Abstract
Aims: To clarify the involvement of matrix metalloproteinase-7 (MMP-7) in cell dissociation and the subsequent invasion of pancreatic cancer cells.
Methods: Western blotting, in vitro invasion assay, immunocytochemistry, and immunohistochemistry were performed in pancreatic cancer cell lines or pancreatic cancer tissue.
Results: The active form of the MMP-7 protein was expressed exclusively in the conditioned medium of dissociated (PC-1.0 and AsPC-1) pancreatic cancer cells, whereas proMMP-7 protein was only detected in the conditioned medium of non-dissociated (PC-1 and Capan-2) cells. Both intracellular and conditioned medium localised MMP-7 was greatly reduced by treatment with the epidermal growth factor receptor (EGFR) inhibitor AG1478 and the mitogen activated protein kinase kinase (MEK) inhibitor U0126 in pancreatic cancer cells. MMP-7 treatment significantly induced the disruption of tight junction (TJ) structures and subsequent cell dissociation, and activation of the EGFR mediated MEK– ERK (extracellular signal regulated protein kinase) signalling pathway in the non-dissociated pancreatic cancer cells. Moreover, the strong in vitro invasiveness of dissociated cells was inhibited by AG1478 and U0126 treatment, whereas the weak invasiveness of non-dissociated cells was apparently induced by MMP-7 treatment. In addition, MMP-7 expression was stronger at the invasive front than at the centre of human pancreatic tumours.
Conclusion: MMP-7 is involved in cell dissociation and the subsequent invasion of pancreatic cancer cells. It induces the disruption of TJ structures and forms a positive feedback loop with activation of the EGFR mediated MEK–ERK signalling pathway.
Keywords: matrix metalloproteinase-7, invasion, signal transduction pathway, tight junction, pancreatic cancer
At the time of diagnosis, pancreatic cancer usually shows extensive local invasion and/or metastasis, precluding a curative surgical resection. Cancer cell dissociation has been shown to be the first and pivotal step in the invasion–metastasis process.1 However, the cellular and molecular mechanisms of invasion and metastasis in pancreatic cancer have not been fully elucidated.
“Matrix metalloproteinase-7 has been implicated in tumour invasion–metastasis and in tumour initiation or growth in gastrointestinal and other cancers”
Two hamster pancreatic cancer cell lines with a different invasion–metastasis potential—the weakly invasive cell line PC-1 and the highly invasive cell line PC-1.0—were established previously.2,3 In our recent research, the epidermal growth factor receptor (EGFR) mediated mitogen activated protein kinase kinase 2 (MEK2)–ERK2 (extracellular signal regulated kinase 2) signal transduction pathway4–6 was implicated in cancer cell dissociation through disrupting the tight junction (TJ) structures in hamster and human dissociated (PC-1.0 and AsPC-1, respectively) and non-dissociated (PC-1 and Capan-2, respectively) pancreatic cancer cell lines.7–9
In addition, matrix metalloproteinase-7 (MMP-7, matrilysin-1) has been implicated in tumour invasion–metastasis and in tumour initiation or growth in gastrointestinal and other cancers.10 Furthermore, positivity for MMP-7, rather than other MMP molecules, significantly correlates with the extent of tumour invasion, lymph node and distant metastasis, and advanced tumour stage in pancreatic carcinoma.11 However, because conventional assays are based on endpoint results, whether MMP-7 is involved in cell dissociation in pancreatic cancer remains unclear.
In our study, the involvement of MMP-7 in TJ disruption, activation of the EGFR mediated MEK–ERK signalling pathway, and the in vitro invasiveness of pancreatic cancer cells were examined to clarify the potential role of MMP-7 in the regulation of cell dissociation and subsequent invasion of pancreatic cancer cells.
MATERIALS AND METHODS
Cell lines and cell culture
We used hamster dissociated (PC-1.0) and non-dissociated (PC-1) pancreatic cancer cell lines. The PC-1 cell line was established from pancreatic ductal/ductular adenocarcinoma induced by BOP (N-nitrosobis(2-oxopropyl) amine) in a Syrian golden hamster.2 The PC-1.0 cell line was established from a subcutaneous tumour produced after inoculation of PC-1 cells.3 The human dissociated (AsPC-1) and non-dissociated (Capan-2) pancreatic cancer cell lines (American Tissue Culture Collection, Rockville, Maryland, USA) were also used. PC-1 and Capan-2 cells grow as island-like colonies, whereas PC-1.0 and AsPC-1 cells grow as single cells.
These cells were incubated in RPMI-1640 (Gibco-BRL, Grand Island, New York, USA), supplemented with 10% fetal bovine serum (Bioserum, Victoria, Australia), 100 units/ml penicillin G, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 to 95% air. The cells were serum starved overnight before experiments.
Antibodies
We used rabbit antihuman MMP-7 (Oncogene Research Products, San Diego, California, USA), occludin and zonula occludens-1 (ZO-1) (Zymed Laboratories Inc, South San Francisco, California, USA), phosphorylated EGFR (p-EGFR), phosphorylated MEK (p-MEK), and phosphorylated ERK (p-ERK) (all Santa Cruz Biotechnology, Santa Cruz, California, USA) polyclonal antibodies in our study. These antibodies are crossreactive with hamster tissues.4–7,9 We also used fluorescein isothiocyanate and rhodamine labelled fluorescence secondary antibodies (Santa Cruz Biotechnology).
Tissue samples
We obtained 37 pancreatic cancer tissue samples at the time of surgery from the department of surgery II, Kumamoto University Hospital from October 1989 to July 2001. The median age of the patients with pancreatic cancer was 63.5 years (range, 35–78); there were 14 men and 23 women. Histologically, the samples consisted of 13 well differentiated, 20 moderately differentiated, and four poorly differentiated adenocarcinomas. All of the tissue samples were histologically examined, and the pathological diagnoses were confirmed.
Preparation of cell lysate and concentration of conditioned medium
We grew the four pancreatic cancer cell lines mentioned earlier in 90 mm dishes. After growing to subconfluent, we replaced the medium with serum free medium and incubated the cells for 36 hours with or without 10μM specific EGFR inhibitor AG1478 (Calbiochem, San Diego, California, USA)12 or 10μM specific MEK inhibitor U0126 (Cell Signaling Technology, Beverly, Massachusetts, USA).13
We then collected the conditioned medium and concentrated it 50 fold. Simultaneously, we lysed the cells using RIPA cell lysis buffer.8 We adjusted the concentration of each sample to 1 mg/ml using the Bio-Rad protein assay kit (Bio-Rad, Anaheim, California, USA).
Western blot analysis
We performed western blotting as described previously.8 In brief, we ran samples of equivalent total protein (20 μg) on a 15% polyacrylamide slab gel. Then we incubated the membranes with primary antibodies overnight at 4°C, after which we incubated the blots with horseradish peroxidase conjugated secondary antibody. We used enhanced chemiluminescence (Santa Cruz Biotechnology) to detect the signals developed on Kodak scientific imaging film (Eastman Kodak Company, Rochester, New York, USA). We used a cell lysate of SW620 cells14 and distilled water as positive and negative controls, respectively.
Immunofluorescent staining and fluorescence intensity analysis
We planted the cells mentioned earlier on the chamber slides and incubated them before the experiment. For the activation study, we incubated the pancreatic cancer cells with 100 ng/ml human purified MMP-7 (a mixture of zymogen and active enzyme; Sigma, St Louis, Missouri, USA) for 36 hours. For the inhibition study, we incubated the cells with 10μM AG1478 or 10μM U0126 for 36 hours.
After incubation, immunofluorescent staining was performed as described previously.4 We prepared the control slides as follows: (1) we processed sections without a primary antibody; (2) we used normal rabbit serum and non-specific rabbit IgG instead of a polyclonal antibody.
Finally, we chose six cells in the image randomly to measure the fluorescence intensity (FI) with the software Fluoview 500 (version 4.3; Olympus, Tokyo, Japan). We used the averages for FI analysis of the expression of MMP-7, p-EGFR, p-MEK, and p-ERK.
In vitro invasion assay
We carried out the in vitro invasion assay using Invasion Chambers (Becton Dickinson Labware, Bedford, Massachusetts, USA) according to the method described previously.15 We incubated the cells for 12 hours at 37°C with or without 10μM AG1478, 10μM U0126, or 100 ng/ml human MMP-7. We determined the number of cells migrating to the lower side of the filter by counting the number of nuclei within an area of 1 mm2 on the grid at a magnification of ×100.
Immunohistochemical analysis
We performed immunohistochemical staining using the avidin–biotin–peroxidase complex technique (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, California, USA), as described previously.4 We prepared the control slides as follows: (1) we processed sections without a primary antibody; (2) we used normal rabbit serum and non-specific rabbit IgG instead of a polyclonal anti-MMP-7 antibody.
Statistical analysis
We analysed the average FI for MMP-7, p-EGFR, p-MEK, and p-ERK in the different experimental groups, and the numbers of pancreatic cancer cells counted in the invasion assay, by the unpaired Student’s t test with the Stat View computerised program (SAS Institute Inc, Cary, North Carolina, USA). We considered p < 0.05 to be significant.
RESULTS
AG1478 and U0126 downregulated both intracellular and conditioned medium localised expression of MMP-7 protein in pancreatic cancer cells
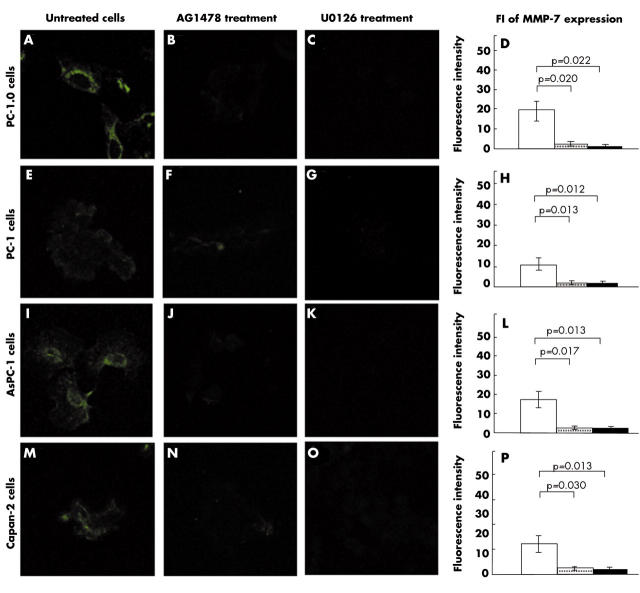
We found constitutive expression of MMP-7 protein in dissociated cells PC-1.0 and AsPC-1 (mean FI, 19.18 (SD, 5.20) and 17.27 (4.26), respectively; fig 1A,I). MMP-7 expression was relatively weak in non-dissociated cells PC-1 and Capan-2 (mean FI, 11.51 (SD, 2.92) and 12.72 (3.54), respectively; fig 1E,M). Furthermore, MMP-7 expression was mainly found in the cytoplasm. The expression of MMP-7 was greatly reduced after treatment with AG1478 or U0126 in dissociated cells PC-1.0 (mean FI, 2.11 (SD, 0.92) and 1.28 (0.49), respectively; p = 0.020 and p = 0.022; fig 1B,C) and AsPC-1 (mean FI, 2.26 (SD, 0.79) and 1.89 (1.25), respectively; p = 0.017 and p = 0.013; fig 1J,K), and in non-dissociated cells PC-1 (mean FI, 2.41 (SD, 1.07) and 2.38 (1.15), respectively; p = 0.013 and p = 0.012; fig 1F,G) and Capan-2 (mean FI, 2.66 (SD, 0.43) and 1.65 (1.37), respectively; p = 0.030 and p 0.013; fig 1N,O). Figure 1 parts D, H, L, and P show the average FI values for MMP-7 expression in the different pancreatic cancer cells.
Figure 1.
Immunofluorescent staining for matrix metalloproteinase-7 (MMP-7) and fluorescent intensity (FI) values in pancreatic cancer cells. Original magnification, ×400. Open bars, untreated cells; shaded bars, AG1478; closed bars, U0126.
The western blotting data confirmed the immunofluorescent staining results (fig 2A,B). Moreover, the western blotting data showed that the active form of the MMP-7 protein (17 kDa in hamster cells and 18 kDa in human cells) was detected only in the conditioned medium of dissociated pancreatic cancer cells PC-1.0 and AsPC-1 (fig 2C,D), whereas the inactive form of the MMP-7 protein (27 kDa in hamster cells and 28 kDa in human cells) was found in the conditioned medium of untreated PC-1 and Capan-2 cells (fig 2C,D). In addition, both intracellular and conditioned medium localised MMP-7 proteins were reduced by treatment with AG1478 or U0126 in all pancreatic cancer cell lines.
Figure 2.
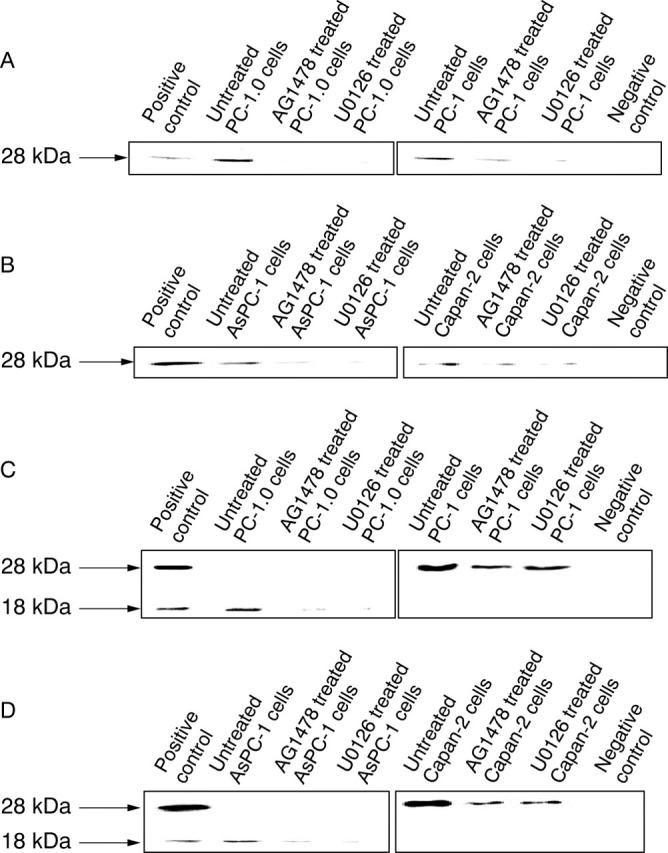
Western blotting for the matrix metalloproteinase-7 (MMP-7) protein in pancreatic cancer cells. The intracellular expression of MMP-7 was inhibited by AG1478 or U0126 treatment in (A) PC-1.0 and PC-1 cells, in addition to (B) AsPC-1 and Capan-2 cells. Inactive and active MMP-7 showed significantly different expression in the conditioned medium between dissociated and non-dissociated pancreatic cancer cells PC-1.0 and PC-1 (C), and AsPC-1 and Capan-2 (D). A lysate of SW620 cells and distilled water were used as positive and negative controls, respectively. The molecular weight is indicated.
MMP-7 induced the disruption of TJ structures and translocation of TJ proteins in non-dissociated pancreatic cancer cells
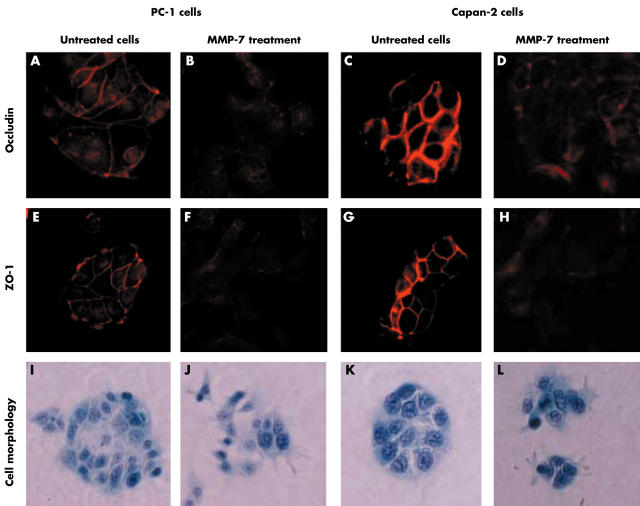
The immunofluorescent staining images showed that occludin (fig 3A,3C) and ZO-1 (fig 3E,G) were exclusively localised to cell–cell junctions in untreated PC-1 and Capan-2 cells. However, after 36 hours of treatment with MMP-7, expression of occludin (fig 3B,D) and ZO-1 (fig 3F,H) was low at cell–cell junctions and they were both translocated to the cytoplasm in PC-1 and Capan-2 cells. In addition, the cell colonies of non-dissociated pancreatic cancer cells PC-1 (fig 3I) and Capan-2 (fig 3K) were disrupted by MMP-7 treatment (fig 3J,L).
Figure 3.
Disruption of tight junction (TJ) structures and translocations of TJ proteins induced by matrix metalloproteinase-7 (MMP-7) in non-dissociated pancreatic cancer cells PC-1 and Capan-2. (A–H) Immunofluorescent staining; (I–L) Papanicolaou’s staining. Original magnification, ×400. ZO1, zonula occludens-1.
MMP-7 induced activation of EGFR mediated MEK–ERK signal transduction in non-dissociated pancreatic cancer cells
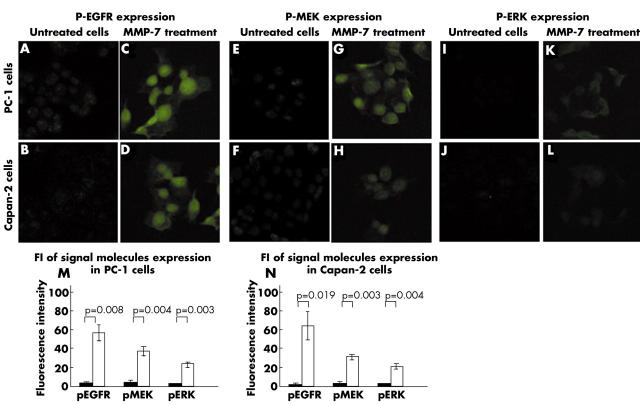
In untreated PC-1 and Capan-2 cells, staining for p-EGFR (mean FI, 3.23 (SD, 0.26) and 2.48 (0.18), respectively; fig 4A,B), p-MEK (mean FI, 4.62 (SD, 1.07) and 2.95 (0.47), respectively; fig 4E,F), and p-ERK (mean FI, 1.93 (SD, 0.46) and 2.15 (0.38), respectively; fig 4I,J) was faint. In contrast, the expression of p-EGFR (mean FI, 56.76 (SD, 8.06) and 63.68 (14.82); p = 0.008 and p = 0.019, respectively; fig 4C,D), p-MEK (mean FI, 37.19 (SD, 4.66) and 30.30 (3.02); p = 0.004 and p = 0.003, respectively; fig 4G,H), and p-ERK (mean FI, 23.18 (SD, 2.39) and 20.11 (2.28); p = 0.003 and p = 0.004, respectively; fig 4K,L) was significantly induced by MMP-7 treatment in PC-1 and Capan-2 cells. Figure 4 parts M and N show the average FI values, respectively.
Figure 4.
Activation of the epidermal growth factor receptor (EGFR) mediated mitogen activated protein kinase kinase (MEK)–ERK (extracellular signal regulated protein kinase) signalling pathway by matrix metalloproteinase-7 (MMP-7) treatment in non-dissociated pancreatic cancer cells PC-1 and Capan-2. Original magnification, ×400. p, phosphorylated. Closed bars, untreated cells; open bars, MMP-7.
In vitro invasiveness of pancreatic cancer cells
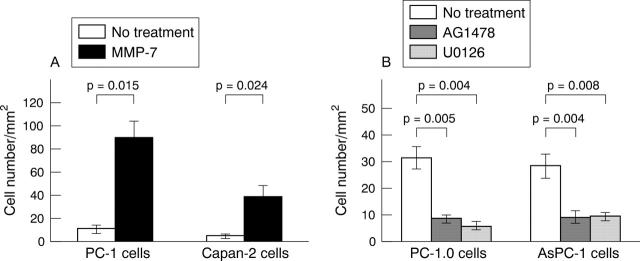
As shown in fig 5A, non-dissociated pancreatic cancer cells PC-1 and Capan-2 were weakly invasive (mean number of invasive cells, 10.7 (SE, 3.1) and 4.7 (1.5), respectively). Surprisingly, the invasive capability was significantly enhanced by 12 hours of treatment with MMP-7 (mean number of invasive cells, 89.3 (SE, 14.0) and 38.3 (10.6); p = 0.015 and p = 0.024, respectively).
Figure 5.
Chemoinvasion of pancreatic cancer cells. (A) Matrix metalloproteinase-7 (MMP-7) treatment greatly enhanced the chemoinvasion of non-dissociated cells PC-1 and Capan-2. (B) AG1478 and U0126 treatment suppressed the chemoinvasion of dissociated cells PC-1.0 and AsPC-1. The invasive cell number is presented as mean (SE). All experiments were done in triplicate.
In contrast, as shown in fig 5B, dissociated cells PC-1.0 and AsPC-1 were strongly invasive (mean number of invasive cells, 31.3 (SE) 4.2 and 28.3 (4.5), respectively). However, their invasive ability was suppressed by AG1478 treatment (mean number of invasive cells, 8.3 (SE) 1.5 and 8.9 (SE) 2.5; p = 0.005 and p = 0.004, respectively) or U0126 treatment (mean number of invasive cells, 5.6 (SE) 1.5 and 9.3 (1.5); p = 0.004 and p = 0.008, respectively).
MMP-7 expression in human pancreatic cancer tissues
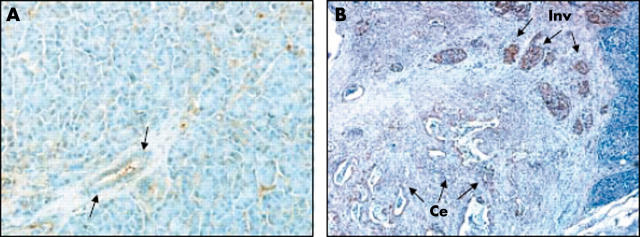
No immunostaining for MMP-7 was seen in non-malignant pancreatic ductal/ductular cells (fig 6A), the cell type from which the pancreatic cancer cell lines used in our current study originated. In pancreatic cancer tissue, MMP-7 expression was seen both at the centre and at the invasive front of pancreatic tumours (fig 6B). Furthermore, MMP-7 expression was significantly stronger at the invasive front than at the centre of the same pancreatic cancer tissue.
Figure 6.
Immunohistochemical expression of matrix metalloproteinase-7 (MMP-7) in human pancreatic cancer tissues. (A) The expression of MMP-7 in non-malignant pancreatic tissues. The arrows show that no MMP-7 expression was seen in the pancreatic ductal/ductular cells. (B) The expression of MMP-7 in pancreatic cancer tissue. The arrows show that the expression of MMP-7 at the invasive front (Inv; upper right) is significantly stronger than at the centre (Ce; lower left) of the tumour. Original magnification, ×100.
DISCUSSION
The MEK–ERK signal transduction pathway is a key intracellular signalling pathway that is involved in diverse cellular activities, including cell proliferation, cell division, cell motility, apoptosis, and others.16 In our previous studies, we found that activation (phosphorylation) of the EGFR mediated MEK–ERK signalling pathway significantly induced cell dissociation in non-dissociated pancreatic cancer cells PC-1 and Capan-2. In contrast, inhibition of this signalling pathway by the EGFR inhibitor AG1478 or MEK inhibitor U0126 induced cell aggregation in dissociated pancreatic cancer cells PC-1.0 and AsPC-1.4–6 In our current study, the conditioned medium localised MMP-7 protein, not the intracellular localised proMMP-7 protein, showed a distinctly different pattern of expression between dissociated and non-dissociated pancreatic cancer cells. Furthermore, both the intracellular and conditioned medium localised MMP-7 proteins were suppressed by AG1478 or U0126 treatment. The invasiveness of non-dissociated cells was also found to be enhanced by MMP-7 treatment. In addition, the stronger expression of MMP-7 at the invasive front compared with the centre of human pancreatic tumours was consistent with the expression pattern of MEK2.4 These results imply that the MMP-7 protein is closely involved in cell dissociation and the subsequent invasion of pancreatic cancer cells. In addition, the EGFR mediated MEK–ERK signalling pathway may closely correlate with MMP-7 expression during cell dissociation and the subsequent invasion of pancreatic cancer cells.
“Matrix metalloproteinase-7 may induce cell dissociation through the disruption of tight junctions and the translocation of tight junction proteins in pancreatic cancer cells”
TJs are known to form continuous circumferential intercellular contacts between epithelial cells and to create a regulated barrier to the movement of paracellular molecules.17 We previously found that the pattern of expression and the distribution of TJ proteins closely correlated with activation of the MEK–ERK signalling pathway during the dissociation of pancreatic cancer cells.7 Our current study demonstrated the disruption of TJ structures and the translocation of TJ proteins from cell–cell junctions to the cytoplasm after the addition of MMP-7 to the culture medium of non-dissociated cells PC-1 and Capan-2. These findings indicate that MMP-7 may induce cell dissociation through the disruption of TJ structures and the translocation of TJ proteins in pancreatic cancer cells.
Interestingly, we found that the addition of MMP-7 to the culture medium of non-dissociated pancreatic cancer cells PC-1 and Capan-2 induced the activation of the EGFR mediated MEK–ERK signalling pathway in these cells. There is evidence that MMP-7 can interact with several cell surface molecules and growth factors (for instance, E-cadherin, β4 integrin, tumour necrosis factor α, and Fas ligand), thereby promoting specific cell functions.18–20 Moreover, MMP-7 also forms a complex with cell surface protein CD44, which leads to shedding of cell surface localised heparin binding epidermal growth factor, which is one of the ligands of EGFR.21 However, whether MMP-7 activates the EGFR mediated MEK–ERK signalling pathway via this mechanism, and the exact EGFR ligand released by MMP-7 are still unclear. Taken together, MMP-7 expression is closely correlated with the activation of EGFR mediated MEK–ERK signalling in the cell dissociation associated with the invasion and metastasis of pancreatic cancer. These molecules possibly form a positive feedback loop, which may facilitate cell dissociation and the subsequent invasion–metastasis process in pancreatic cancer.
Take home messages.
Matrix metalloproteinase-7 (MMP-7) is closely involved in cell dissociation and the subsequent invasion of pancreatic cancer cells
MMP-7 can induce cell dissociation in pancreatic cancer through activating epidermal growth factor receptor (EGFR) mediated mitogen activated protein kinase kinase (MEK)–ERK (extracellular signal regulated protein kinase) signalling, and through inducing the disruption of tight junction (TJ) structures and the translocation of TJ proteins
MMP-7 and EGFR mediated MEK–ERK signalling possibly form a positive feedback loop to induce and sustain cell dissociation, which is directly related to the high potential for invasion and metastasis seen in pancreatic cancer
In summary, MMP-7 is closely involved in cell dissociation and subsequent invasion of pancreatic cancer cells. MMP-7 can induce cell dissociation in pancreatic cancer through activating EGFR mediated MEK–ERK signalling, and through inducing the disruption of TJ structures and the translocation of TJ proteins. MMP-7 and the EGFR mediated MEK–ERK signalling pathway possibly form a positive feedback loop to induce and sustain cell dissociation, which is directly related to the high potential for invasion and metastasis in pancreatic cancer. Controlling the expression of MMP-7 will contribute to the development of a new clinical therapeutic method based on anti-invasion and antimetastasis in pancreatic cancer.
Figure 7.

Abbreviations
EGFR, epidermal growth factor receptor
ERK, extracellular signal regulated protein kinase
FI, fluorescence intensity
MEK, mitogen activated protein kinase kinase
MMP-7, matrix metalloproteinase-7
p-, phosphorylated
TJ, tight junction
ZO-1, zonula occludens-1
REFERENCES
- 1.Torimura T, Ueno T, Kin M, et al. Autocrine motility factor enhances hepatoma cell invasion across the basement membrane through activation of β1 integrins. Hepatology 2001;34:62–71. [DOI] [PubMed] [Google Scholar]
- 2.Egami H, Takiyama Y, Cano M, et al. Establishment of hamster pancreatic ductal carcinoma cell line (PC-1) producing blood group-related antigens. Carcinogenesis 1989;10:861–9. [DOI] [PubMed] [Google Scholar]
- 3.Egami H, Tomioka T, Tempero M, et al. Development of intrapancreatic transplantable model of pancreatic duct adenocarcinoma in Syrian golden hamsters. Am J Pathol 1991;138:557–61. [PMC free article] [PubMed] [Google Scholar]
- 4.Tan X, Egami H, Kamohara H, et al. Involvement of the mitogen-activated protein kinase kinase 2 in the induction of cell dissociation in pancreatic cancer. Int J Oncol 2004;24:65–73. [PubMed] [Google Scholar]
- 5.Tan X, Egami H, Ishikawa S, et al. Relationship between the expression of extracellular signal-regulated kinase 1/2 and the dissociation of pancreatic cancer cells: involvement of ERK1/2 in the dissociation status of cancer cells. Int J Oncol 2004;24:815–20. [PubMed] [Google Scholar]
- 6.Tan X, Egami H, Ishikawa S, et al. Relationship between activation of epidermal growth factor receptor and cell dissociation in pancreatic cancer. Int J Oncol 2004;25:1303–9. [PubMed] [Google Scholar]
- 7.Tan X, Tamori Y, Egami H, et al. Analysis of invasion–metastasis mechanism in pancreatic cancer: involvement of tight junction transmembrane protein occludin and MEK/ERK signal transduction pathway in cancer cell dissociation. Oncol Rep 2004;11:993–8. [PubMed] [Google Scholar]
- 8.Tan X, Egami H, Ishikawa S, et al. Arrangement of expression and distribution of tight junction protein claudin-1 in cell dissociation of pancreatic cancer cells. Int J Oncol 2004;25:1567–74. [PubMed] [Google Scholar]
- 9.Tan X, Egami H, Ishikawa S, et al. Zonula occludens-1 (ZO-1) redistribution is involved in the regulation of cell dissociation in pancreatic cancer cells. Dig Dis Sci 2005;50:1402–9. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto H, Itoh F, Adachi Y, et al. Relation of enhanced secretion of active matrix metalloproteinases with tumour spread in human hepatocellular carcinoma. Gastroenterology 1997;112:1290–6. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Itoh F, Iku S, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol 2001;19:1118–27. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XF, Liu ZC, Xie BF, et al. EGFR tyrosine kinase inhibitor AG1478 inhibits cell proliferation and arrests cell cycle in nasopharyngeal carcinoma cells. Cancer Lett 2001;169:27–32. [DOI] [PubMed] [Google Scholar]
- 13.Favata MF, Horiuchi KY, Manos EJ. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 1998;273:18623–32. [DOI] [PubMed] [Google Scholar]
- 14.Seiler N, Schneider Y, Gosse F, et al. Polyploidisation of metastatic colon carcinoma cells by microtubule and tubulin interacting drugs: effect on proteolytic activity and invasiveness. Int J Oncol 2004;25:1039–48. [PubMed] [Google Scholar]
- 15.Kurizaki T, Egami H, Hirota M, et al. Characterization of cancer cell dissociation factor in a highly invasive pancreatic cancer cell line. Cancer 1995;75:1554–61. [DOI] [PubMed] [Google Scholar]
- 16.Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol 1999;71:479–500. [DOI] [PubMed] [Google Scholar]
- 17.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963;17:375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 2001;114:111–18. [DOI] [PubMed] [Google Scholar]
- 19.Von Bredow DC, Nagle RB, Bowden GT, et al. Cleavage of beta 4 integrin by matrilysin. Exp Cell Res 1997;236:341–5. [DOI] [PubMed] [Google Scholar]
- 20.Haro H, Crawford HC, Fingleton B, et al. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest 2000;105:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Cancer Biol 2000;10:415–33. [DOI] [PubMed] [Google Scholar]