the form of a hypothesis that tumour growth is angiogenesis dependent.4 This hypothesis suggested that tumour cells and vascular endothelial cells within a neoplasm may constitute a highly integrated ecosystem, and that endothelial cells may be switched from a resting state to a rapid growth phase by a “diffusible” chemical signal produced by the tumour cells. The biological mechanisms underlying the angiogenetic process have not yet been completely clarified, but several positive regulators have been identified. Vascular endothelial growth factor (VEGF) is a well characterised angiogenetic factor and is known to play a crucial role in endothelial cell proliferation and migration and, consequently, new vessel development.
“Increased vascular density has been shown to correlate with a higher incidence of metastasis and a worse prognosis in cancer”
Because tumour growth has been associated with angiogenesis,5 studies on the relation between angiogenesis and cell proliferation parameters in different types of tumour have been performed, generating conflicting results.6,7,8,9,10
The association between increased VEGF expression and in vivo tumour development has been attributed to VEGF involvement in the angiogenic process.11 However, the direct impact of VEGF expression on tumour growth kinetics is not clearly defined.
Recent experimental results have shown that some oncogenes are involved in the regulation of angiogenesis,12 and interesting data have been reported on the genetic inactivation of p53 in cancer cells: loss of wild-type p53 function contributes to activation of the angiogenic switch in tumours.13 In our recent study, in the same colon cancer series, we demonstrated a highly significant correlation between VEGF expression, microvessel count, and p53 tumour expression.14
To gain further insight into the effects of microvessel density and VEGF expression in colon cancer, we investigated their relation with tumour proliferation and ploidy status in a series of 50 colon cancers. In addition, we studied the possible association with p53 protein expression, which is known to regulate genes involved in the angiogenic process and has been linked to proliferation and apoptosis.14,15
MATERIAL AND METHODS
Patients
Surgical specimens of colon carcinomas (n = 57), from patients with primary colon carcinoma, excluding those with multiple or metachronous cancer, diagnosed from January 1998 to August 2001, were consecutively selected from the archives of the service of pathology at Campus Bio-Medico, Rome, Italy.
The patients’ ages ranged from 33 to 90 years (average, 65); 40 were male and 17 were female. No patient had received chemotherapy or radiotherapy before surgery.
The pathological features were classified using the UICC-TNM classification. Tumours were graded according to the World Health Organisation classification criteria as well, moderately, or poorly differentiated.
The specimens were fixed in 10% neutral buffered formaldehyde and embedded in paraffin wax. Based on the quality of the morphological preservation of all available haematoxylin and eosin stained slides of the surgical specimen sections, we selected one paraffin wax block for each case. Consecutive 3 μm sections were re-cut from each block for immunohistochemical studies. Furthermore, 50 μm sections, from the same paraffin wax blocks used for immunohistochemistry, were obtained for flow cytometric studies.
Immunohistochemical staining
Immunohistochemical staining was performed by the streptavidin–biotin method. Sections were dewaxed, and immunostaining was performed using a polyclonal rabbit antibody against the VEGF protein (A-20; Santa Cruz Biotechnology, Santa Cruz, California, USA), a monoclonal mouse antibody against the p53 protein (clone DO7; Dakocytomation A\S, Glostrup, Denmark), and a rabbit antibody against von Willebrand factor (VWF; Dakocytomation A\S).
Slides were examined by two independent pathologists (GP, EC) blinded to each other’s work and with no prior knowledge of the clinical and pathological parameters. For each colon carcinoma, staining was evaluated at the invasive edge of the tumour and at least 1000 cells were observed. The staining score was evaluated as the percentage of stained cells out of the total number of cell evaluated. For the purpose of our study and on the basis of previous experience, staining for p53 and VEGF was defined as positive when > 10% of tumour cells were stained and negative when none or ⩽ 10% of tumour cells were stained. Furthermore, those tumours defined as VEGF positive on the basis of the percentage ratio of stained cells were re-graded according to the intensity of staining using a scale of 1–3, with 1 representing mild, 2 moderate, and 3 strong intensity of staining.16 Arbitrarily, tumour staining graded between 0 and 1 was defined as low intensity and between 2 and 3 as high.
Determination of the microvessel count was performed by hot spot analysis according to the Weidner method. Details of the immunohistochemical analysis and its interpretation are provided in our previous study.14
Flow cytometry
For flow cytometric studies, nuclear suspensions were prepared using a modified version of the method of Hedley et al,17 and were incubated for 30 minutes in propidium iodide solution. The S phase fraction (SPF) was evaluated in each tumour as the percentage of cells in S phase and cases were categorised at low or high SPF according to a 15% cutoff value. This value represents the mean SPF value in our series of colon carcinomas studied.
The DNA index (DI) was calculated as the ratio of the modal value of the DNA histogram of the tumour sample to that of the reference cells. By definition, diploid tumours have a DI of 1.0, and a single G0–G1 peak at 2n. Samples with a second peak distinct from the diploid 2n peak and with a corresponding 4n peak that show a DI > 1.0 were classified as aneuploid; those with a DI of 1.9–2.1 were tetraploid, although they were analysed as aneuploid.18 Cell kinetic measurements were performed on combined populations of diploid and aneuploid cells. Data were acquired with Flomax Software 2.0 and DNA histograms were analysed by multicycle for windows 3.0.
Statistical analysis
Fisher’s test was used to examine the relation of clinicopathological parameters and VEGF expression (positive v negative) with SPF and ploidy status. In addition, the Mann Whitney U test for non-parametric independent variables was used to assess the association between SPF and ploidy status and VEGF expression (classified as high v low), and to determine whether there was a significant difference between microvessel count, ploidy status, p53, and SPF. p Values < 0.05 were regarded as significant. SPSS software (version 10.00; SPSS, Chicago, Illinois, USA) was used for statistical analysis.
RESULTS
SPF, ploidy status, and clinicopathological parameters
An evaluable DNA histogram was obtained from 50 of the 57 samples studied. Seven tumour samples were not evaluated because of a paucity of lesions. The mean SPF was 15% (range, 3.5–37.7%). Twenty eight tumours had an SPF ⩽ 15% and 22 tumours had an SPF > 15% (table 1).
Table 1.
Correlation between flow cytometric and clinicopathological parameters
| Clinicopathological parameters | N | High SPF | p Value | Aneuploid | p Value | ||
| N | % | N | % | ||||
| Age | |||||||
| ⩽50 | 4 | 2 | 50.0% | 0.596 | 2 | 50.0% | 1.000 |
| >50 | 46 | 20 | 43.5% | 28 | 60.9% | ||
| T | |||||||
| 1 | 1 | 1 | 100% | 0.994 | 1 | 100% | 0.791 |
| 2 | 9 | 4 | 44.4% | 6 | 66.7% | ||
| 3 | 36 | 16 | 44.4% | 21 | 58.3% | ||
| 4 | 4 | 1 | 25.0% | 2 | 50.0% | ||
| N | |||||||
| 0 | 20 | 10 | 50.0% | 0.567 | 12 | 60.0% | 1.000 |
| 1, 2 | 30 | 12 | 40.0% | 18 | 60.0% | ||
| M | |||||||
| 0 | 37 | 17 | 45.9% | 0.751 | 23 | 62.2% | 0.744 |
| 1 | 13 | 5 | 38.5% | 7 | 53.8% | ||
| Grade | |||||||
| 1 | 2 | 1 | 50.0% | 0.892 | 2 | 100.0% | 0.496 |
| 2 | 38 | 16 | 42.1% | 22 | 57.9% | ||
| 3 | 10 | 5 | 50.0% | 6 | 60.0% | ||
p Values were assessed by Fisher’s test.
The DI was calculated as the ratio of the modal value of the DNA histogram of the tumour sample to that of the reference cells. By definition, tumours with DI = 1.0 were classified as diploid and tumours with a DI > 1.0 as aneuploid. Twenty tumours were diploid and 30 were aneuploid (table 1).
No association was found between flow cytometric data and the clinicopathological parameters considered (table 1).
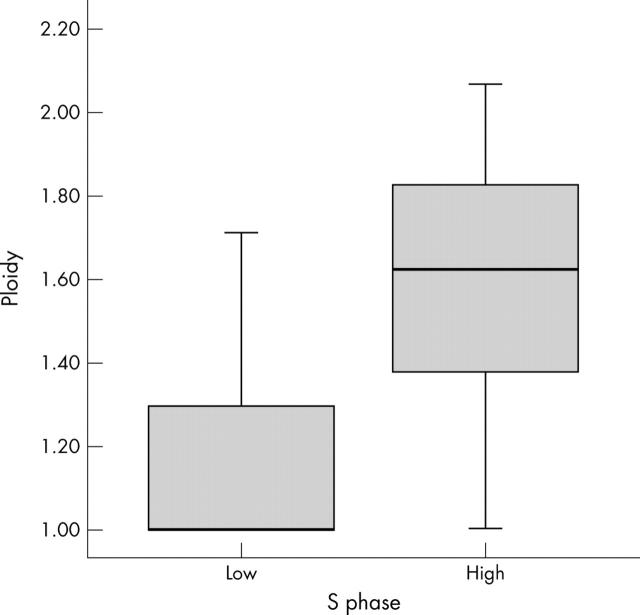
A positive correlation was found between SPF and ploidy status. The DI was significantly higher in high SPF tumours than in low SPF ones (median, 1 (interquartile range (IQR), 1–1.32) v 1.62 (1.38–1.81); Mann Whitney test, p < 0.0001; fig 1).
Figure 1.
Relation between S phase (low, ⩽ 15; high, > 15) and ploidy in colon cancer. Mann-Whitney test, p < 0.0001.
Correlation of SPF and ploidy with angiogenesis and p53 expression
SPF showed a positive correlation with the microvessel count. The microvessel count in high SPF tumours was significantly higher than in low SPF ones (median, 142 (IQR, 122–159) v 112 (87.8–142); Mann Whitney test, p = 0.036; table 2; fig 2).
Table 2.
Correlation between angiogenesis and flow cytometric parameters
| Flow cytometric parameters | N (%) | MC | p Value* | VEGF staining | p Value† | ||
| Median | IQR | Positive/total | Positive (%) | ||||
| SPF | |||||||
| ⩽15 | 28 (56%) | 112 | 87.8–142 | 0.036 | 22/28 | 78.5% | 0.439 |
| >15 | 22 (44%) | 142 | 122–159 | 20/22 | 90.9% | ||
| Ploidy | |||||||
| Diploid | 20 (40%) | 115 | 87.5–124 | 0.003 | 16/20 | 80.0% | 0.697 |
| Aneuploid | 30 (60%) | 142 | 125–161 | 26/30 | 86.6% | ||
*Mann-Whitney test; †Fisher’s test.
MC, microvessel count; SPF, S phase fraction; VEGF, vascular endothelial growth factor.
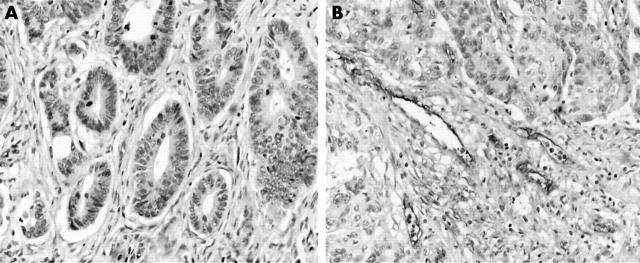
Figure 2.
Representative immunohistochemical results from colon cancer. Immunostaining for von Willebrand factor (VWF) with rabbit anti-VWF antibody (Dakocytomation) of newly formed capillary vessels. (A) Tumour with low microvessel count, (B) tumour with high microvessel count (original magnification, ×200).
There was no significant correlation between SPF and VEGF expression (Fisher’s test, p = 0.439; table 2). Moreover, no correlation was found between SPF and VEGF, stratified according staining intensity (low v high VEGF expression; Mann-Whitney test, p = 0.072; table 3).
Table 3.
Correlation of VEGF score with SPF and ploidy status
| VEGF expression | N | SPF | p Value | Diploid | Aneuploid | p Value | |
| <15% | >15% | ||||||
| Low | 16 | 11 (69%) | 5 (31%) | 10 (62.5%) | 6 (37.5%) | ||
| 0.072 | 0.027 | ||||||
| High | 34 | 14 (41%) | 20 (59%) | 10 (29%) | 24 (71%) | ||
*p Values were assessed by the Mann-Whitney test.
SPF, S phase fraction; VEGF, vascular endothelial growth factor.
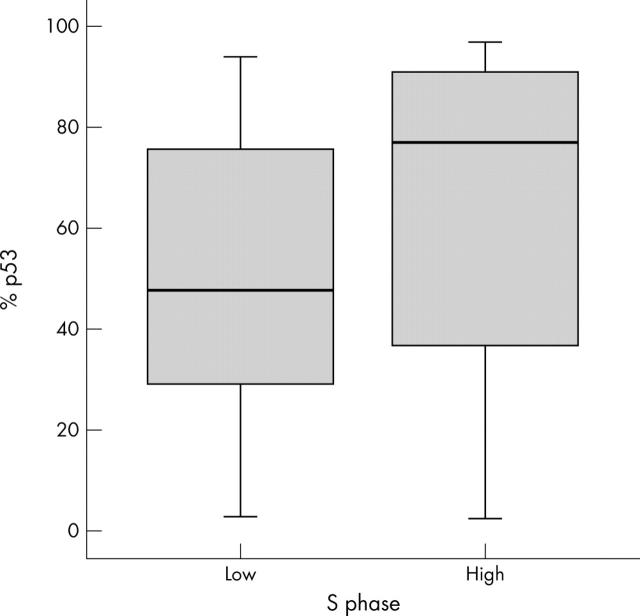
In addition, the percentage of p53 positive cells in high SPF tumours was significantly higher than in low SPF tumours (median, 77.4 (IQR, 39.3–90.5) v 47.8 (29.3–75.9); Mann Whitney test, p = 0.041; fig 3).
Figure 3.
Relation between S phase (low, ⩽ 15; high, > 15) and p53 expression. Mann-Whitney test, p = 0.041.
Ploidy status showed a positive correlation with microvessel count. The microvessel count in aneuploid tumours was significantly higher than in diploid tumours (median, 142 (IQR, 125–161) v 115 (87.5–124); Mann Whitney test, p = 0.003).
There was no significant correlation between ploidy status and VEGF expression (Fisher’s test, p = 0.697; table 2), although there was a positive correlation between ploidy status and VEGF, stratified according to intensity of staining (low v high VEGF expression; Mann-Whitney test, p = 0.027; table 3).
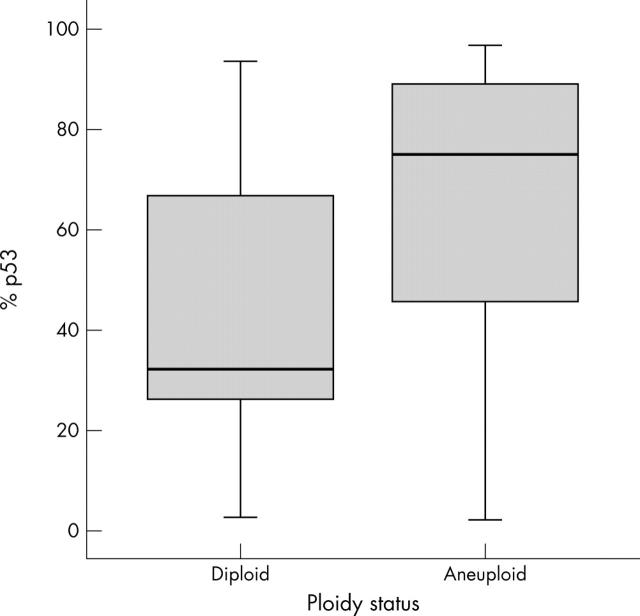
In addition, the percentage staining for p53 in aneuploid tumours was significantly higher than in diploid ones (median, 75.2 (IQR, 45.6–88.7) v 32 (27.6–63.9); Mann Whitney test, p = 0.004; fig 4).
Figure 4.
Relation between ploidy status and p53 expression. Mann-Whitney test, p = 0.004.
DISCUSSION
In an attempt to evaluate the impact of angiogenesis and the angiogenetic factor VEGF on tumour growth, we investigated the relation between microvessel count, VEGF expression, proliferation, and ploidy status in a set of 50 colon cancers. Moreover, we also examined whether p53 expression correlated with tumour proliferation and ploidy status.
Angiogenesis was assessed by immunodetection of the endothelial cell marker VWF, which is more specific than CD31 or CD34 because CD31 showed inferior staining performance compared with VWF19 and CD34 showed immunostaining of lymphatic vessels.20 Furthermore, recently, it has been shown that the VWF positive microvessel count may be considered to be an independent prognostic marker for patients with colorectal cancer and may help to identify patients with an unfavourable prognosis.21 Hot spot analysis was performed using the Weidner method because when counted this way the microvessel count has been shown to correlate with patient outcome.22,23
To assess tumour proliferation status, we evaluated the S phase fraction by flow cytometry. The technical issues of validity and reproducibility related to prognostically useful methods of measuring tumour cell proliferation are a matter of controversy, but according to recent studies flow cytometric SPF is the most useful tumour cell proliferation marker to assess disease prognosis.24,25
Because VEGF exhibits angiogenic activity and increased VEGF has been related to in vivo tumour development,5 we examined the impact of VEGF expression and microvessel count on tumour growth.
In our series, we failed to demonstrate an association between VEGF expression and tumour proliferation, whereas a significant positive correlation was found between SPF and microvessel count. The median microvessel count in high SPF tumours was significantly higher than in low SPF ones (p = 0.036).
Our results are in agreement with a study by Zhang et al, in which transfection of a full length cDNA encoding the shortest isoform of VEGF (VEGF121) into MCF-7 cells showed that, in vitro, the growth rate of MCF-7 wild-type cells was indistinguishable from that of VEGF121 transfected MCF-7, although subcutaneous implantation of transfected cells formed faster growing tumours in vivo because of intense neovascularisation.11 Moreover, Takahashi et al showed that VEGF receptors were not present on tumour epithelial cells, so that VEGF probably has no direct mitogenic effect on colon cancer cells.26
“The vascular endothelial growth factor protein may be an indirect mediator of tumour growth in colon cancer by microvessel proliferation induction”
These findings support the idea that tumour cell proliferation occurs in part because the growth of new tumour vessels allows for exchange of nutrients, oxygen, and waste products in a crowded cell population for which simple diffusion is inadequate. Moreover, the almost significant correlation (p = 0.072) between tumour cell proliferation and VEGF expression indicates that these two parameters are not directly correlated and any increase in S phase associated with VEGF expression is probably the result of its angiogenic activity.
In a recent study, we found an association between angiogenesis and p53 overexpression in colon cancer.14 In the same series, a significant association was found between p53 overexpression and aneuploidy (p = 0.004). This result is consistent with other reports,27,28 suggesting that wild-type p53 function may be required to maintain diploidy.29 Furthermore, wild-type p53 can prevent replication of damaged DNA and DNA re-replication that can lead to aneuploidy.30
Take home messages.
Microvessel density was closely correlated with proliferation, suggesting that tumour growth may be stimulated by vascular supply in colon cancer
The lack of a correlation between tumour cell proliferation and vascular endothelial growth factor (VEGF) expression indicates that they may be regulated by separate mechanisms
The positive correlation between microvessel density, VEGF expression, and ploidy status provides more evidence that genetic alterations are involved in tumour angiogenesis
Taking into account the involvement of angiogenesis in tumour genetic alteration, we examined the relation between angiogenesis and DNA ploidy, because the tumour aneuploidy status reflects molecular genetic abnormalities.31,32 In our present study, a positive correlation was found between ploidy status and microvessel count (p = 0.003). Moreover, a positive correlation was found between DNA ploidy and VEGF expression. In fact, 24 of 34 colon cancers with high VEGF expression were aneuploid in flow cytometric studies, whereas only six of 16 colon cancers with low VEGF expression were aneuploid (p = 0.027). Mutated p53, which is associated with increased aneuploidy and increased VEGF, may form a common link to these findings.14,27,28
In conclusion, our present study provides evidence that microvessel density is closely correlated with S phase fraction in colon cancer, thereby showing that tumour growth may be stimulated by vascular supply. Therefore, the VEGF protein may be an indirect mediator of tumour growth in colon cancer by microvessel proliferation induction. Furthermore, the association between aneuploidy, high microvessel count, and VEGF expression is more evidence to suggest a direct relation between DNA alteration and the angiogenesis process.
Further prospective studies will be needed to confirm the clinical value of measuring the combination of angiogenesis with S phase fraction and ploidy status in colon cancer. Perhaps in the future, the combination of clinical and tumour related parameters in colon cancer will lead to a grading system with better prognostic and therapeutic value.
Acknowledgments
We thank F D’Ingiullo, A Innocenzi, and G Lescarini, for technical support and Dr M Crapulli for useful collaboration.
Abbreviations
DI, DNA index
IQR, interquartile range
SPF, S phase fraction
VEGF, vascular endothelial growth factor
VWF, von Willebrand factor
Presented in part to the 46th Annual Meeting of the Italian Cancer Society, Pisa, October 24–27, 2004.
REFERENCES
- 1.Folkman J, Klagsbrun M. Angiogenic factors. Science 1987;235:442–7. [DOI] [PubMed] [Google Scholar]
- 2.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 3.Macchiarini P, Fontanini G, Hardin MJ, et al. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 1992;340:145–6. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182–6. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y, Arii S, Mori A, et al. Enhancement of angiogenesis, tumor growth, and metastasis by transfection of vascular endothelial growth factor into LoVo human colon cancer cell line. Clin Cancer Res 2000;6:622–30. [PubMed] [Google Scholar]
- 6.Mattern J, Koomagi R, Volm M. Biological characterization of subgroups of squamous cell lung carcinomas. Clin Cancer Res 1999;5:1459–63. [PubMed] [Google Scholar]
- 7.Matsushima H, Goto T, Hosaka Y, et al. Correlation between proliferation, apoptosis, and angiogenesis in prostate carcinoma and their relation to androgen ablation. Cancer 1999;85:1822–7. [DOI] [PubMed] [Google Scholar]
- 8.Charpin C, Devictor B, Bergeret D, et al. CD31 quantitative immunocytochemical assays in breast carcinomas. Correlation with current prognostic factors. Am J Clin Pathol 1995;103:443–8. [DOI] [PubMed] [Google Scholar]
- 9.Giatromanolaki A, Koukourakis MI, Kakolyris S, et al. Vascular endothelial growth factor, wild-type p53, and angiogenesis in early operable non-small cell lung cancer. Clin Cancer Res 1998;4:3017–24. [PubMed] [Google Scholar]
- 10.Turner HE, Nagy Z, Gatter KC, et al. Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumor behaviour. Br J Cancer 2000;82:1441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang HT, Craft P, Scott PA, et al. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst 1995;87:213–19. [DOI] [PubMed] [Google Scholar]
- 12.Dameron KM, Volpert OV, Tainsky MA, et al. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 1994;265:1582–4. [DOI] [PubMed] [Google Scholar]
- 13.Ravi R, Mookerjee B, Bhujwalla ZM, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 14.Perrone G, Vincenzi B, Santini D, et al. Correlation of p53 and bcl-2 expression with vascular endothelial growth factor (VEGF), microvessel density (MVD) and clinico-pathological features in colon cancer. Cancer Lett 2004;208:227–34. [DOI] [PubMed] [Google Scholar]
- 15.Gorgoulis VG, Zacharatos P, Kotsinas A, et al. Altered expression of the cell cycle regulatory molecules pRb, p53 and MDM2 exert a synergetic effect on tumor growth and chromosomal instability in non-small cell lung carcinomas (NSCLCs). Mol Med 2000;6:208–37. [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi Y, Tucker SL, Kitadai Y, et al. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg 1997;132:541–6. [DOI] [PubMed] [Google Scholar]
- 17.Hedley DW, Friedlander ML, Taylor IW, et al. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem 1983;31:1333–5. [DOI] [PubMed] [Google Scholar]
- 18.Hiddemann W, Schumann J, Andreef M, et al. Convention on nomenclature for DNA cytometry. Committee on nomenclature, Society for Analytical Cytology. Cancer Genet Cytogenet 1984;13:181–3. [DOI] [PubMed] [Google Scholar]
- 19.Siitonen SM, Haapasalo HK, Rantala IS, et al. Comparison of different immunohistochemical methods in the assessment of angiogenesis: lack of prognostic value in a group of 77 selected node-negative breast carcinomas. Mod Pathol 1995;8:745–52. [PubMed] [Google Scholar]
- 20.Vermeulen PB, Gasparini G, Fox SB, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer 1996;32A:2474–84. [DOI] [PubMed] [Google Scholar]
- 21.Lackner C, Jukic Z, Tsybrovskyy O, et al. Prognostic relevance of tumour-associated macrophages and von Willebrand factor-positive microvessels in colorectal cancer. Virchows Arch 2004;445:160–7. [DOI] [PubMed] [Google Scholar]
- 22.Bosari S, Lee AK, DeLellis RA, et al. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 1992;23:755–61. [DOI] [PubMed] [Google Scholar]
- 23.de Jong JS, van Diest PJ, Baak JP. Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology 2000;36:306–12. [DOI] [PubMed] [Google Scholar]
- 24.Pinto AE, Andre S, Pereira T, et al. Prognostic comparative study of S-phase fraction and Ki-67 index in breast carcinoma. J Clin Pathol 2001;54:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparini G, Boracchi P, Verderio P, et al. Cell kinetics in human breast cancer: comparison between the prognostic value of the cytofluorimetric S-phase fraction and that of the antibodies to Ki-67 and PCNA antigens detected by immunocytochemistry. Int J Cancer 1994;57:822–9. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Kitadai Y, Bucana CD, et al. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995;55:3964–8. [PubMed] [Google Scholar]
- 27.Nasif WA, El-Emshaty HM, Tabll A, et al. Immunoreactivity evaluation of mutant p53 gene product with DNA ploidy pattern in colorectal carcinoma. Hepatogastroenterology 2004;51:1001–6. [PubMed] [Google Scholar]
- 28.Offerhaus GJ, De Feyter EP, Cornelisse CJ, et al. The relationship of DNA aneuploidy to molecular genetic alterations in colorectal carcinoma. Gastroenterology 1992;102:1612–19. [DOI] [PubMed] [Google Scholar]
- 29.Cross SM, Sanchez CA, Morgan CA, et al. A p53-dependent mouse spindle checkpoint. Science 1995;267:1353–6. [DOI] [PubMed] [Google Scholar]
- 30.Fukasawa K, Choi T, Kuriyama R, et al. Abnormal centrosome amplification in the absence of p53. Science 1996;271:1744–7. [DOI] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Hart J, Michelassi F, et al. Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma. Clin Cancer Res 1995;1:1103–10. [PubMed] [Google Scholar]
- 32.Dey P. Aneuploidy and malignancy: an unsolved equation. J Clin Pathol 2004;57:1245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]