The patient was a 70 year old woman. A tumour in liver segment 8 arose in a background of cirrhotic liver with chronic hepatitis C and reached a size of 6.0 cm in six months. The patient’s serum concentration was raised (17101 ng/ml), and the tumour was suspected to be hepatocellular carcinoma (HCC) based on various image findings. An extended liver anterior segmentectomy was performed, and serum α fetoprotein returned to normal immediately after surgery.
Although the macroscopic findings were compatible with conventional HCC (fig 1A), the histology of the tumour was atypical—the tumour cells mainly formed irregular tubular structures filled with a bloody/serous or bloody fluid (fig 1B), and small tubular or acinar-like structures were also found (fig 1C). Solid structures were seen in a small portion of the tumour (fig 1D), and massive bleeding was also seen. The tumour cells had abundant eosinophilic granular cytoplasm and round nuclei with moderate variations in size and shape. The typical trabecular pattern was not seen, and no evidence of desmoplastic stroma, extracapsular proliferation, vascular invasion, or Alcian blue/periodic acid Schiff positive mucin was seen. In addition, a typical moderately differentiated HCC (measuring 1.0 cm) with trabecular pattern was also found.
Figure 1.
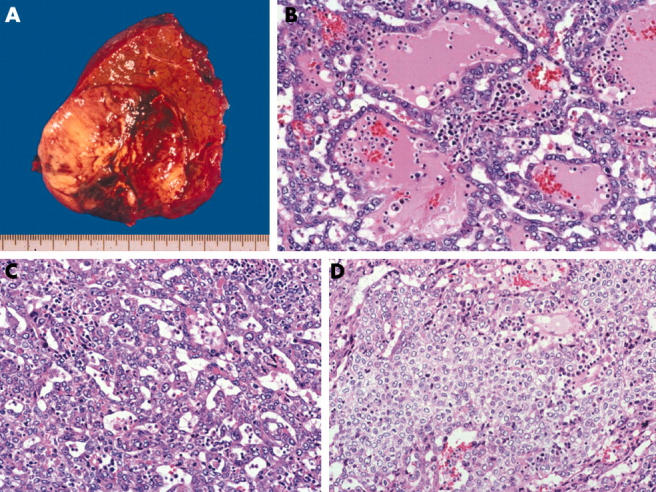
(A) Macroscopically, the nodular S8 tumour has a fibrous capsule. The cut surface of the tumour is well circumscribed, expanded, and yellow/white to dark red in colour. (B) The tumour cells have abundant eosinophilic granular cytoplasm and round nuclei with moderate variations in size and shape. The irregular trabecular structures are filled with a bloody/serous or bloody fluid without mucin production and desmoplastic tissue. The typical trabecular pattern was not seen (haematoxylin and eosin (H&E) stain, original magnification, ×200). (C) Small tubular or acinar-like patterns are also visible (H&E stain; original magnification, ×200). (D) Solid structures are seen in a small portion of the tumour (H&E stain; original magnification, ×200).
Immunohistochemical examination revealed that the tumour cells showed diffuse and strong reactivity for vimentin and pankeratin (AE1/3), focal reactivity for α fetoprotein and HepPar 1, and negativity for calretinin, Wilms’ tumour 1 protein, c-kit, CD34, cytokeratin 7, cytokeratin 19, cytokeratin 20, low molecular weight cytokeratin (CAM5.2), epithelial membrane antigen, chromogranin A, synaptophysin, neurone specific enolase, carcinoembryonic antigen, CA125, 2A2, 2G10, and 4C4. The tumour cells had a high proliferative activity, scoring 60% on the MIB-1 labelling index.
All candidate tumour types with the exception of HCC (cholangiocellular carcinomas, metastatic adenocarcinomas, primary malignant mesotheliomas, carcinoid tumours, and germ cell tumours) were ruled out clinically and histologically. Pseudoglandular formation is a common histological manifestation of HCC, and pelioid-type HCC shows large vascular lakes within the tumour, mimicking peliosis hepatis. Therefore, we consider this tumour to resemble such types of HCC.
Recently, intermediate liver carcinomas and hepatic stem cell malignancies have been reported.1,2 However, an apparent stem cell component was not prominent in the present tumour, and the negativity for c-kit, the hypochromatic nuclei, and the absence of desmoplastic stroma were not compatible with these types of tumours.
The reticular-like pattern suggested a yolk sac tumour, and an association between hepatitis C virus infection and yolk sac tumours has been suggested.3 However, specific features, such as Schiller-Duval bodies, a cystic pattern, and hyaline globules, were not detected. In addition, the tumour was immunohistochemically negative for 2A2, 2G10, and 4C4, which have been reported to be specific to yolk sac tumours.
A strong reactivity for vimentin is associated with metastatic HCCs or sarcomatous HCCs,4,5 indicating a highly malignant form of HCC. Clinically, this tumour showed rapid growth and a high proliferative activity of 60% as assessed by the MIB-1 labelling index.
Considering the various findings described above, we finally diagnosed this tumour as an unusual type of HCC with poorly differentiated features presenting with a high degree of malignancy. Thirteen months after surgery, a new tumour was detected in liver segment 2 and percutaneous ethanol injection therapy was performed.
The patient gave informed consent for this letter to be published.
References
- 1.Haeryoung K, Chanil P, Kwang-Hyup H, et al. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol 2004;40:298–304. [DOI] [PubMed] [Google Scholar]
- 2.Theise ND, Yao JL, Harada K, et al. Hepatic “stem cell” malignancies in adults: four cases. Histopathology. 2003: 43;263–71,. [DOI] [PubMed]
- 3.Morinaga S, Nishiya H, Inafuku T. Yolk sac tumor of the liver combined with hepatocellular carcinoma. Arch Pathol Lab Med 1996;120:687–90. [PubMed] [Google Scholar]
- 4.Hu L, Lau SH, Tzang CH, et al. Association of vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene 2004;23:298–302. [DOI] [PubMed] [Google Scholar]
- 5.Kakizoe S, Kojiro M, Toshiro N. Hepatocellular carcinoma with sarcomatous change. Clinicopathologic and immunohistochemical studies of 14 autopsy cases. Cancer 1987;59:310–16. [DOI] [PubMed] [Google Scholar]


