Abstract
Aims: To evaluate the potential use of the immunohistochemical expression of telomerase and the measurement of its activity as diagnostic tools in the uterine cervix.
Methods: The fluorescent telomeric repeat amplification protocol (TRAP) assay was used to evaluate telomerase activity in a series of 43 cervical scrapings. Twenty five cases were cytologically classified as inflammatory, and/or metaplastic, and/or acanthotic, and 18 cases presented cell alterations compatible with mild, moderate, or severe cervical intraepithelial neoplasia (CIN). Immunohistochemistry was performed on a retrospective series of 86 archival, paraffin wax embedded blocks using a recently developed anti-hTERT (human telomerase reverse transcriptase) monoclonal antibody.
Results: Telomerase activity was expressed as arbitrary enzymatic units (AEU). Median values were 38.0 AEU for inflammatory non-dysplastic cell specimens, 33.5 AEU for CIN I, 41.0 AEU for CIN II, and 28.0 AEU for CIN III. The median percentage of immunoreactive dysplastic cells, as detected by immunohistochemistry, was significantly (p = 0.024) lower in CIN I (45%) than in more severe dysplastic (CIN II 70%, CIN III 80%) lesions. In contrast, no differences were seen in the enzymatic activity detected by the TRAP assay among the different dysplastic lesions.
Conclusions: These data indicate that, using a molecular extra situ method, the telomerase activity of inflammatory and non-dysplastic elements masks the expected differences between mild and severe dysplasia. Conversely, an in situ approach permits the accurate identification of telomerase positive dysplastic cells.
Keywords: cervical dysplasia, immunohistochemistry, telomerase, telomeric repeat amplification protocol assay
Cervical cancer, which in most cases has been shown to develop from the benign precursor, cervical intraepithelial neoplasia (CIN), is a cause of death in women worldwide,1 and the second leading cause of cancer death after breast cancer for women aged between 20 and 39 years.2
“The role of telomerase in precancerous lesions and its relation with disease progression remain to be defined”
The clinical evolution of this disease depends on the degree of cell alteration. Specifically, most low grade/CIN I lesions will regress, whereas in the longterm, 12–40% of high grade/CIN II–III lesions progress to squamous cell carcinoma.3,4
Results from molecular analyses confirm that human papillomavirus (HPV) is the major risk factor for cervical cancer.5 Although HPV DNA transcripts or protein products have been identified in more than 95% of cervical carcinomas,1,6,7 HPV infection alone is thought to be insufficient for the development of invasive carcinoma.
Cervical cytology remains the most effective screening test since its introduction by Dr George Papanicolau in the 1930s. Studies from the Nordic countries (World Health Organisation 2003) have shown that well organised cytology screening programmes reduce mortality from cervical cancer by approximately 60%.
Criteria for the diagnosis of CIN vary somewhat among pathologists, and the degree of intraepithelial neoplasia is essentially determined on the basis of mitotic activity, immature cell proliferation, and nuclear atypia.
Telomerase has been shown to be reactivated in premalignant lesions and in different tumour types,8 and some studies have shown that telomerase activity is higher in tumour tissue compared with normal tissue,9,10 but its role in precancerous lesions and its relation with disease progression remain to be defined.
Telomerase consists of three subunits: an RNA component, which acts as a template for DNA replication,11 a telomerase associated protein12 of unknown function, and the human telomerase reverse transcriptase (hTERT), which is responsible for its catalytic activity.13,14
The aim of our present study was to analyse the relation between the degree of morphological alteration and telomerase activity and expression. Enzymatic activity was evaluated by a quantitative fluorescent telomeric repeat amplification assay and by a newly developed anti-hTERT antibody.
PATIENTS AND METHODS
Case series
Because we expected that most prospectively collected scraping samples would have a cytological referral of inflammatory and/or non-pathological cervical cells, and would therefore not be followed by removal of bioptic material for histological diagnosis, a retrospective independent series of paraffin wax embedded histologically assessed dysplastic lesions was used for the immunohistochemical analysis.
The investigations were performed after approval by a local ethical committee and all patients gave their informed written consent. Cervical scrapings were collected consecutively from 43 women presenting for cervical screening. Part of this material was sent to the cytology laboratory for evaluation and the remainder was placed in test tubes containing phosphate buffered saline (PBS) and sent to the molecular biology laboratory, where it was immediately spun down and stored at −80°C until it was used for telomeric repeat amplification protocol (TRAP) analysis.
Cytological features were classified according to the 1991 Bethesda system as normal/benign reactive changes, squamous cell abnormalities, and glandular cell abnormalities.15 The immunohistochemical analysis was carried out on a retrospective series of 86 dysplastic biopsies fixed in 10% formalin and embedded in paraffin wax. There were 27 CIN I, 32 CIN II, and 27 CIN III lesions.
TRAP assay
We used a modified version16,17 of the method described previously.9,18
Telomerase products were evaluated on fluorescence electropherograms and the area underlying the different peaks was calculated.
Protein concentrations corresponding to 10, 30, 100, 300, 1000, and 3000 cells of a human bladder cancer line (MCR)16,17 were analysed in each assay and an internal standard was used. To obtain quantitative evaluations, the areas of each sample were also normalised to the internal telomerase assay standard peak. Telomerase activity was expressed in arbitrary enzymatic units (AEU) as a continuous variable in all the analyses. Two successive determinations were carried out for each sample and when variations were greater than 15%, as seen in about 10% of cases, a third analysis was performed.
Immunohistochemical assay
Paraffin wax embedded sections (4 μm thick) were mounted on positive charged slides (Bio Optica, Milan, Italy), dewaxed in xylene, rehydrated twice in 100% alcohol for two minutes, in 90% alcohol for one minute, and rinsed in 70% alcohol for one minute. Endogenous peroxidase was quenched in 3% H2O2 for 10 minutes. Antigen retrieval was carried out by incubating the sections in citrate buffer (pH 6) at 98.5°C for 45 minutes. After washing for five minutes in PBS, non-specific sites were blocked in 1% bovine serum albumin in PBS for 20 minutes.
A new monoclonal antibody obtained by immunisation with a fragment of cDNA and directed against the hTERT polypeptide sequence of the telomerase enzyme (tel 3 36-10; DIESSE Diagnostica Senese, Siena, Italy; distributed by Alexis Corporation, Lausanne, Switzerland) was used. The clone was purified by chromatography on Sepharose–protein G.
The monoclonal antibody was diluted to a final concentration of 40 μg/ml using background reducing components (Dako Corporation, Carpinteria, California, USA) and placed in contact with tissue for one hour at room temperature.
The slides were washed twice in PBS-Tween (0.05%), incubated for 30 minutes with antirat biotinylated secondary antibody (Dako Corporation), diluted 1/300, washed again in PBS-Tween (0.05%), and incubated in streptavidin–peroxidase conjugate (LSAB+ kit; Dako Corporation) for 15 minutes. The final enzymatic reaction was developed to a brown stain with diaminobenzidine/hydrogen peroxidase chromogen solution (DAB+, liquid substrate–chromogen solution; Dako Corporation) for five minutes.
Sections were rinsed in deionised water, cell nuclei were counterstained blue by haematoxylin, and the slides were mounted in Eukitt (Bio Optica).
Paraffin wax embedded MCF-7 human breast cancer cells were used as positive controls and differentiated striated muscle tissue served as negative controls.
hTERT expression was determined semiquantatively as the percentage of immunopositive epithelial dysplastic cells within the total dysplastic area. Cells were considered positive in the presence of brown cytoplasmic and/or nuclear staining.
STATISTICAL ANALYSIS
The χ2 test was performed for each CIN subgroup and was used to compare telomerase activity in the presence or absence of koilocytosis. Only p values less than 0.05 were considered significant.
RESULTS
Telomerase activity by TRAP
Of the 43 cervical scrapings submitted to TRAP assay, 25 were cytologically classified as containing inflammatory and/or metaplastic and/or acanthotic cells only (non-dysplastic cells), whereas in six, seven, and five specimens, cytological features suggestive of mild, moderate, and severe dysplastic alterations were found, respectively. The median value of telomerase activity was similar for the three different CIN subgroups (33.5, 41.0, and 28.0 AEU for CIN I, II, and III, respectively), and not significantly different from that seen in non-dysplastic samples (38.0 AEU).
Wider ranges were seen in samples with inflammatory elements and narrower and almost overlapping ranges (0–65 AEU) were found in samples presenting mild, moderate, and severe dysplastic cell alterations. No association was found with the presence of koilocytosis (data not shown).
Telomerase expression by immunohistochemistry
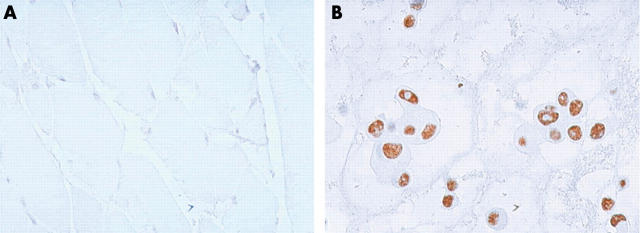
MCF-7 cells (positive control) were always immunopositive. Conversely, differentiated striated muscle tissue (negative control) was always negative for telomerase expression (fig 1).
Figure 1.
hTERT (human telomerase reverse transcriptase) immunohistochemistry (original magnification, ×400). (A) Striated muscle tissue (negative control), (B) MCF-7 cell line (positive control).
hTERT was expressed in the epithelial basal layers of both normal cervical tissue and dysplastic lesions (fig 2). Morphological and immunocytochemical analyses showed that inflammatory cells, when present, were always positive, and dysplastic cells seen throughout the entire thickness of the intraepithelial lesions were almost always strongly positive. Nuclear staining was seen in almost all the cells but was also frequently associated with cytoplasmic immunoreactivity.
Figure 2.
(A) Normal cervical epithelium with hTERT (human telomerase reverse transcriptase) immunopositivity in the basal cell layer and inflammatory elements (original magnification, ×400). (B) Cervical intraepithelial neoplasia I (CIN I) lesion: immunopositivity was also present in more superficial cell layers (original magnification, ×400). (C) CIN III lesion: immunopositivity was diffusely present in dysplastic cells throughout the whole thickness of the cervical epithelium (original magnification, ×400). (D) CIN III lesion with koilocytosis (original magnification, ×400).
The median percentage of immunoreactive cells was significantly (p = 0.024) lower in mild (45%) than in moderate (70%) or severe (80%) dysplastic lesions, even though the ranges of values overlapped. Moreover, the median percentage of immunoreactive cells in the presence of koilocytosis was double that seen in the absence of koilocytosis in CIN I, but not in CIN II–III lesions (table 1).
Table 1.
Immunohistochemical evaluation of human telomerase reverse transcriptase
| Overall series | Without koilocytosis | With koilocytosis | ||||
| No. of cases | % Positive cells Median (range) | No. of cases | % Positive cells Median (range) | No. of cases | % Positive cells Median (range) | |
| CIN I | 27 | 45 (0–95) | 10 | 30 (0–80) | 17 | 60 (0–95) |
| CIN II | 32 | 70 (0–95) | 8 | 75 (0–90) | 24 | 70 (5–95) |
| CIN III | 27 | 80 (0–95) | 10 | 87 (0–95) | 17 | 75 (20–95) |
CIN, cervical intraepithelial neoplasia.
DISCUSSION
The identification of new tumour specific markers is one of the most important goals for the non-invasive early detection of cancer. With regard to the uterine cervix, for the diagnosis of invasive and pre-invasive lesions, cytology is considered the gold standard, but investigations have been carried out to assess the usefulness of some molecular markers in identifying such lesions. In particular, previous reports suggested that telomerase reactivation may be involved in cancer development, and it therefore seemed important to clarify the role of telomerase expression in cervical dysplastic lesions.
Telomerase activation and arrest of telomere shortening appear to be involved with HPV infection, HPV associated immortalisation, and carcinogenesis of the uterine cervix.
“An important limitation of the TRAP assay is the rate of potential false positive results as a result of the presence of non-dysplastic telomerase positive elements, such as inflammatory, acanthotic, and metaplastic cells”
In the present study, we found that telomerase activity, detected by TRAP on smear specimens, was similar in normal cell epithelium and different dysplastic lesions. Our results do not agree with those obtained by other authors on fresh or frozen histologically confirmed dysplastic tissue,19–22 whereas they do agree with those obtained on cervical scrapings.23–25
However, an important limitation of the TRAP assay is the rate of potential false positive results as a result of the presence of non-dysplastic telomerase positive elements, such as inflammatory, acanthotic, and metaplastic cells, which may affect the information on the enzymatic activity of dysplastic cells. The use of a new monoclonal antibody developed in our laboratories for the immunohistochemical detection of in situ telomerase expressing cells showed, as expected, higher telomerase expression in CIN II and III than in CIN I lesions. This difference was independent of the presence of koilocytosis.
It is noteworthy that hTERT immunoreactivity was seen not only in the nucleus, but also in the cytoplasm of dysplastic cells. This finding, also reported by others,26 is somewhat unexpected, but has been hypothetically explained.26,27
Based on our results, the TRAP assay does not seem to be suitable for determining telomerase activity in dysplastic cervical scrapings, whereas an in situ approach using a monoclonal antibody capable of accurately attributing positivity to different cells appears to be a specific and more accurate method for evaluating differences in telomerase expression. Whether or not telomerase is activated by HPV at an early or late stage of the dysplastic process remains unclear, but we found no relation between HPV infection, demonstrated by the presence of koilocytosis, and telomerase expression.
Take home messages.
Using the telomeric repeat amplification protocol assay, the telomerase activity of inflammatory and non-dysplastic elements masks the expected differences between mild and severe cervical dysplasia, whereas in situ immunohistochemistry permitted the accurate identification of telomerase positive dysplastic cells
Telomerase activity was already present in mild dysplastic lesions, so that telomerase analysis does not help to increase the already high accuracy of cytological examination
In conclusion, we found that telomerase expression (detected by immunohistochemistry) and enzymatic activity (determined by the TRAP assay) were already present in mild dysplastic lesions. Thus, it could be hypothesised that telomerase reactivation parallels the dysplastic process from its earliest manifestation. For this reason, telomerase analysis does not help to increase the already high accuracy of cytological examination, as also recently reported by others.28,29 It remains to be determined whether the fraction of telomerase expressing dysplastic cells is related to the evolution of dysplastic lesions and to a greater chance of progression towards an invasive phenotype. This aspect needs to be verified in follow up studies.
Acknowledgments
We thank Professor R Silvestrini for her invaluable scientific contribution, A Lega for technical assistance, and G Tierney for editing the manuscript. This work was supported by Istituto Oncologico Romagnolo, Forlì, Italy and by Consiglio Nazionale della Ricerca/Ministero della Istruzione, Università e Ricerca (CNR/MIUR-Progetto Strategico ‘Oncologia’), grant numbers CU03.00393 and 03.00073.ST97, Rome, Italy.
Abbreviations
AEU, arbitrary enzymatic units
CIN, cervical intraepithelial neoplasia
HPV, human papillomavirus
hTERT, human telomerase reverse transcriptase
PBS, phosphate buffered saline
TRAP, telomeric repeat amplification protocol
REFERENCES
- 1.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide prospective. International biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst 1995;87:796–802. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin 1999;49:8–31. [DOI] [PubMed] [Google Scholar]
- 3.Pinto AP, Crum CP. Natural history of cervical neoplasia: defining progression and its consequence. Clin Obstet Gynecol 2000;43:352–62. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell MF, Tortolero-Luna G, Wright T, et al. Cervical human papillomavirus infection and intraepithelial neoplasia: a review. J Natl Cancer Inst Monogr 1996;21:17–25. [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342–50. [DOI] [PubMed] [Google Scholar]
- 6.Arends MJ, Buckey CH, Wells M. Aetiology, pathogenesis, and pathology of cervical neoplasia. J Clin Pathol 1998;51:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sano T, Oyama T, Kashiwabara K, et al. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 1998;153:1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takakura M, Kyo S, Kanaya T, et al. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res 1998;58:1558–61. [PubMed] [Google Scholar]
- 9.Kim NW, Piatyszek MA, Rainey WE, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011–15. [DOI] [PubMed] [Google Scholar]
- 10.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer 1997;33:787–91. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Funk WD, Wang SS, et al. The RNA component of human telomerase. Science 1995;269:1236–41. [DOI] [PubMed] [Google Scholar]
- 12.Harrington L, McPhail T, Mar V, et al. A mammalian telomerase-associated protein. Science 1997;275:973–7. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura TM, Morin GB, Chapman KB, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997;277:955–9. [DOI] [PubMed] [Google Scholar]
- 14.Meyerson M, Counter CM, Eaton EN, et al. hEST2, the putative human telomerase catalytic subunit gene, is upregulated in tumor cells and during immortalization. Cell 1997;90:785–95. [DOI] [PubMed] [Google Scholar]
- 15.Kurman RJ, Solomon D. The Bethesda system for reporting cervical/vaginal cytologic diagnoses: definitions, criteria, and explanatory notes for terminology and specimen adequacy. New York: Springer-Verlag, 1994.
- 16.Fedriga R, Gunelli R, Nanni O, et al. Telomerase activity detected by quantitative assay in bladder carcinoma and exfoliated cells in urine. Neoplasia 2001;3:446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchini MA, Bravaccini S, Medri L, et al. Urine telomerase: an important marker in the diagnosis of bladder cancer. Neoplasia 2004;6:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res 1995;23:3794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyo S, Takakura M, Tanaka M, et al. Telomerase activity in cervical cancer is quantitatively distinct from that in its precursor lesions. Int J Cancer 1998;79:66–70. [DOI] [PubMed] [Google Scholar]
- 20.Pao CC, Tseng CJ, Lin CY, et al. Differential expression of telomerase activity in human cervical cancer and cervical intraepithelial neoplasia lesions. Clin Oncol 1997;15:1932–7. [DOI] [PubMed] [Google Scholar]
- 21.Snijders PJ, van Duin M, Walboomers JM, et al. Telomerase activity exclusively in cervical carcinomas and a subset of cervical intraepithelial neoplasia grade III lesions: strong association with elevated messenger RNA levels of its catalytic subunit and high-risk human papillomavirus DNA. Cancer Res 1998;58:3812–18. [PubMed] [Google Scholar]
- 22.Mutirangura A, Sriuranpong V, Termrunggraunglert W, et al. Telomerase activity and human papillomavirus in malignant, premalignant and benign cervical lesions. Br J Cancer 1998;78:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reesink-Peters N, Helder MN, Wisman GB, et al. Detection of telomerase, its components, and human papillomavirus in cervical scrapings as a tool for triage in women with cervical dysplasia. J Clin Pathol 2003;56:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisman GB, Hollema H, de Jong S, et al. Telomerase activity as a biomarker for (pre)neoplastic cervical disease in scrapings and frozen sections from patients with abnormal cervical smear. J Clin Oncol 1998;16:2238–45. [DOI] [PubMed] [Google Scholar]
- 25.Jarboe EA, Liaw KL, Thompson LC, et al. Analysis of telomerase as a diagnostic biomarker of cervical dysplasia and carcinoma. Oncogene 2002;21:664–73. [DOI] [PubMed] [Google Scholar]
- 26.Kyo S, Masutomi K, Maida Y, et al. Significance of immunological detection of human telomerase reverse transcriptase: re-evaluation of expression and localization of human telomerase reverse transcriptase. Am J Pathol 2003;163:859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K, Hodes RJ, Weng NP. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol 2001;166:4826–30. [DOI] [PubMed] [Google Scholar]
- 28.Ngan HY, Cheung AN, Liu SS, et al. Telomerase assay and HPV 16/18 typing as adjunct to conventional cytological cervical cancer screening. Tumour Biol 2002;23:87–92. [DOI] [PubMed] [Google Scholar]
- 29.Cheung AN, Chiu PM, Tsun KL, et al. Chromosome in situ hybridisation, Ki-67, and telomerase immunocytochemistry in liquid based cervical cytology. J Clin Pathol 2004;57:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]