Abstract
Background: Extensive intestinal metaplasia (EIM) has been reported in gastrectomies from patients dwelling in the Pacific and Atlantic basins.
Aims: To compare all the results in an attempt to explain the findings.
Method: All sections from 3421 gastrectomies were reviewed at various hospitals: 1946 in the Atlantic and 1475 in the Pacific basin. Sections with EIM showed IM encompassing one or more entire low power field (⩾5 mm in length/section) in one or more section.
Results: In the Atlantic basin, EIM was present in 18.8% (153 of 814) of specimens with intestinal carcinoma (IC) and in 10.3% (65 of 630) of those with diffuse carcinoma (DC). In the Pacific basin, EIM was found in 62.9% (412 of 655) of gastrectomies with IC and in 33.3% (160 of 481) of those with DC. The numbers of specimens with EIM were significantly higher in the Pacific than in the Atlantic basin for both carcinoma phenotypes, particularly among elderly patients (⩾60 years).
Conclusions: The proportion of gastrectomies with EIM was higher among populations at a higher gastric cancer risk than in those with a lower cancer risk. EIM was mostly associated with IC rather than DC or with miscellaneous gastric diseases (841 control gastrectomies) in both basins. The proportion of gastrectomies with EIM was significantly higher in Vancouver than in New York and in Santiago de Chile than in Buenos Aires, even though these populations reside at approximately the same geographical latitude, but in different basins. Environmental factors seem to accelerate the evolution of EIM.
Keywords: gastric, intestinal metaplasia, carcinoma, gastrectomy
Gastric carcinoma is a leading cause of cancer related death worldwide. In many countries bordering the Pacific basin, the gastric mucosa continues to be the main target for cancer development.1 In contrast, populations bordering the Atlantic basin have experienced a rapid decrease in the incidence of cancer of the stomach (mainly in the corpus and antrum2). Although the cause(s) of this significant reduction remains unclear, it has become apparent that the improvement in food preservation has greatly contributed to the decrease.3
“The mechanism(s) whereby intestinal metaplasia evolves into a neoplastic lesion remains poorly understood”
In recent years, several studies have suggested that Helicobacter pylori4 may be the principal agent responsible for setting in motion the cascade of histological alterations involved in the progression of chronic gastritis to carcinoma, via intestinal metaplasia (IM) and epithelial dysplasia.5 That evolution has been recently demonstrated experimentally in H pylori infected Mongolian gerbils.6
The mechanism(s) whereby IM evolves into a neoplastic lesion remains poorly understood. Many authors think that the precancerous potential of IM is related to the histological/histochemical constituents of the mucin contained in columnar and goblet cells,7,8 irrespective of their extension into the gastric mucosa. Surprisingly, much less attention has been given in the literature to the possibility that extended mucosal areas with IM could be more important than small islands of histochemically altered mucins in gastric cells. Because multiple gastric biopsies are not sufficient to assess the extension of IM in individual patients,9 other methods have been proposed to explore that important parameter. Some authors mapped the distribution of IM at gross examination by registering the colorimetric reaction of the gastric mucosa challenged with alkaline phosphatase. However, using that colorimetric method Stemmermann and Hayashi,10 and more recently Kumagae,11 found false positive and false negative results. Thus, the colorimetric test appears to be an unreliable predictor of the true histological distribution of IM in entire gastrectomy specimens.
With the aid of morphometry12,13 and image analysis,14–17 we previously evaluated the extent of IM in haematoxylin and eosin (H&E) stained sections and in histochemically (Alcian blue (pH 2.5) and high iron diamine) treated sections from entire gastrectomy specimens. In those reports, only a few cases were investigated for research purposes, because the methods were highly time consuming and impractical for evaluating a large number of gastrectomies.
Several years ago we developed a semiquantitative, more practical method of assessing the presence of small islands or “spots” of IM and of extensive mucosal areas of IM in H&E stained sections.18 This method was used to investigate sections from gastrectomies carried out at hospitals bordering the Atlantic19 and the Pacific20–23 basins. The studies were done separately and a comprehensive comparative study between the results obtained in the two basins has never been carried out.
Because of the continued concern about the importance of IM in gastric carcinogenesis,24–27 we thought that it would be interesting to compare the results of the extent of IM obtained in the two basins. We thought that a comparison between the results in the two disparate geographical regions might enable the possible influence of environmental factors upon the development of extensive IM (EIM) in the gastric mucosa to be explored.
The following questions were regarded essential for this comparative study:
Is the distribution of EIM similar in both basins?
Is EIM age dependent in patients dwelling in different basins?
Is EIM more frequent in a certain phenotype of gastric cancer?
Is EIM more frequent in gastric cancer than in patients with miscellaneous gastric diseases?
Does the number of sections available in individual basins influence the presence of EIM?
This comparative work summarises the results obtained over a period of 18 years at various hospitals located at the rim of the Pacific and Atlantic basins.
MATERIALS AND METHODS
Between 1981 and 1998, 3421 gastrectomies were reviewed at various hospitals located at the rim of the Atlantic19 and the Pacific20–22 basins. Of the 1946 gastrectomies reviewed in the Atlantic basin, 1444 had gastric carcinoma and 502 miscellaneous gastric diseases (peptic ulcers, gastrointestinal stromal tumours, lymphomas, or carcinoid tumours). Of the 1475 gastrectomies studied in the Pacific basin, 1136 had gastric carcinoma and 339 miscellaneous gastric diseases.
All cases were collected during an approximately 10 year period at the respective hospitals. Sections were stained with H&E. Some cases were also stained with Alcian blue (pH 2.5) and high iron diamine.16
Histological classification of carcinomas
Carcinomas were classified according to the predominant histological type into intestinal (IC) and diffuse (DC) carcinomas. According to the recommendations of Ming,28 mixed carcinomas were classified as DC.
Assessing IM
All filed histological sections were reviewed at low power magnification (×4 objective). Sections were classified into those having “spotty” IM or EIM, as reported elsewhere.18 Specimens considered to have spotty IM were those with one or more small island of IM (fig 1) at low power (⩾ 5 mm in length/section) in one or more section. Specimens classified as EIM were those with IM in one or more entire (fig 2) low power field (⩾ 5 mm in length/section) in one or more section. When present, EIM was localised to the antrum, or the antrum and fundic mucosa. The presence of EIM in the fundic mucosa exclusively was not seen in carcinoma, except in one patient from Stockholm, who had pernicious anaemia and gastric carcinoma.
Figure 1.
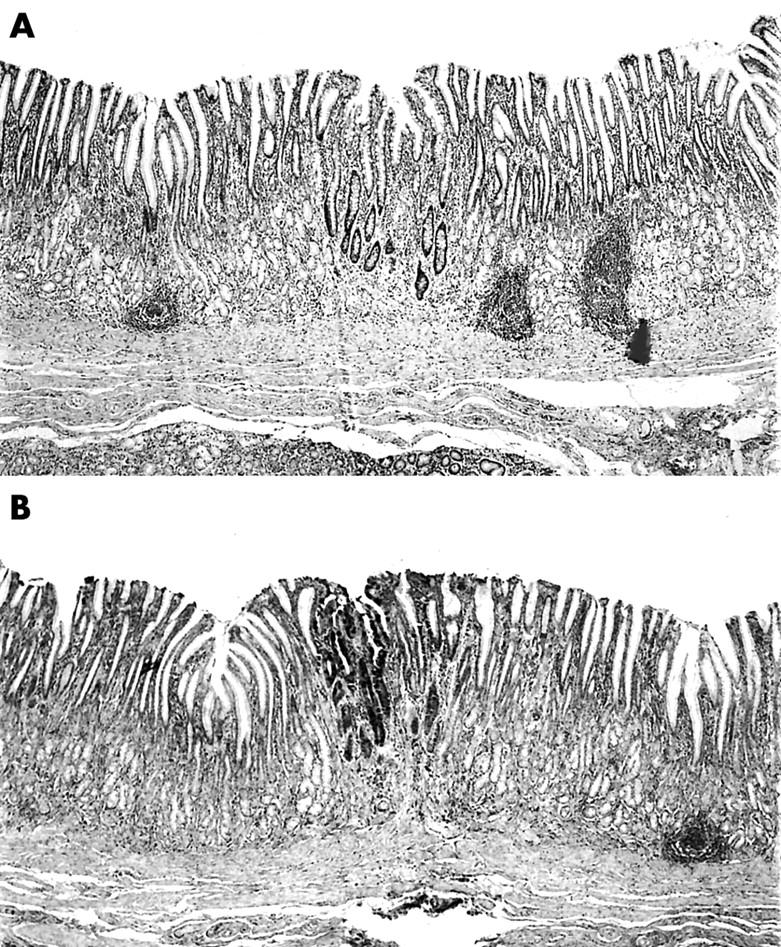
(A) Section from a gastrectomy specimens showing a “spot” with intestinal metaplasia (haematoxylin and eosin stain; original magnification, ×4). (B) Section from a gastrectomy specimen showing a spot with intestinal metaplasia (Alcian blue stain, pH 2.5; original magnification, ×4).
Figure 2.
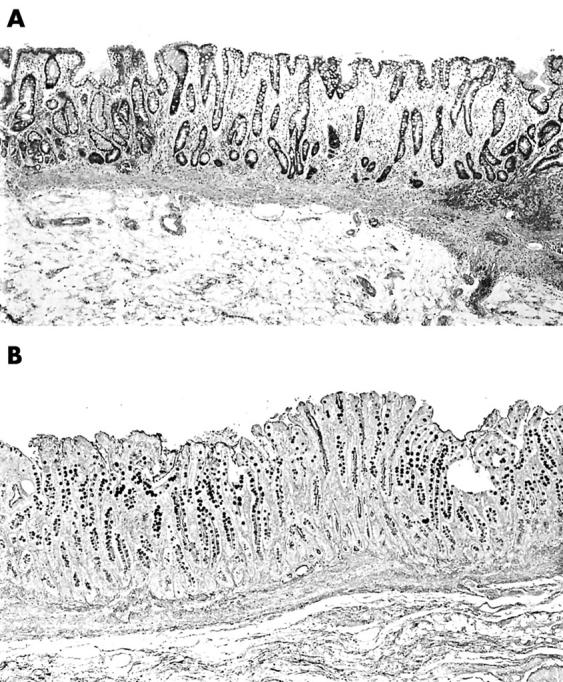
(A) Section from a gastrectomy specimen showing intestinal metaplasia in the entire low power field (haematoxylin and eosin stain; original magnification, ×4). (B) Section from a gastrectomy specimen showing intestinal metaplasia in the entire low power field (Alcian blue stain, pH 2.5, without counterstain; original magnification, ×4)
Because IM usually increases with age,28 we compared specimens with EIM among elderly patients (⩾ 60 years of age) dwelling in disparate geographical regions within the two basins.
Statistical analysis
The non-parametric Wilcoxon test and ANOVA analysis were performed using StatView Version 4.5 software (Abacus Concepts, Berkeley, California, USA). Significance was defined as p < 0.05.
RESULTS
Gastric cancer phenotypes
Of the1444 gastric carcinomas reviewed in the Atlantic basin, 56.4% (n = 814) were ICs and the remaining 43.6% (n = 630) were DCs. Of the 1136 gastric carcinomas reviewed in the Pacific basin, 57.7% (n = 655) were ICs and the remaining 42.3% (n = 481) were DCs.
The difference between the number of cases with IC and DC in the two basins was significant (p < 0.05).
EIM in gastrectomies with carcinoma
Table 1 shows that in the Atlantic Basin 15.0% (218 of 1444) of the gastrectomy specimens with carcinoma had EIM. EIM was found in 18.8% (153 of 814) of the gastrectomies with IC and in 10.3% (65 of 630) of those with DC. The difference was significant (p < 0.05).
Table 1.
The frequency of gastrectomies having EIM among 1444 specimens with carcinoma reviewed in the Atlantic basin
| City | EIM in IC | EIM in DC | Gastrectomy with EIM | Elderly patients (⩾60 years) with carcinoma | EIM in elderly patients (⩾60 years) with carcinoma |
| New York | 33/344 (9.6%) | 15/236 (6.4%) | 48/580 (8.3%) | IC: 284/344 (82.6%) | IC: 31/284 (10.9%) |
| DC: 180/236 (76.2%) | DC 13/180 (7.2%) | ||||
| London | 22/65 (33.8%) | 8/56 (14.3%) | 30/121 (24.8%) | IC: 52/65 (80.0%) | IC: 20/52 (38.5%) |
| DC: 38/56 (67.8%) | DC: 7/38 (18.4%) | ||||
| Reykjavik | 43/141 (30.5%) | 23/160 (14.4%) | 66/301 (21.9%) | IC: 120 /141 (85.1%) | IC: 48/120 (40.0%) |
| DC: 104/160 (65.0%) | DC: 20/104 (19.2%) | ||||
| Florence | 28/116 (24.1%) | 10/70 (14.3%) | 38/186 (20.4%) | IC: 103/116 (88.8%) | IC: 27/103 (26.2%) |
| DC: 43/70 (61.4%) | DC: 8/43 (18.6%) | ||||
| Mexico City | 6/25 (24.0%) | 0/3 (0%) | 6/28 (21.4%) | IC: 4/25 (16.0%) | IC: 1/4 (16.7%) |
| DC: 0/3 (0%) | DC: 0/0 (0%) | ||||
| Buenos Aires | 12/76 (15.8%) | 7/69 (10.1%) | 19/145 (13.1%) | IC: 40/76 (52.6%) | IC: 10/40 (25%) |
| DC: 25/69 (36.2%) | DC: 5/25 (20.0%) | ||||
| Stockholm | 9/47 (19.1%) | 2/36 (5.6%) | 11/83 (13.3%) | IC: 38/47 (80.5%) | IC: 8/38 (21.0%) |
| DC: 21/36 (58.3%) | DC: 2/21 (9.5%) | ||||
| Total | 153/814 (18.8%) | 65/630 (10.3%) | 218/1444 (15.0%) | IC: 641/814 (78.7%) | IC: 145/641 (22.6%) |
| DC: 411/630 (65.2%) | DC: 55/411 (13.4%) |
DC, diffuse carcinoma; EIM, extensive intestinal metaplasia; IC, intestinal carcinoma.
Table 2 shows that in the Pacific basin 50.3% (572 of 1136) of the gastrectomies with carcinoma had EIM. EIM was found in 62.9% (412 of 655) of the gastrectomies with IC and in 33.3% (160 of 481) of those with DC. The difference was significant (p < 0.05).
Table 2.
The frequency of gastrectomies having gastric EIM among 1136 specimens with carcinoma reviewed in the Pacific basin
| City | EIM in IC | EIM in DC | Gastrectomy with EIM | Elderly patients (⩾60 years) with carcinoma | EIM in elderly patients with carcinoma |
| Tokyo* | 143/209 (68.4%) | 76/184 (41.3%) | 219/393 (55.7%) | IC 126/204 (61.8%) | IC: 91/126 (72.2%) |
| DC: 96/176 (54.5%) | DC: 54/95 (56.8%) | ||||
| Matsuyama | 115/168 (68.4%) | 29/73 (39.7%) | 144/241 | IC: 101/168 (60.1%) | IC: 78/101 (77.2%) |
| DC: 46/73 (63.0%) | DC: 28/46 (60.8%) | ||||
| Honolulu | 56/95 (58.9%) | 18/48 (37.5%) | 74/143(32.9%) | IC: 57/95 (60.0%) | IC: 39/57 (68.4%) |
| DC: 24/48 (50.0%) | DC: 12/24 (50.0%) | ||||
| Auckland/Otahuhu | 29/74 (39.1%) | 18/75 (24.0%) | 47/149 (31.5%) | IC: 57/74 (77.0%) | IC: 26/57 (55.3%) |
| DC: 37/75 (49.3%) | DC: 16/37 (43.2%) | ||||
| Santiago | 51/64 (79.7%) | 9/45(20.0%) | 60/109 (55.0%) | IC: 34/64 (53.1) | IC: 29/34 (85.3%) |
| DC: 22/45 (48.9%) | DC: 8/22 (36.4%) | ||||
| Vancouver | 18/45 (40.0%) | 10/56 (17.9%) | 28/101 (27.7%) | IC: 38/45 (84.4%) | IC: 17/38 (44.7%) |
| DC: 39/56 (69.6%) | DC: 4/39 (10.3%) | ||||
| Total | 412/655 (62.9%) | 160/481 (33.3%) | 572/1136 (50.3%) | IC: 413/655 (63.0%) | IC: 280/413 (67.8%) |
| DC: 264/481 (54.9%) | DC: 122/264 (46.4%) |
*48 additional gastrectomy specimens12,13 were not included in a previous report.23
The number of specimens with EIM was significantly higher in the Pacific than in the Atlantic basin for both carcinoma phenotypes (p<0.05).
DC, diffuse carcinoma; EIM, extensive intestinal metaplasia; IC, intestinal carcinoma.
EIM in the elderly with carcinoma
Table 1 shows that in the Atlantic basin 19.1% (200 of 1052) of the gastrectomies among elderly patients (⩾ 60 years of age at surgery) had EIM. EIM was found in 22.6% (145 of 641) of the elderly with IC and in 13.4% (55 of 411) of those with DC.
Table 2 shows that in the Pacific basin 59.5% (402 of 676) of the gastrectomies among the elderly had EIM. EIM was present in 67.8% (280 of 413) of the elderly patients with IC and in 46.4% (122 of 263) of those with DC.
The numbers of specimens having EIM among the elderly was significantly higher in those with IC than those with DC in both basins (p ⩽ 0.05).
Cancer specimens without IM
No IM was seen in 25.0% (361 of 1444) of the specimens with carcinoma reviewed in the Atlantic basin and in 10.8% (123 of 1136) of the specimens with carcinoma reviewed in the Pacific basin. The difference was significant (p < 0.05).
Number of sections/gastrectomy in cases with carcinoma
In the Atlantic basin a total number of 15215 sections (mean, 10.5 sections/gastrectomy; range, 3–18) were reviewed in the 1444 specimens with gastric carcinoma. The highest mean number of sections/gastrectomy was recorded in Stockholm (31.2) and the lowest in Buenos Aires (2.4).
In the Pacific basin a total number of 23 881 sections (mean, 21.0 sections/gastrectomy; range, 9–67) were reviewed in the 1136 specimens with gastric carcinoma. The highest mean number of sections/gastrectomy was recorded in Tokyo and Matsuyama (43.0) and the lowest in Auckland/Otahuhu (8.9).
Significantly more sections were available at hospitals in the Pacific basin than in the Atlantic basin (p < 0.05).
Miscellaneous gastric diseases
EIM in gastrectomy specimens with miscellaneous diseases
The mean per cent of cases with EIM in miscellaneous gastric diseases in the Atlantic basin was 7.2% (36 of 502) and in the Pacific basin the figure was 10.6% (36 of 339). The difference between the two basins was not significant (p < 0.6).
Miscellaneous diseases without IM
In the Atlantic basin IM was not seen in 63.3% (318 of 502) of the specimens with miscellaneous gastric diseases. In the Pacific basin 32.7% (111 of 339) of the specimens showed no IM. Significantly more specimens with miscellaneous gastric diseases had no IM in the Atlantic than in the Pacific basin (p < 0.05).
Number of sections/gastrectomy in patients with miscellaneous diseases
In the 502 specimens with miscellaneous diseases collected in the Atlantic basin, 4237 sections (mean, 8.4 sections/gastrectomy; range, 3–14) were reviewed. In the 339 specimens with miscellaneous diseases seen in the Pacific basin, 4318 sections (mean, 12.9 sections/gastrectomy; range, 7–36) were reviewed.
Significantly more sections were reviewed in the Pacific than in the Atlantic basin (p < 0.05).
DISCUSSION
We found that the proportion of gastrectomies with carcinoma showing EIM was higher among populations with a higher gastric cancer risk (Japanese and Chileans in the Pacific basin) than in those with a lower cancer risk (North Americans, Mexicans, and Swedes in the Atlantic basin). The percentage of cases with EIM was significantly higher in specimens with IC than in those with DC in all cities investigated, indicating that EIM is mostly associated with the development of gastric carcinoma of that particular phenotype in both basins. Nonetheless, 13% of the specimens in elderly patients with DC in the Atlantic basin, in addition to 46% of elderly patients with DC in the Pacific basin, also had EIM. Thus, the possibility that EIM may also precede the development of a substantial number of gastric carcinomas of diffuse type (particularly in high cancer incidence areas) should be entertained. This possibility has not received much attention in the literature.
It should be stressed that in the Atlantic basin 25.0% of the gastrectomies with carcinoma had no IM and that 7.2% of the specimens with miscellaneous diseases showed EIM. As a comparison, 10.8% of the specimens with carcinoma in the Pacific basin had no IM, whereas 10.6% of gastrectomies with miscellaneous diseases displayed EIM. These results clearly indicate that the growing tumour does not elicit EIM, and that some carcinomas evolve in the absence of IM. Whether patients with miscellaneous gastric diseases having EIM are at future risk of developing gastric carcinoma could not be answered by our study.
To investigate the validity of the results obtained in patients with carcinoma, one important confounding factor was explored; namely, the number of sections obtained for each gastrectomy specimen. The mean number of sections obtained in specimens with carcinoma was higher in the Pacific basin (mean, 21.0 sections/specimen) than in the Atlantic basin (mean, 10.5). Consequently, it is possible that the higher number of sections obtained at some hospitals bordering the Pacific basin may have contributed to detecting a higher proportion of specimens with EIM. However, the higher number of sections in specimens from cities with a high gastric cancer incidence cannot totally explain the differences found, because a high proportion of specimens with EIM was found in cities with a relatively low number of sections/specimen (table 2). Environmental factors could be an explanation. In this respect, it has been postulated that environmental carcinogens encourage the development of gastric carcinoma.2,3,29–32 Hot food and/or hot fluids, bacteria, or viruses could induce chronic inflammatory changes in susceptible individuals and subsequently IM, thus rendering the gastric mucosa more vulnerable to environmental carcinogens. It is not unconceivable that environmental carcinogens might alter the gastric mucosa and contribute to the development of EIM before cancer ensues. Because gastric carcinoma is more frequent in the Pacific basin, it is possible that environmental carcinogens are either more powerful in that area, or that for unknown reasons the gastric mucosa of their inhabitants is more vulnerable to carcinogens. Ethnicity appears not to influence the presence of EIM; specimens from populations of different ethnicity dwelling in the same oceanic basin, such as Chileans and Japanese had the highest frequency of EIM in the entire survey.
Some authors have reported that IM is found in biopsies taken exclusively from endoscopically abnormal areas,33 whereas other authors recommend harvesting gastric biopsies from pre-established mucosal sites.34 In this respect, the Sydney system35 for the grading of gastritis has provided practical guidelines for optimal biopsy sampling of the gastric mucosa. However, using the Sydney system’s recommendations, El-Zimaity and Graham9 found that IM was missed in more than 50% of biopsies from “Sydney” sites in patients with confirmed gastric IM on multiple site sampling. They showed that the detection of IM increased from 48% to 75% when the biopsy sites were changed from the Sydney system.9 In addition, they concluded that the minimum number of biopsies needed to identify IM should probably be eight, and emphasised that current and future studies using the Sydney system as a basis for detecting gastric IM are probably not reliable.9 More recently Khakoo and colleagues36 and Dursun and colleagues37 also found that the endoscopic division of the Sydney classification was unable to clarify the reporting of gastritis and IM. From the above, it appears that sampling gastric biopsies from endoscopically abnormal areas,33,38 or from pre-established mucosal sites,34 may be insufficient for calculating the prevalence of IM and estimating the possible cancer risk of IM in long follow up studies.39
The conflicting results may partly be explained by confounding factors, such as the focal distribution of IM in many cases, and by the difficulty in diagnosing IM at gross examination, as shown by Stemmermann and Hayashi10 and by Kumagae et al.11 Another confounding factor not mentioned in the literature is the difference in proficiency among endoscopists in various countries in detecting gastric IM. Because endoscopists are instrumental in providing the material for histological evaluation, it is conceivable that the conflicting findings at histology could partly be explained by differences in the skill of endoscopists in detecting IM areas. Moreover, the protocols received at the pathology department are often incomplete, providing no information on whether the biopsies were taken from a single “spot”, from one of many spots, or from one or several extensive mucosal areas. The difficulty in calculating the risk of IM in biopsy specimens can be exemplified as follows: patients showing at histology either a minimal spot of IM (with or without histochemically abnormal mucins7,8) or with extensive IM areas will be classified as having IM. The question arises: which patients are at risk of developing carcinoma; those with a single, minimal spot of IM, perhaps with histochemically abnormal mucins, or those with extensive IM? At this stage, it should be pointed out that recently the importance of the histochemical constituents of the mucin in IM in gastric biopsies7,8 has repeatedly been challenged.39–41 Hence, the limitations of gastric biopsies in assessing: (1) the extension of gastric IM and (2) the possible cancer risk in long term follow up studies have been emphasised in our study.
Therefore, we planned our present investigation with an awareness of the limitations of gastric biopsies in assessing the magnitude of IM in the gastric mucosa in individual patients. For that purpose, gastrectomy specimens with carcinoma and with miscellaneous diseases (control cases) were investigated. We soon realised that the method had several pitfalls because the size of the resected specimens varied with the topographical location of the tumour, with the size of the tumour removed, and with the resection technique used by individual surgeons within the same hospital, between different hospitals, and between different countries. In addition, the number of blocks taken from the resected specimens by pathologists at various hospitals also varied. Nevertheless, despite these limitations, the method described herein enabled us to compare the prevalance of gastric IM in populations dwelling in disparate geographical regions at the rim of the Atlantic and the Pacific basins.
It should be mentioned that this work was initiated in 1981—before the discovery of H pylori by Warren and Marshall.42 In our subsequent studies, we did not investigate H pylori, mainly because H pylori specific stains were not performed in the hospitals contributing to this survey. In addition, the organism is known to be absent in cases with IM for a variety of reasons, namely: alterations in the pH of the mucosa (from neutral mucin to acid mucins secreted by goblet cells), mucosal atrophy, and mucosal hypoacidity (often found in patients with gastric cancer). Another factor known to influence the presence of H pylori after resection is the flushing of the specimen with saline, followed (in the past) by the intense light exposure required for photographic documentation. This leads to autolytic necrosis (as a result of a heating and drying effect) of the superficial cell layer of the mucosa, where H pylori usually resides.
“The differences in the prevalence of extensive intestinal metaplasia in gastrectomies appear to be unrelated to ethnicity but linked to the patient’s geographical habitat”
Shousha and colleagues43 found a significantly higher prevalence of IM in gastric biopsies from British patients than in those from Yemeni patients, despite the fact that the Yemeni patients had a significantly higher prevalence of H pylori. Our own studies of gastric biopsies from elderly Swedish patients with H pylori infection44 showed only occasional foci of IM. It has been shown that a particular combination of bacterial and host genotypes is required to define high cancer risk in H pylori infected individuals.45 More recent developments have shown that there are ethnic differences in H pylori induced gastritis between Asian and Western populations.46 Working with a transgenic mouse system we recently found that gastric tumours concurred with antral IM.47 Apparently, particular alterations in the genome in transgenic mice are able to induce gastric IM without the participation of H pylori.48 Thus, the relation between H pylori and the development of IM has not yet been fully clarified. The mere presence of H pylori is not sufficient to infer that these bacteria are responsible for gastric carcinogenesis. In retrospect, and based on the aforementioned pitfalls in the handling and processing of resected specimens, and on more recent knowledge, searching for H pylori in our survey would probably have led to questionable results and to unreliable suppositions.
In the Pacific basin, a similarly high prevalence of EIM was found in gastrectomies from Japanese and Chilean individuals (who have different ethnicities). In the Atlantic basin, populations such as those of London and Florence had a similar prevalence of cases with EIM to that of Iceland (a population with a purer ethnicity). However, these populations in the Atlantic basin had a much lower percentage of cases with EIM than was seen in Japan or in Chile in the Pacific basin. Interestingly, the proportion of gastrectomies with EIM was significantly higher in Vancouver than in New York and in Santiago de Chile than in Buenos Aires, despite the fact that these populations reside at approximately the same geographical latitude, but in different basins. Hence, the differences appear to be unrelated to ethnicity but linked to the patient’s geographical habitat.
Take home messages.
The following differences in the occurrence of gastric intestinal metaplasia (IM) were found in our survey between dwellers of the Pacific and the Atlantic basins
The proportion of gastrectomies with extensive IM (EIM) was significantly higher in the Pacific than in the Atlantic basin, despite the fact that patients undergoing surgery for gastric carcinoma were older in the Atlantic than in the Pacific basin
The proportion of specimens with EIM among the elderly was significantly higher in those with intestinal carcinoma (IC) than in those with diffuse carcinoma (DC)
EIM in the Pacific basin was more widely spread in the gastric mucosa from individual specimens with IC than those with DC
The proportion of gastrectomies with EIM in Vancouver was higher than in New York and in Santiago de Chile than in Buenos Aires, despite the fact that those populations reside at approximately the same geographical latitude, but in different basins
IM was totally absent in nearly 22% of specimens with carcinoma, but it was present in 11% of specimens with miscellaneous gastric diseases, strongly suggesting that EIM is not initiated by a synchronously growing carcinoma
The Japanese (the population with the highest incidence of gastric cancer) had atypical mitoses in areas with EIM far from the tumour. This important histological parameter has been ignored in discussions aimed at disclosing the gastric cancer risk of IM. It remains to be elucidated whether atypical mitoses are one of the alterations required for the evolution of EIM towards gastric dysplasia and carcinoma in populations at risk
A pertinent question would be: does EIM per se increase the gastric cancer risk or are other parameters also involved? During the course of our work, we noticed atypical mitoses in gastrectomies from Japanese patients.49,50 These were found in areas with EIM far from the carcinoma. Because atypical mitoses reflect mutated cells, it appears that cellular mutation(s) are important in the evolution of EIM towards dysplasia/carcinoma in the Japanese. Those speculations fit well with the hypothesis of gastric carcinogenesis proposed by Correa,51 who suggested that mutagenic carcinogens in the microenvironment trigger the sequence of cellular mutations leading to gastric carcinoma.
Acknowledgments
This study was supported by grants from the Yamagiwa-Yoshida Foundation, Japan; the Japan Society for the Promotion of Science; the Swedish Academy of Sciences; the Swedish Society of Medicine; the Cancer Society, Stockholm; and the Karolinska Institute and Hospital, Stockholm, Sweden. We thank Professor J Rosai, Department of Pathology, Memorial Hospital, New York, USA; Professor D Antognoli, Harvard University, Boston, Massachusetts, USA; Professor J Jass, Department of Pathology, Auckland University, Auckland, New Zealand; Professor G Zampi, Dipartimento di Patologia Umana ed Oncologia, Universitá Degli Study Di Firenze, Florence, Italy; Dr I Filipe, Department of Pathology, Guy’s Hospital, London, UK; Dr G Stemmermann, Department of Pathology, Kuakini General Hospital, Honolulu, Hawaii, USA; Dr T Hirota, Department of Pathology, National Cancer Institute, Tokyo, Japan; Dr T Kitagawa, Cancer Institute, Tokyo, Japan; Dr Y Kato, Department of Pathology, Cancer Institute, Tokyo, Japan; Drs PA de Ruiz and J Jesssurum, Hospital General de Méjico, Mexico City, Mexico; and Dr R Hojman, Centro de Gastroenterologia, Buenos Aires, Argentina for allowing the review of sections from the gastrectomy specimens processed at their respective departments.
Abbreviations
DC, diffuse carcinoma
EIM, extensive intestinal metaplasia
H&E, haematoxylin and eosin
IC, intestinal carcinoma
IM, intestinal metaplasia
REFERENCES
- 1.Parkin D, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer 1993;54:594–606. [DOI] [PubMed] [Google Scholar]
- 2.Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol 1996;23:281–91. [PubMed] [Google Scholar]
- 3.Harrison LE, Zhang ZF, Karpeh M, et al. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma. Cancer 1997;80:1021–8. [PubMed] [Google Scholar]
- 4.Correa P, Fox J, Fontham E, et al. Helicobacter pylori and gastric carcinoma. Cancer 1990;66:2569–74. [DOI] [PubMed] [Google Scholar]
- 5.Genta R, Rugge M. Gastric precancerous lesions: heading for an international consensus. Gut 1999;45 (suppl 1) :15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruta F, Ota R, Kenta M, et al. Role of N-methyl-N-nitrosourea in the induction of intestinal metaplasia and gastric adenocarcinoma in Mongolian gerbils infected with Helicobacter pylori. Scand J Gastroenterol 2001;36:283–90. [DOI] [PubMed] [Google Scholar]
- 7.Filipe I, Barbatis C, Sandley A, et al. Expression of intestinal mucin antigens in the gastric epithelium and its relationship with malignancy. Hum Pathol 1988;19:19–26. [DOI] [PubMed] [Google Scholar]
- 8.Jass J, Filipe M. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology 1979;3:191–9. [DOI] [PubMed] [Google Scholar]
- 9.El-Zimaity H, Graham D. Evaluation of gastric biopsy site and number for identification of H pylori or intestinal metaplasia: role of the Sydney system. Hum Pathol 1999;30:72–7. [DOI] [PubMed] [Google Scholar]
- 10.Stemmermann G, Hayashi T. Intestinal metaplasia of the gastric mucosa: a gross and microscopic study of its distribution in various disease states. J Natl Cancer Inst 1968;41:627–34. [PubMed] [Google Scholar]
- 11.Kumagae Y. Enzyme histochemical studies of intestinal metaplasia in the human stomach, with special reference to electron microscopy. Jpn Soc Digest Syst 1979;30:17–30. [PubMed] [Google Scholar]
- 12.Rubio CA, Kato Y, Sugano H, et al. Intestinal metaplasia of the stomach. I-Quantitative analysis in gastric peptic ulcer and in incipient adenocarcinoma in Japanese patients. Anticancer Res 1985;5:435–40. [PubMed] [Google Scholar]
- 13.Rubio CA, Hirota T, Itabashi M, et al. Quantitation of gastric intestinal metaplasia by morphometry in Japanese patients. Jpn J Cancer Res 1992;83:495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubio CA, Porwit A, Rodensjö M. Method to quantitate intestinal metaplasia of stomach by image analysis. J Clin Pathol 1988;41:799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio CA, Saraga E, Lindhom J. Improved method for mapping gastric intestinal metaplasia using selective histochemical morphometry. Anal Quant Cytol Histol 1990;12:122–6. [PubMed] [Google Scholar]
- 16.Rivera F, Rubio CA. Quantitative studies of the extension of gastric intestinal metaplasia in gastrectomy specimens from Swedish patients. Eur J Gastroenterol Hepatol 1993;5:521–5. [Google Scholar]
- 17.Rubio CA, Matthies M, Itabashi M, et al. Image quantitation of intestinal metaplasia in entire gastrectomy specimens from Swedish and Japanese patients. Jpn J Cancer Res 1996;87:662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubio CA, Jass JR, King A. Gastric cell phenotypes and intestinal metaplasia in Polynesian and non-Polynesian residents of New Zealand. J Environ Pathol Toxicol Oncol 1994;13:243–9. [PubMed] [Google Scholar]
- 19.Rubio CA, Jonasson JG, Filipe I, et al. Gastric carcinomas of intestinal type concur with distant changes in the gastric mucosa. A multicenter study in the Atlantic basin. Anticancer Res 2001;21:813–18. [PubMed] [Google Scholar]
- 20.Rubio CA, Kato Y, Yanagisawa A, et al. Cardia carcinomas of intestinal type are associated with histologic changes in the gastric mucosa. Gastric Cancer 1999;2:215–20. [DOI] [PubMed] [Google Scholar]
- 21.Rubio CA, Pisano R, Llorens P, et al. A comparative study between the gastric mucosa of Chileans and other dwellers of the Pacific basin. Jpn J Cancer Res 1996;87:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio CA, Owen DA. Comparative study between the gastric mucosa of Western Canadians and other dwellers of the Pacific basin. Anticancer Res 1998;18:2463–70. [PubMed] [Google Scholar]
- 23.Rubio CA, Hirota T, Itabashi M, et al. Extended intestinal metaplasia. A survey of 1392 gastrectomies from dwellers of the Pacific basin. Anticancer Res 2004;24:3185–92. [PubMed] [Google Scholar]
- 24.Pellicano R, Fagoonee S, Palestro G, et al. Intestinal metaplasia, dysplasia, gastric cancer and Helicobacter pylori: epidemiological observations. Minerva Med 2005;96:1–10. [PubMed] [Google Scholar]
- 25.Faller G, Kirchner T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch 2005;446:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka T, Tsukamoto T, Mizoshita T, et al. Coexistence of gastric- and intestinal-type endocrine cells in gastric and intestinal mixed intestinal metaplasia of the human stomach. Pathol Int 2005;55:170–9. [DOI] [PubMed] [Google Scholar]
- 27.Leung WK, Sung JJ. Related pathogenesis of pre-neoplastic lesions of the stomach: targets for prevention. Dig Dis 2004;22:306–12. [DOI] [PubMed] [Google Scholar]
- 28.Ming S-C. Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer 1998;1:31–50. [DOI] [PubMed] [Google Scholar]
- 29.Muñoz N, Correa P, Cuello C, et al. Histological types of gastric carcinoma in high- and low-risk areas. Int J Cancer 1971;8:809–18. [DOI] [PubMed] [Google Scholar]
- 30.Laurén P, Nevalainen T. Epidemiology of intestinal and diffuse types of gastric carcinoma. A time trend study in Finland with comparison between studies from high- and low-risk areas. Cancer 1993;71:2926–33. [DOI] [PubMed] [Google Scholar]
- 31.Weisburger JH. Can cancer risks be altered by changing nutritional traditions? Cancer 1998;83:1278–81. [DOI] [PubMed] [Google Scholar]
- 32.Mirvish S. The etiology of gastric cancer. Intragastric nitrosamide formation and other theories. J Natl Cancer Inst 1983;71:631–47. [PubMed] [Google Scholar]
- 33.Whiting J, Sigurdsson A, Rowlands D, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 2002;50:378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misiewicz J, Tygat G, Goodwin C, et al. The Sydney system: a new classification of gastritis. Working Party Reports 1990:1–10.
- 35.Dixon M, Genta R, Yardley J, et al. Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 36.Khakoo S, Lobo A, Shepherd N, et al. Histological assessment of the Sydney classification of endoscopic gastritis. Gut 1994;35:1172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dursun M, Yilmaz S, Yukselen V, et al. Evaluation of optimal gastric mucosal biopsy site and number for identification of Helicobacter pylori, gastric atrophy and intestinal metaplasia. Hepatogastroenterology 2004;51:1732–5. [PubMed] [Google Scholar]
- 38.Rubio CA, Befrits R. Gastric intestinal metaplasia. J Clin Pathol 2004;57:894–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rameshar K, Danders D, Hopwwood D. Limited value of type III intestinal metaplasia in predicting risk of gastric carcinoma. J Clin Pathol 1987;40:1287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ectors N, Dixon M. The prognostic value of sulphomucin positive intestinal metaplasia in the development of gastric cancer. Histopathology 1986;10:1271–7. [DOI] [PubMed] [Google Scholar]
- 41.Petersson F, Borch K, Franzén E. Prevalence of subtypes of intestinal metaplasia in the general population and in patients with autoimmune chronic atrophic gastritis. Scand J Gastroenterol 2002;37:262–6. [DOI] [PubMed] [Google Scholar]
- 42.Warren J, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983;1:1273–5. [PubMed] [Google Scholar]
- 43.Shousha S, el-Sherif A, el-Guneid A, et al. Helicobacter and intestinal metaplasia: comparison between British and Yemeni patients. Am J Gastroenterol 1993;88:1373–6. [PubMed] [Google Scholar]
- 44.Befrits R, Granström M, Rylander R, et al. Helicobacter pylori in 205 consecutive endoscopy patients. Scand J Infect Dis 1993;25:185–91. [DOI] [PubMed] [Google Scholar]
- 45.Figuereiredo C, Machado J, Pharoah P, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst 2002;94:1680–7. [DOI] [PubMed] [Google Scholar]
- 46.Lee I, Lee H, Kim M, et al. Ethnic difference of Helicobacter pylori gastritis: Korean and Japanese gastritis is characterized by male- and antrum-predominant acute foveolitis in comparison with American gastritis. World J Gastroenterol 2005;11:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson P, McGuire J, Rubio CA, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci U S A 2002;88:9990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubio CA, Andersson P, Hanberg A. Gastric intestinal metaplasia in transgenic mice. Gastroenterology 2003;125:996–7. [DOI] [PubMed] [Google Scholar]
- 49.Rubio CA, Kato Y, Kitagawa T. Atypical mitosis in gastric intestinal metaplasia in Japanese patients. Jpn J Cancer Res 1993;84:493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubio CA, Kato Y, Kitagawa T. Frequency of atypical mitosis in the intestinal metaplasia of the gastric mucosa in Japanese patients. Jpn J Cancer Res 1994;85:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:1319–26. [PubMed] [Google Scholar]


