Abstract
Background: Helicobacter pylori associated gastric cancer arises via a multistage process, with atrophic gastritis being the precursor lesion. Helicobacter pylori is typically acquired in childhood, yet little is known of the prevalence of atrophic gastritis in childhood.
Aim: To study atrophic gastritis among children from countries with high gastric cancer incidence.
Methods: Sections from topographically mapped gastric biopsy specimens from children undergoing clinically indicated endoscopy in Korea and Colombia were evaluated using visual analogue scales. Atrophy was defined as loss of normal glandular components, including replacement with fibrosis, intestinal metaplasia (IM), and/or pseudopyloric metaplasia of the corpus (identified by the presence of pepsinogen I in mucosa that was topographically corpus but phenotypically antrum).
Results: One hundred and seventy three children, 58 from Korea (median age, 14 years) and 115 from Colombia (median age, 13 years), were studied. Helicobacter pylori was present in 85% of Colombian children versus 17% of Korean children (p<0.01). Atrophic mucosa near the antrum–corpus border was present in 16% of children, primarily as pseudopyloric metaplasia (31%, IM; 63%, pseudopyloric metaplasia; 6%, both). The median age of children with corpus atrophy was 15 (range, 7–17) years.
Conclusion: Gastric atrophy occurs in H pylori infected children living in countries with high gastric cancer incidence. Identification and characterisation of the natural history of H pylori gastritis requires targeted biopsies to include the lesser and greater curve of the corpus, starting just proximal to the anatomical antrum–corpus junction, in addition to biopsies targeting the antrum and cardia.
Keywords: children, gastritis, atrophy
Infection with Helicobacter pylori is the most common identifiable cause of gastritis in children and adults.1–3 The pathogenesis of H pylori associated gastric cancer is thought to proceed through a series of steps of increasingly damaged gastric mucosa.4–8 Although H pylori infection is typically acquired in childhood, there are few data regarding the prevalence of atrophy or intestinal metaplasia (precancerous changes) in the stomachs of children. Helicobacter pylori gastritis has been described in the antrum, and less frequently in the corpus, of children. In contrast, mucosal atrophy in children is either rare or under-recognised, and when it has been identified it has not been well characterised.1,9–16 Our current study examined the prevalence of H pylori gastritis and atrophic gastric mucosa among children from two countries where gastric cancer is common—Korea, a recently developed country, and Bogota, Colombia, a developing country. Bogota is an area with a medium to high risk for gastric cancer.
“Infection with Helicobacter pylori is the most common identifiable cause of gastritis in children and adults”
MATERIALS AND METHODS
Patients
Our study population consisted of children already scheduled for endoscopy for the evaluation of chronic abdominal pain at two centres: Guru Hospital Endoscopic in Seoul, Korea, and Hospital San Juan de Dios Universidad Nacional in Bogotá, Colombia. Both centres were experienced in evaluating patients for H pylori and used a standard protocol for obtaining gastric mucosal biopsies. The protocols were approved by the local ethical committees of the Hospital San Juan de Dios and Guro Hospital, Korea University College of Medicine, Seoul, Korea.
Endoscopy
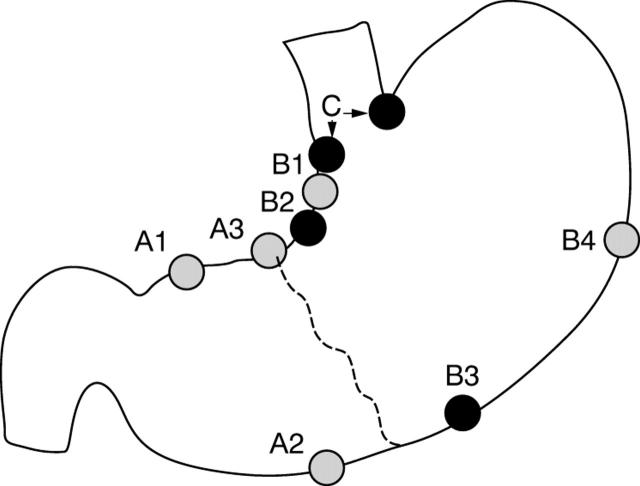
Two endoscopists performed all procedures in Bogotá, Colombia (OG and HC), and one endoscopist in Seoul Korea (JK). Mucosal biopsy specimens were obtained from predetermined locations. A special effort was made to examine five predetermined and targeted sites in all patients (including two antral sites (A2, A3), three corpus sites (B2, B3, and B4), and the cardia site (C); fig 1). The endoscopists were experienced in gastric mapping using the same protocol for adults and had previously received training in biopsy site placement by the use of an instructional video. In practice, the angularis biopsy was taken at the junction of the antrum and corpus on the lesser curve. The antrum–corpus junction on the greater curvature was taken as the beginning of the first longitudinal fold. The cardia was defined as the mucosa immediately distal to the normally located Z line. A paediatric endoscope was used in all cases (Pentax FG23H; approximately 7 mm diameter); local topical xylocaine pharyngeal anaesthesia was used in children older than 7 years and intravenous sedation with midazolam for younger children.
Figure 1.
Mucosal biopsy specimens were obtained from five to six (median, five) predetermined locations. A special effort was made to examine five predetermined sites in all patients, namely: A2, A3, B2, B3, and B4. Grey circles correspond to those recommended by the updated Sydney system. Corpus atrophy, when present, was identified just proximal to the normal antrum–corpus junction (dashed line).
Pathology
Each specimen was placed in a separate bottle of formalin, labelled as to site of origin, and later processed in Houston, Texas, USA. All specimens were embedded in paraffin wax, cut at 4 μm, and serial sections were stained with the El-Zimaity triple strain.17 Two pathologists (OR and HE-Z) reviewed all specimens independently. Each specimen was scored using a visual analogue scale from 0 (absent/normal) to 5 (maximal intensity) for H pylori, active inflammation, and intestinal metaplasia.16 In addition, the type of epithelium (antral, oxyntic, or transitional) was recorded. Atrophy was defined as the loss of normal glands with and without replacement by fibrosis, intestinal metaplasia, and/or pseudopyloric metaplasia (also called pyloric metaplasia or mucous metaplasia). Pseudopyloric metaplasia was identified by the presence of mucosa that was phenotypically antrum, but was from an anatomical region where corpus would be expected, and glands stained positive for the marker for corpus epithelium, pepsinogen I (PGI) (fig 2).18 Areas with lymphoid follicles were excluded from the analysis (for example, if a lymphoid follicle located in the lamina propria above muscularis mucosae occupied half of the biopsy, only the other half of the biopsy was considered when evaluating atrophy).18–20 After the results were analysed, all slides with disagreements about the presence or absence of H pylori or atrophy were re-examined jointly and a consensus was reached on the score.
Figure 2.
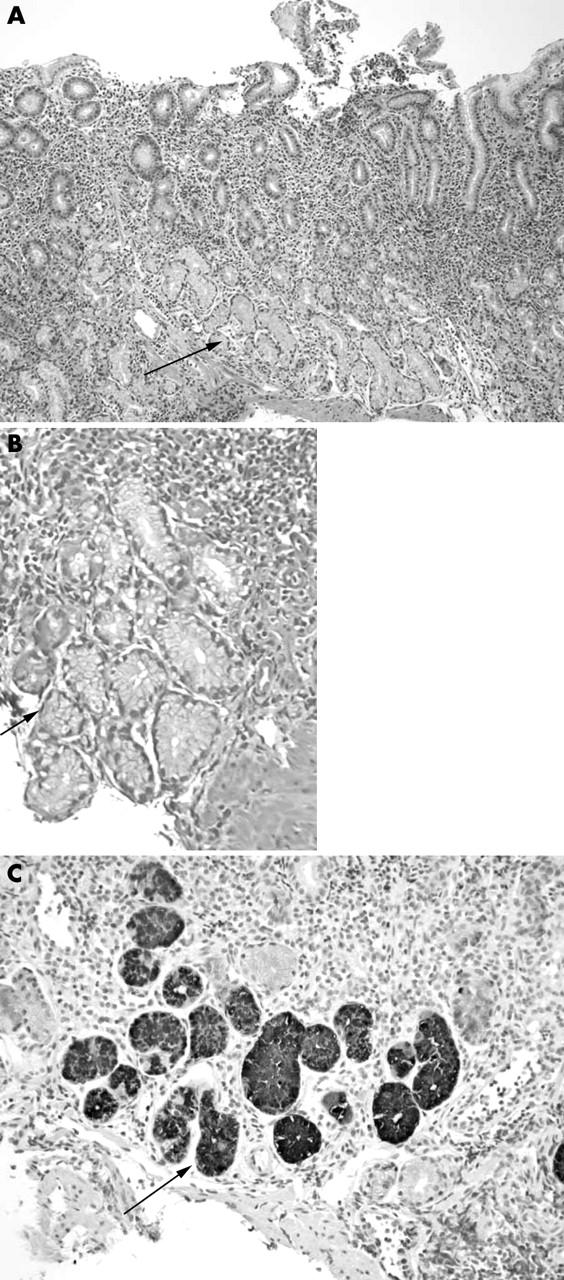
Pseudopyloric metaplasia (arrow) in a 9 year old child as identified by the presence of mucosa that was phenotypically antrum, stained positive for pepsinogen I (PGI), and was anatomically in a region where corpus would be expected. Replacement of oxyntic mucosa with mucous type glands starts as early as 9 years in Colombia. (A) El-Zimaity triple stain; original magnification, ×10. (B) El-Zimaity triple stain; original magnification, ×40. (C) Anti-PGI immunohistochemistry; original magnification, ×10.
Separation of antrum from corpus based on the presence of PGI
PGI is localised to the gastric corpus, primarily within chief cells, and has been used to identify pseudopyloric metaplasia, (that is, to separate what was anatomically corpus but histologically antrum from true antrum).18 To control for PGI immunostaining, biopsies from the anatomical antrum were used as negative controls. It has previously been shown that biopsies from the antrum and transitional mucosa at the normal antrum–corpus junction were negative for PGI.18 For immunophenotyping, 4 μm thick sections were stained using a modified streptavidin–biotin complex method. Slides were pretreated by steam for 20 minutes in a Black and Decker steamer in 10 mmol/litre citrate buffer (pH 6.0), followed by cooling for 20 minutes. The following reagents were used in sequential steps at 36°C: endogenous peroxidase inhibitor, protein blocker, PGI primary antibody for one hour (anti-PGI; Biogenesis, Kingston, New Hampshire, USA), biotinylated secondary antibody, avidin–biotin complex with horseradish peroxidase, and 3,3’-diaminobenzidine tetrahydrochloride. Slides were counterstained with haematoxylin.
Analyses
Scores were entered into a database and analysed using Stata/SE8 (College Station, Texas, USA). Fisher’s exact or, when appropriate, the χ2 test (both two tailed) were used for comparison of proportions. The Mann-Whitney non-parametric test was used to compare numerical data from the ordered categories of the scoring system. Significant differences and associations were determined by p values of less than 0.05.
RESULTS
We examined gastric mucosal biopsies from 173 children (58 from Korea and 115 from Colombia; table 1). Our comparative study comprised a total of 944 biopsy specimens (236 slides from Korea and 708 slides from Colombia).
Table 1.
Clinical features of the patients
| Korea | Colombia | |
| Number | 58 | 115 |
| Age (years) | 8–18 (mean/median, 14) | 4–18 (mean, 12/median, 13) |
| Sex | 31 F, 27 M | 58 F, 57 M |
Forty four biopsy specimens that were judged to be too small for adequate evaluation were not included in this comparison. They were deemed non-evaluable biopsies and formed 3% of the biopsies from Korea (eight of 244) and 5% of those from Colombia (36 of 744). Twenty three were from the antrum, 10 from the corpus, and 11 from the cardia.
In Korean children, the mean/median number of evaluable biopsies was four, with a range of three to six; biopsy sites A3, A2, B3, and B4 were typically available for analysis. In Colombian children, the mean number of biopsies was six (median, seven; range, two to seven) for each child. Biopsy sites C, B2, B3, B4, A1, A3, and A4 were usually available for analysis.
Histological features
Helicobacter pylori infection was present in 107 children (61.8%) including 10 (17%) Korean children and 97 (84%) Colombian children (p < 0.01). Mucosal atrophy was present in 16 children from Colombia (14% overall; 95% confidence interval, 8% to 22%; 16% of those with active H pylori infections). Atrophy was not present in the 58 Korean children (0%; 95% confidence interval, 0% to 6%; p = 0.002). All Colombian children with atrophy were actively infected with H pylori. The presence of atrophy was evenly distributed with regard to the Colombian endoscopist performing the procedure. Intestinal metaplasia was identified in the antrum in four children (table 2). Corpus atrophy was manifest by intestinal metaplasia in six (four in the antrum and two in the cardia) and pseudopyloric metaplasia in 11 (one child had both intestinal metaplasia and pseudopyloric metaplasia). Pseudopyloric metaplasia was present in distal biopsies obtained from the lower corpus on the lesser and greater curvature (biopsy sites labelled B2 or B3) (fig 1). Atrophy was not identified in biopsies taken more proximal on the greater curvature mid-corpus (biopsies labelled B4). The median age of children with atrophic gastritis was 15 years (range, 7–17); the youngest child with gastric atrophy was 9 years old (table 2). Colombian children with gastric atrophy had a higher median score for acute and chronic inflammatory cells (p < 0.003) than Korean children and Colombian children with no atrophy. Helicobacter pylori infection, acute inflammation in the antrum and corpus, and chronic inflammation in the antrum were highly correlated with the presence of atrophy (p = 0.01). Using logistic regression and partial correlations, and controlling for all the other variables, chronic inflammation in the corpus was a predictor for atrophy (p = 0.02).
Table 2.
Details of atrophy in Colombian children
| Case | Age (years) | Sex | IM* | PPM* | Location† |
| 1 | 9 | F | 1 | 0 | A |
| 2 | 9 | F | 2 | 1 | A, B3 |
| 3 | 12 | F | 0 | 1 | B3 |
| 4 | 16 | F | 1 | 0 | A |
| 5 | 15 | M | 0 | 1 | B2 |
| 6 | 17 | M | 1 | 0 | C |
| 7 | 15 | M | 0 | 1 | C |
| 8 | 15 | M | 3 | 0 | C |
| 9 | 15 | M | 0 | 3, 2 | B3, B2 |
| 10 | 15 | F | 0 | 2 | B2 |
| 11 | 15 | F | 0 | 1 | B2 |
| 12 | 12 | F | 0 | 1 | B2 |
| 13 | 12 | M | 1 | 0 | A |
| 14 | 10 | F | 0 | 1 | B2 |
| 15 | 10 | M | 0 | 3 | C, B3 |
| 16 | 14 | M | 0 | 1 | B2 |
*Scored on a visual analogue scale of 0–5; †when atrophy is present in more than one biopsy, the first figure relates to IM and the second to PPM. For example, patient 2 has IM (score 2) in the antrum and PPM (score 1) at location B3.
IM, intestinal metaplasia; PPM, pseudopyloric metaplasia.
DISCUSSION
Most reported studies of the histological features of H pylori infection in children have used either random biopsies12 or a small number of targeted biopsies,2,9,11,21 taken primarily from the antrum.5,22–27 In these studies, the identification of atrophy focused on the presence of intestinal metaplasia or was ill defined.9 Our study used targeted biopsies of both the antrum and corpus and included methods that could histologically separate phenotypical antrum into true antrum or pseudopyloric metaplasia of corpus mucosa.18
The most common presentation of corpus atrophy was as pseudopyloric metaplasia. Intestinal metaplasia was found in the corpus of six children and, if intestinal metaplasia had been used as the sole criterion for the presence of atrophy, the frequency of atrophy would have been greatly underestimated. These data are consistent with recent studies showing that atrophy in H pylori gastritis presents as either intestinal metaplasia or pseudopyloric metaplasia.18 The pattern of atrophy in the form of pseudopyloric metaplasia has been seen previously in gastric remnants after distal gastrectomy with gastroenteric anastomosis,28 and has been shown to increase with age.29 For example, Oi and Oshida reported that benign gastric ulcers occurred in mucosa with pyloric-type glands found proximal to the normal border zone (antrum–corpus junction).30
Our study emphasises the importance of biopsy site location for identifying the presence of corpus atrophy. In children, atrophy was only identified in biopsies taken near the normal antrum–corpus junction (B2 and B3), which is consistent with the notion that atrophy progresses as an advancing antrum–corpus border (border zone), with proximal expansion, replacing fundic gland mucosa with atrophic gastritis as pseudopyloric metaplasia.18,25,31 The atrophic border extends more rapidly along the lesser curve than the greater curvature, so that locations high on the greater curvature are among the last to show atrophy.25,30,31 Thus, in children, the location of the antral–corpus border would be expected to be nearer to the normal anatomical border,29 so that identification of atrophy requires biopsies be taken close to the normal antrum–corpus junction.
“If intestinal metaplasia had been used as the sole criterion for the presence of atrophy, the frequency of atrophy would have been greatly underestimated”
We also identified intestinal metaplasia and pseudopyloric metaplasia in the cardia. Interestingly, the cardia has been shown previously to be a high yield zone for H pylori32; this has now been confirmed in other paediatric populations.33 Taken together, the cardia (in addition to biopsies taken just proximal to the antrum–corpus junction) might be a good target site for biopsy in children. We did not identify gastric mucosal atrophy among the Korean children studied. One possible reason is the lower number of biopsies examined and failure to target the cardia specifically in Korean children. The number of non-evaluable biopsies is also higher in the paediatric population, which further reduced the chance of identifying all patients with gastric atrophy.34
The presence of a higher density of mucosal mononuclear cells was a predictor for the presence of gastric atrophy in Colombian children with atrophy, suggesting that a high density of mononuclear cells that infiltrate deep into the lamina propria may be a precursor to atrophy. This conclusion is also consistent with studies showing that acid suppression leads to a rapid progression of the inflammatory reaction deeper within the pit, and involving the proliferative zone after treatment.35
The Sydney system and updated Sydney system36 were primarily designed to provide standardisation in the reporting of gastric biopsies. The system suggested five standardised biopsy locations, but was not designed for research purposes, or to identify the changes in relation to the mucosal dynamics that occur in the pathogenesis of gastric cancer. However, the Sydney system has proved to be useful for its original purposes—for example, the grading of atrophy using a visual analogue scale—and the biopsy sites provide reliable identification of H pylori infection.10,37,38 Unfortunately, the sites recommended by the Sydney system can identify corpus atrophy only when it is extensive.11,38,39 In addition, failure to separate antral and corpus biopsies makes identification of suspected pseudopyloric metaplasia difficult or impossible.
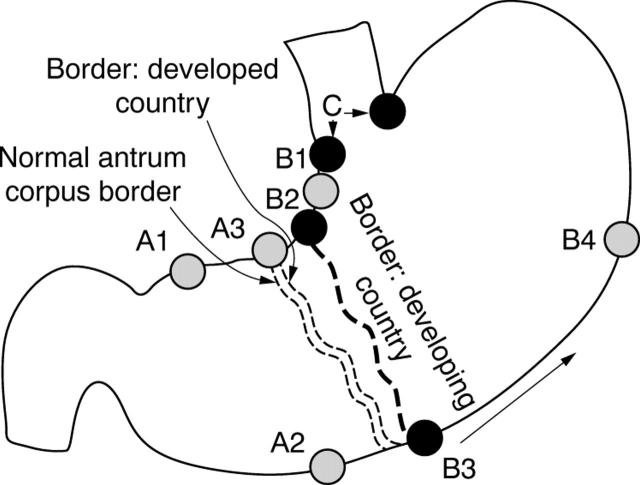
We used both anatomical site and PGI staining to distinguish phenotypic antrum (misdirected or mislabelled biopsy) from atrophic corpus mucosa. Although it is theoretically possible to stain all phenotypical antral biopsies for PGI and thus identify which ones were actually from the corpus, this would be an extremely inefficient approach, and would still require that the biopsy sites encompass likely locations for the presence of atrophy. The recommended biopsy sites for research studies designed to identify the presence, pattern, or changes in atrophic gastritis over time or after a therapeutic intervention must be carefully selected to ensure that they encompass the advancing atrophic border. Therefore, the number and sites chosen will depend on the average degree and severity of atrophic gastritis expected in the population. For example, in a developed country with a low incidence of gastric carcinoma, the atrophic front (atrophic border) in infected adults will probably be close to the normal antrum–corpus junction (normal border zone), similar to that seen in Colombian children. In contrast, the atrophic front (atrophic border) is expected to be more proximal in developing countries, in countries with a high incidence of gastric carcinoma (fig 3), and within particular groups in developed countries that have a higher incidence of gastric carcinoma, including the socially and economically disadvantaged,40,41 with the atrophic border advancing further proximally with age.29
Figure 3.
The atrophic border location differs between different countries and between different groups within a country (it is more proximal in countries/ethnic groups with a higher risk of gastric carcinoma).
These data suggest that research studies in gastric cancer should use standardised biopsy site locations with respect to the question being asked (presence of H pylori versus presence of atrophy). Use of a standardised reporting system such as the updated Sydney system is useful for biopsy specimens, particularly because it promotes the use of a visual analogue scale to score mucosal findings. For research purposes, we suggest the use of a six point scale,17 because it provides finer gradation than the four point scale used for reporting clinical specimens.36 Thus, use of the Sydney system recommended biopsy sites, failure to target biopsy sites to encompass the likely sites of advancing atrophic border, and relying on intestinal metaplasia instead of intestinal metaplasia and pseudopyloric metaplasia will probably result in incomplete and possibly misleading results in studies designed to characterise the natural history of H pylori gastritis in populations, over time, or after a therapeutic intervention in relation to gastric cancer.42
Take home messages.
Gastric atrophy occurs in Helicobacter pylori infected children living in countries with a high incidence of gastric cancer
Identification and characterisation of the natural history of H pylori gastritis requires targeted biopsies to include the lesser and greater curve of the corpus, starting just proximal to the anatomical junction of the antrum and corpus, in addition to biopsies targeting the antrum and the cardia
Gastric atrophy can be identified among H pylori infected children living in countries with a high incidence of gastric cancer. Identification and characterisation of the natural history of H pylori gastritis requires multiple targeted biopsies to include the corpus proximal to the anatomical junction of the antrum and corpus, in addition to the antrum and cardia.
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. Dr JG Kim is deceased.
Abbreviations
PGI, pepsinogen I
REFERENCES
- 1.Dohil R, Hassall E, Jevon G, et al. Gastritis and gastropathy of childhood. J Pediatr Gastroenterol Nutr 1999;29:378–94. [DOI] [PubMed] [Google Scholar]
- 2.Hassall E, Dimmick JE. Unique features of Helicobacter pylori disease in children. Dig Dis Sci 1991;36:417–23. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K. Chronological transition of the fundic–pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology 1972;63:584–92. [PubMed] [Google Scholar]
- 4.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 5.Correa P, Cuello C, Duque E. Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst 1970;44:297–306. [PubMed] [Google Scholar]
- 6.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 1995;19 (suppl 1) :S37–43. [PubMed] [Google Scholar]
- 7.Vineis P, Thomas T, Hayes RB, et al. Proportion of lung cancers in males, due to occupation, in different areas of the USA. Int J Cancer 1988;42:851–6. [DOI] [PubMed] [Google Scholar]
- 8.Fontham ET, Pickle LW, Haenszel W, et al. Dietary vitamins A and C and lung cancer risk in Louisiana. Cancer 1988;62:2267–73. [DOI] [PubMed] [Google Scholar]
- 9.Guarner J, Bartlett J, Whistler T, et al. Can pre-neoplastic lesions be detected in gastric biopsies of children with Helicobacter pylori infection? J Pediatr Gastroenterol Nutr 2003;37:309–14. [DOI] [PubMed] [Google Scholar]
- 10.Torrente F, Anthony A, Heuschkel RB, et al. Focal-enhanced gastritis in regressive autism with features distinct from Crohn’s and Helicobacter pylori gastritis. Am J Gastroenterol 2004;99:598–605. [DOI] [PubMed] [Google Scholar]
- 11.Camorlinga-Ponce M, Aviles-Jimenez F, Cabrera L, et al. Intensity of inflammation, density of colonization and interleukin-8 response in the gastric mucosa of children infected with Helicobacter pylori. Helicobacter 2003;8:554–60. [DOI] [PubMed] [Google Scholar]
- 12.Kim KM, Oh YL, Ko JS, et al. Histopathology and expression of Ki-67 and cyclooxygenase-2 in childhood Helicobacter pylori gastritis. J Gastroenterol 2004;39:231–7. [DOI] [PubMed] [Google Scholar]
- 13.Schiffman MH, Pickle LW, Fontham E, et al. Case–control study of diet and mesothelioma in Louisiana. Cancer Res 1988;48:2911–15. [PubMed] [Google Scholar]
- 14.Oi M, Oshida K, Sugimura S. The location of gastric ulcer. Gastroenterology 1968;54 (suppl) :740–1. [PubMed] [Google Scholar]
- 15.Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol 1988;83:504–9. [PubMed] [Google Scholar]
- 16.Falk RT, Pickle LW, Fontham ET, et al. Life-style risk factors for pancreatic cancer in Louisiana: a case–control study. Am J Epidemiol 1988;128:324–36. [DOI] [PubMed] [Google Scholar]
- 17.El-Zimaity HM, Ota H, Scott S, et al. A new triple stain for Helicobacter pylori suitable for the autostainer: carbol fuchsin/Alcian blue/hematoxylin-eosin. Arch Pathol Lab Med 1998;122:732–6. [PubMed] [Google Scholar]
- 18.El-Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 2002;94:1428–36. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Correa P, Dixon MF, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther 2002;16:1249–59. [DOI] [PubMed] [Google Scholar]
- 20.Correa P, Fontham E, Chen V, et al. Diet, nutrition, and cancer. J La State Med Soc 1988;140:43–9. [PubMed] [Google Scholar]
- 21.Campbell DI, Warren BF, Thomas JE, et al. The African enigma: low prevalence of gastric atrophy, high prevalence of chronic inflammation in West African adults and children. Helicobacter 2001;6:263–7. [DOI] [PubMed] [Google Scholar]
- 22.Ozen H, Dinler G, Akyon Y, et al. Helicobacter pylori infection and recurrent abdominal pain in Turkish children. Helicobacter 2001;6:234–8. [DOI] [PubMed] [Google Scholar]
- 23.Carpentieri DF, Wenner W, Liquornik K, et al. Significance of lymphoid follicles and aggregates in gastric mucosa of children. Pediatr Dev Pathol 2000;3:177–9. [DOI] [PubMed] [Google Scholar]
- 24.Korzon M, Sikorska-Wisniewska G, Jankowski Z, et al. Clinical and pathological importance of cagA-positive Helicobacter pylori strains in children with abdominal complaints. Helicobacter 1999;4:238–42. [DOI] [PubMed] [Google Scholar]
- 25.Begue RE, Gonzales JL, Correa-Gracian H, et al. Helicobacter pylori infection in children with abdominal ailments in a developing country. Am J Med Sci 1997;314:279–83. [DOI] [PubMed] [Google Scholar]
- 26.Aquino RT, Franken RA, Lancellotti CL, et al. [Cardiomyopathy associated with acromegaly. A case report.] Arq Bras Cardiol 1988;50:197–201. [PubMed] [Google Scholar]
- 27.De Stefani E, Correa P, Oreggia F, et al. Black tobacco, wine and mate in oropharyngeal cancer. A case–control study from Uruguay. Rev Epidemiol Sante Publique 1988;36:389–94. [PubMed] [Google Scholar]
- 28.Savage A, Jones S. Histological appearances of the gastric mucosa 15–27 years after partial gastrectomy. J Clin Pathol 1979;32:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Zimaity HMT, Gutierrez O, Kim JG, et al. Geographic differences in the distribution of intestinal metaplasia in duodenal ulcer patients. Am J Gastroenterol 2001;96:666–72. [DOI] [PubMed] [Google Scholar]
- 30.Oi M, Oshida K. The association of esophageal, gastric, and duodenal ulcers; case report. Gastroenterology 1959;36:57–9. [PubMed] [Google Scholar]
- 31.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969;3:87–97. [Google Scholar]
- 32.Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc 1994;40:342–5. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli O, Hassall E, D’Armiento F, et al. Inflammation of the gastric cardia in children with symptoms of acid peptic disease. J Pediatr 2003;143:520–4. [DOI] [PubMed] [Google Scholar]
- 34.Gillett P, Hassall E. Pediatric gastrointestinal mucosal biopsy. Special considerations in children. Gastrointest Endosc Clin N Am 2000;10:669–712, vi-vii. [PubMed] [Google Scholar]
- 35.Graham DY, Opekun AR, Yamaoka Y, et al. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther 2003;17:193–200. [DOI] [PubMed] [Google Scholar]
- 36.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 37.el-Zimaity HM, al-Assi MT, Genta RM, et al. Confirmation of successful therapy of Helicobacter pylori infection: number and site of biopsies or a rapid urease test. Am J Gastroenterol 1995;90:1962–4. [PubMed] [Google Scholar]
- 38.El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney system. Hum Pathol 1999;30:72–7. [DOI] [PubMed] [Google Scholar]
- 39.El-Zimaity HM, Ramchatesingh J, Saeed MA, et al. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol 2001;54:679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 2004;101:3–27. [DOI] [PubMed] [Google Scholar]
- 41.Gill S, Shah A, Le N, et al. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a Canadian cancer center. J Clin Oncol 2003;21:2070–6. [DOI] [PubMed] [Google Scholar]
- 42.Kimura K, Satoh K, Taniguchi Y, et al. Some personal comments on the Sydney system for the classification of chronic gastritis. J Gastroenterol 1994;29 (suppl 7) :114–19. [PubMed] [Google Scholar]