Abstract
A 45 year old man presented with abdominal pain, loss of appetite, and significant weight loss over a period of about four weeks. Imaging of the abdomen showed a mass in the region of the head of the pancreas. In view of the size of the mass and the clinical picture, a Whipple’s procedure was performed. Histological evaluation of the pancreatic tumour showed an adenosquamous carcinoma (predominantly composed of squamous carcinoma), which was extensively infiltrative with perineural invasion and involvement of peripancreatic lymph nodes. Areas of pancreatic intraepithelial neoplasia grade III and merging of the squamous and adenocarcinoma components were evident. Unusual histological features that characterised this case included a pronounced acantholytic pattern within the squamous carcinoma component, and the presence of both osteoclastic and pleomorphic giant cells. Giant cells have not been documented previously in association with an adenosquamous carcinoma. Although an acantholytic pattern has been noted in squamous carcinomas in other sites, this is the first report of such a pattern in an adenosquamous carcinoma of the pancreas.
Keywords: pancreas, adenosquamous carcinoma, giant cells, osteoclasts
Adenosquamous carcinoma of the pancreas is a rare aggressive subtype of pancreatic adenocarcinoma with a worse prognosis than the usual type of ductal adenocarcinoma. It has been called adenoacanthoma1 and mucoepidermoid carcinoma,1,2 and accounts for 1–4% of all exocrine malignancies of the pancreas.3 It is characterised by both ductal adenocarcinoma and squamous carcinoma within the same tumour. We describe an unusual adenosquamous carcinoma with a dominant squamous component displaying acantholytic foci and both pleomorphic and osteoclastic giant cells—histological features not described previously. In addition, the pertinent literature on adenosquamous carcinoma of the pancreas is reviewed for comparative purposes.
CASE REPORT
This rare tumour occurred in a 45 year old man who was previously well and presented with constant epigastric pain, loss of appetite, and a 20 pound weight loss over four weeks. Abdominal ultrasound and computerised tomography scan showed a mass in the head of the pancreas measuring up to 6 cm in size with dilatation of the main pancreatic ducts. A fine needle aspiration biopsy was done, which revealed malignant squamous cells. A decision was made to perform a Whipple’s procedure on the patient.
PATHOLOGY
On gross examination of the resection specimen a mass occupied most of the pancreas. The mass was firm, dark brown in colour, and extended into the wall of the duodenum, ampulla of Vater, and the common bile duct in the region of the ampulla. However, the tumour appeared grossly clear of all local resection margins.
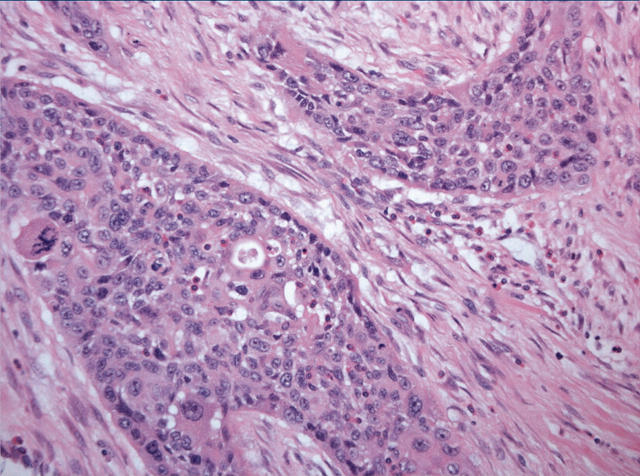
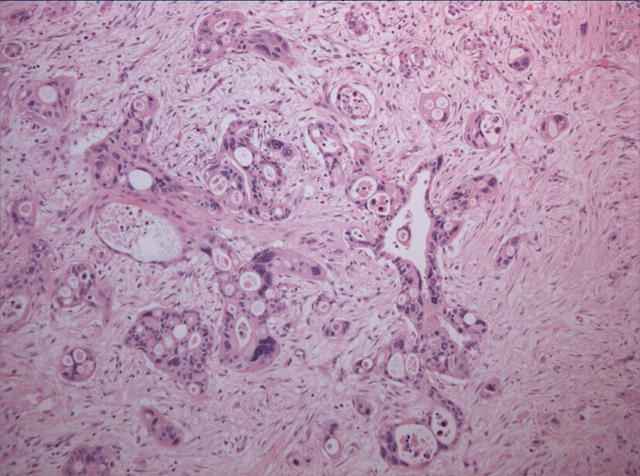
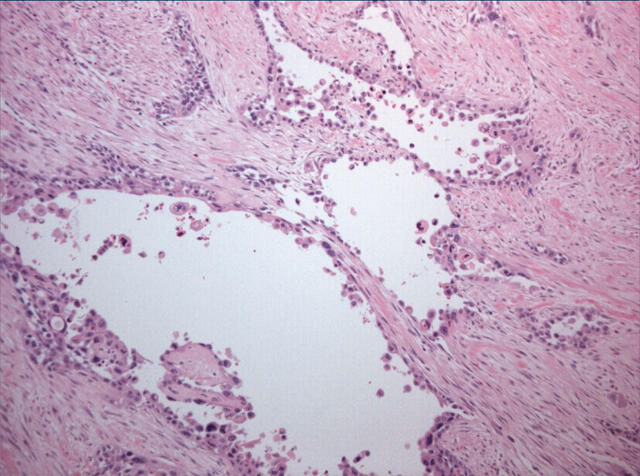
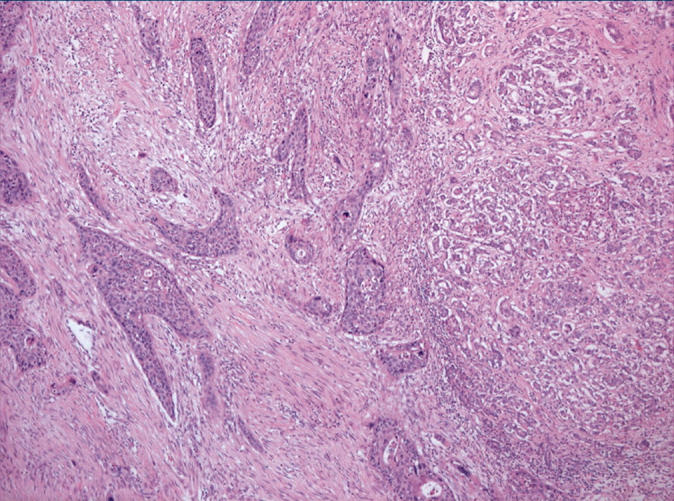
Histological examination showed a highly invasive tumour arranged in sheets, islands, and single cells, which infiltrated the pancreas diffusely and involved the wall of the duodenum, in addition to the common bile duct, primarily in the area of the ampulla of Vater. The stomach was not involved by tumour. The islands were made up of squamous cells with a high nuclear to cytoplasmic ratio, prominent nucleoli, and abundant eosinophilic cytoplasm (fig 1). Single, dyskeratotic cells were noted infiltrating the stroma and within the tumour islands (fig 2). In some areas, we noted up to 30 mitoses/10 high power fields, including abnormal forms. Glandular differentiation was present in about 10% of the tumour (fig 3). The malignant glands were lined by either obvious columnar cells or vacuolated appearing cells. Intracytoplasmic mucin was confirmed with a mucicarmine stain. In other areas, pseudoglandular spaces with acantholytic squamous cells were noted (fig 4). Large areas of the tumour showed this particular feature. These areas simulated invasive adenocarcinoma, but careful examination showed a lining of squamous cells and pronounced acantholysis of malignant squamoid cells. Merging of the squamous and glandular components was seen in some areas, and in other areas glands lined by both malignant columnar and squamous cells were seen. Another focal but prominent feature was the presence of both osteoclastic and tumour giant cells. The osteoclastic giant cells were scattered individually within the stroma and within close proximity to tumour islands (fig 5A). These cells were associated with areas of haemorrhage but were also scattered randomly within the stroma. Pleomorphic tumour giant cells were seen in association with the infiltrating squamous carcinoma, in addition to invading singly within the stroma (fig 6). The tumour elicited an intense desmoplastic stromal reaction and displayed extensive perineural invasion. Focal areas of high grade pancreatic intraepithelial neoplasia were seen intimately associated with the squamous carcinoma. Five of the 21 retrieved lymph nodes were positive for metastatic tumour, which was composed of squamous carcinoma only.
Figure 1.
Low power image demonstrating adenosquamous carcinoma (mainly squamous component) eliciting a desmoplastic stromal response.
Figure 2.
Under higher magnification, cytological atypia with pleomorphism and mitoses are clearly seen. Focal glandular differentiation is also noted.
Figure 3.
A more obvious adenocarcinoma component was present in only 10% of the tumour.
Figure 4.
Large areas of the squamous carcinoma were typified by an acantholytic pattern, with loss of cohesion of tumour cells and extrusion into pseudoglandular spaces.
Figure 5.
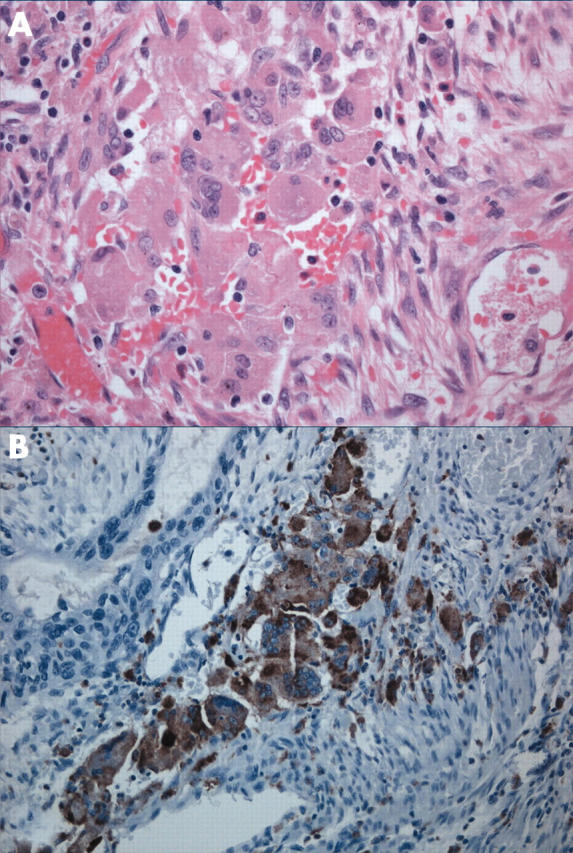
(A, B) Osteoclastic-like giant cells were noted within the stroma. These cells had bland nuclear features and were strongly positive for CD68 (B).
Figure 6.
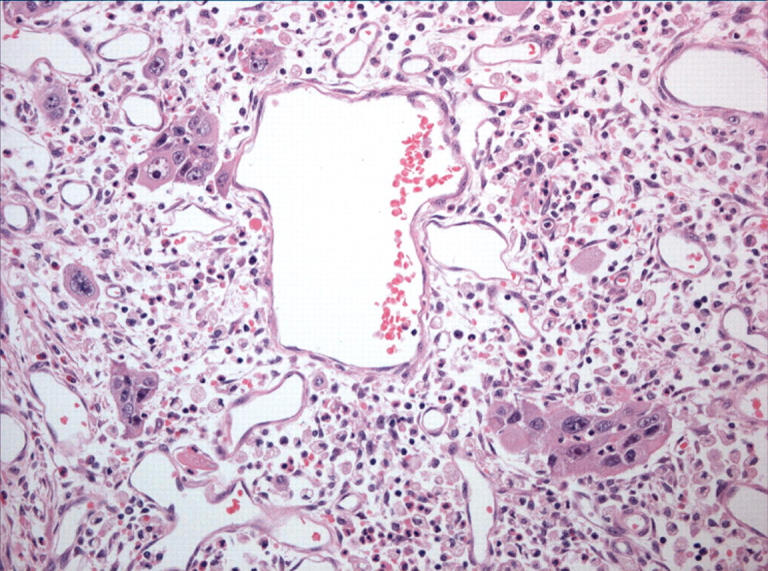
The pleomorphic tumour giant cells, in contrast, were malignant in appearance with pronounced nuclear atypia.
The osteoclastic giant cells stained positively for CD68 (fig 5B), whereas the pleomorphic tumour giant cells were negative.
DISCUSSION
There were two histological features in our case of pancreatic adenosquamous carcinoma that have not been reported previously in the English language literature. The first feature is multiple areas of acantholytic or pseudoglandular squamous carcinoma, which superficially mimic infiltrating adenocarcinoma. The other feature is the large number of pleomorphic giant cells and giant cells with osteoclast-like features, which were scattered singly or within the islands of the tumour cells. Adenosquamous carcinoma of the pancreas is thought to be a variant of typical ductal carcinoma, but with a worse prognosis. The presence of associated grade III pancreatic intraepithelial neoplasia in this tumour supports the notion that both components originate from a ductal precursor lesion. A dismal five year survival rate of 3–5% is reported.4,5 Making a diagnosis of adenosquamous carcinoma on fine needle aspiration and/or needle biopsy before surgery can be difficult because of sampling errors related to the distribution and relative predominance of the two components.6,7 However, the presence of squamous cells should almost always point to a diagnosis of adenosquamous carcinoma, because the presence of a pure squamous cell carcinoma of the pancreas is exceptionally rare, despite occasional case reports of the entity.8,9 Pure squamous carcinomas of the pancreas should only be diagnosed after thorough and exhaustive sampling, and even then a small focus of adenocarcinoma may be overlooked. Kardon et al published the largest series of adenosquamous carcinomas of the pancreas.10 They reviewed 25 cases, all of which showed biphasic malignant components—well to poorly differentiated adenocarcinoma and a well to poorly differentiated squamous cell carcinoma. The two types of carcinoma were present in variable proportions in their series and only a few of the tumours were predominantly squamous in differentiation. Most metastases reported in the literature were from the adenocarcinomatous component and not the squamous component. In our case, however, the metastatic deposits were all squamous carcinoma. The two unusual histological features seen in our case of adenosquamous carcinoma (the coexistence of osteoclastic and pleomorphic giant cells and the acantholysis within the squamous carcinoma component) have not been documented previously. Acantholytic or pseudoglandular squamous carcinoma is well recognised and described in other sites. Superficially, it resembles an adenocarcinoma, but closer scrutiny shows the squamous nature of the tumour. No particular biological or prognostic relevance is associated with this particular morphological variant of squamous carcinoma.
“The presence of squamous cells should almost always point to a diagnosis of adenosquamous carcinoma, because the presence of a pure squamous cell carcinoma of the pancreas is exceptionally rare”
Osteoclast giant cell carcinoma or undifferentiated carcinoma with osteoclast giant cells of the pancreas is a distinct entity, and to the best of our knowledge no overlap between this entity and adenosquamous carcinoma has been described.
Undifferentiated or anaplastic carcinoma of the pancreas, a subtype of ductal carcinoma, constitutes 7% of non-endocrine pancreatic malignancies.11 Pleomorphic giant cell carcinoma, osteoclastic giant cell tumour, sarcomatoid carcinoma, and carcinosarcoma are four histological variants thought to occur within the rubric of undifferentiated pancreatic carcinoma. Osteoclastic giant cell tumour appears to have the best prognosis of these four subtypes, although it is still a highly aggressive tumour, with death invariably occurring within a year of diagnosis. This entity is different morphologically from anaplastic carcinoma with pleomorphic giant cells, although they occasionally coexist and do show overlap in morphology and biology. Microscopically, this unusual subtype consists of osteoclast-like giant cells, sometimes admixed with pleomorphic multinucleated large cells; histiocyte-like mononuclear cells; atypical mononuclear cells, the nucleus of which resembles pleomorphic large cells; and ductal carcinoma cells.12–14 The osteoclastic cells are deemed to be non-neoplastic and morphologically resemble the giant cells encountered in giant cell tumour of bone.13,15 The osteoclast-like giant cells stain for CD68 and lysozyme—which are histiocytic markers—are keratin negative and k-ras mutations have not been demonstrated in these cells. In contrast, the pleomorphic large cells are CD68 negative and often have k-ras mutations. The histiocyte-like mononuclear cells are CD68 positive, keratin positive, and often have k-ras mutations. Atypical mononuclear cells are CD68 negative and also often have k-ras mutations. Pancreatic duct adenocarcinoma cells are CD68 negative and have the same k-ras mutations as the pleomorphic large cells and mononuclear cells. Given the bland morphology, lack of mitotic figures, immunophenotype, and absence of k-ras mutations, the osteoclast giant cells are thought to be reactive in nature and of monocyte/histiocyte derivation, recruited into the tumour by chemotactic factors elaborated by the malignant tumour cells. In our case, the osteoclast giant cells stained for CD68, in keeping with the fact that they are of histiocytic lineage. Mixed pleomorphic and osteoclastic giant cell tumours of the pancreas have been described.16,17 Adenosquamous carcinomas with osteoclast-like giant cells in other sites apart from the pancreas have also been documented. These include the oesophagus,18 gallbladder,19 and kidney.20 In addition, a ductal type adenocarcinoma of the pancreas with osteoclastic giant cells has been reported.21
Take home messages.
We describe a 45 year old man who presented with adenosquamous carcinoma of the pancreas with unusual histological features
These histological features included a prominent acantholytic pattern within the squamous carcinoma component, together with the presence of pleomorphic and osteoclastic giant cells
Giant cells have not been documented previously in association with adenosquamous carcinoma and, although an acantholytic pattern has been noted in squamous carcinomas in other sites, this is the first report of such a pattern in an adenosquamous carcinoma of the pancreas
This case highlights an unusual constellation of histological features: an adenosquamous carcinoma of the pancreas displaying a prominent acantholytic pattern, together with the presence of pleomorphic and osteoclastic giant cells.
The patient gave full permission for this case report to be published
REFERENCES
- 1.Cihak RW, Kawashima T, Steer A. Adenoacanthoma (adenosquamous carcinoma) of the pancreas. Cancer 1972;29:1133–40. [DOI] [PubMed] [Google Scholar]
- 2.Sommers SC, Meissner WA. Unusual carcinomas of the pancreas. Arch Pathol Lab Med 1954;58:101–11. [PubMed] [Google Scholar]
- 3.Madura JA, Jarman BT, Doherty MG, et al. Adenosquamous carcinoma of the pancreas. Arch Surg 1999;134:599–603. [DOI] [PubMed] [Google Scholar]
- 4.Aranha GV, Yong S, Olson M. Adenosquamous carcinoma of the pancreas. Int J Pancreatol 1999;26:85–91. [DOI] [PubMed] [Google Scholar]
- 5.Baylor SM, Berg JW. Cross-classification and survival characteristics of 5,000 cases of cancer of the pancreas. J Surg Oncol 1973;5:335–58. [DOI] [PubMed] [Google Scholar]
- 6.Wilczynski SP, Valente PT, Atkinson BF. Cytodiagnosis of adenosquamous carcinoma of the pancreas. Use of intraoperative fine needle aspiration. Acta Cytol 1984;28:733–6. [PubMed] [Google Scholar]
- 7.Smit W, Mathy JP, Donaldson E. Pancreatic cytology and adenosquamous carcinoma of the pancreas. Pathology 1993;25:420–2. [DOI] [PubMed] [Google Scholar]
- 8.Sears HF, Kim Y, Strawitz J. Squamous cell carcinoma of the pancreas. J Surg Oncol 1980;14:261–5. [DOI] [PubMed] [Google Scholar]
- 9.Brayko CM, Doll DC. Squamous cell carcinoma of the pancreas associated with hypercalcemia. Gastroenterology 1982;83:1297–9. [PubMed] [Google Scholar]
- 10.Kardon DE, Thompson LD, Przygodzki RM, et al. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol 2001;14:443–51. [DOI] [PubMed] [Google Scholar]
- 11.Sun AP, Ohtsuki Y, Liang SB, et al. Osteoclast-like giant cell tumor of the pancreas with metastases to gallbladder and lymph nodes. A case report. Pathol Res Pract 1998;194:587–94. [DOI] [PubMed] [Google Scholar]
- 12.Fischer HP, Altmannsberger M, Kracht J. Osteoclast-type giant cell tumour of the pancreas. Virchows Arch A Pathol Anat Histopathol 1988;412:247–53. [DOI] [PubMed] [Google Scholar]
- 13.Sakai Y, Kupelioglu AA, Yanagisawa A, et al. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol 2000;31:1223–9. [DOI] [PubMed] [Google Scholar]
- 14.Molberg KH, Heffess C, Delgado R, et al. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer 1998;82:1279–87. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RD, Michelassi F, Montag AG. Osteoclast-like giant cell tumor of the pancreas: immunophenotypic similarity to giant cell tumor of bone. Hum Pathol 1991;22:618–22. [DOI] [PubMed] [Google Scholar]
- 16.Gatteschi B, Saccomanno S, Bartoli FG, et al. Mixed pleomorphic-osteoclastic tumor of the pancreas: light microscopical, immunohistochemical, and molecular biological studies. Int J Pancreatol 1995;18:169–75. [DOI] [PubMed] [Google Scholar]
- 17.Loya AC, Ratnakar KS, Shastry RA. Combined osteoclastic and pleomorphic giant cell tumour of the pancreas: a rarity. An immunohistochemical analysis and review of the literature. J Pancreas 2004;5:220–4. [PubMed] [Google Scholar]
- 18.Tanimura T, Yatsuka K, Tachibana K, et al. A polypoidal adenosquamous cell carcinoma of the esophagus with a pseudosarcomatous stromal reaction. Gan No Rinsho 1990;36:728–32. [PubMed] [Google Scholar]
- 19.Grosso LE, Gonzalez JG. Stromal osteoclastic-like giant cells in an adenosquamous carcinoma of the gallbladder. Hum Pathol 1992;23:703–6. [DOI] [PubMed] [Google Scholar]
- 20.Sekido Y, Satoh F, Usui Y, et al. Sarcomatoid carcinoma of the renal pelvis. Pathol Int 2000;50:562–7. [DOI] [PubMed] [Google Scholar]
- 21.Newbould MJ, Benbow EW, Sene A, et al. Adenocarcinoma of the pancreas with osteoclast-like giant cells: a case report with immunocytochemistry. Pancreas 1992;7:611–15. [DOI] [PubMed] [Google Scholar]