Abstract
Vav proteins belong to the family of guanine-nucleotide-exchange factors for the Rho/Rac family of small G-proteins. In addition, they serve as important adapter proteins for the activation of PLCγ (phospholipase Cγ) isoforms by ITAM (immunoreceptor tyrosine-based activation motif) receptors, including the platelet collagen receptor GPVI (glycoprotein VI). Vav proteins are also regulated downstream of integrins, including the major platelet integrin αIIbβ3, which has recently been shown to regulate PLCγ2. In the present study, we have investigated the role of Vav family proteins in filopodia and lamellipodia formation on fibrinogen using platelets deficient in Vav1 and Vav3. Wild-type mouse platelets undergo a limited degree of spreading on fibrinogen, characterized by the formation of numerous filopodia and limited lamellipodia structures. Platelets deficient in Vav1 and Vav3 exhibit reduced filopodia and lamellipodia formation during spreading on fibrinogen. This is accompanied by reduced αIIbβ3-mediated PLCγ2 tyrosine phosphorylation and reduced Ca2+ mobilization. In contrast, the G-protein agonist thrombin stimulates full spreading of control and Vav1/3-deficient platelets. Consistent with this, stimulation of F-actin (filamentous actin) formation and Rac activation by thrombin is not altered in Vav-deficient cells. These results demonstrate that Vav1 and Vav3 are required for optimal spreading and regulation of PLCγ2 by integrin αIIbβ3, but that their requirement is by-passed upon G-protein receptor activation.
Keywords: fibrinogen, integrin αIIbβ3, phospholipase Cγ2 (PLCγ2), platelet spreading, signalling, Vav
Abbreviations: AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride; BAPTA-1/AM, bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester); CHO, Chinese-hamster ovary; DIC, differential interference contrast; F-actin, filamentous actin; Gads, Grb2-related adaptor downstream of Shc; GEF, guanine-nucleotide-exchange factor; GPVI, glycoprotein VI; GST, glutathione S-transferase; ITAM, immunoreceptor tyrosine-based activation motif; LAT, linker for activation of T-cells; PAK, p21-activated kinase; PI3K, phosphoinositide 3-kinase; PLCγ, phospholipase Cγ; SH, Src homology; SLP-76, SH2 domain-containing leucocyte protein of 76 kDa; TCR, T-cell receptor
INTRODUCTION
Platelets are small anucleate cells that circulate in a quiescent state in the vasculature. Following vascular damage, platelets are recruited to the site of injury and undergo explosive activation to stem the loss of blood from the wound. Following tethering, platelets are activated by prolonged close proximity of the platelet immunoglobulin receptor GPVI (glycoprotein VI) with subendothelial collagen (reviewed in [1]). GPVI stimulates an increase in the affinity of the major platelet integrin αIIbβ3 for its ligands, vWF (von Willebrand factor) and fibrinogen. Outside-in signalling by the integrin contributes to spreading over the subendothelial matrix and recruitment of additional platelets which become cross-linked by fibrinogen, thereby generating a vascular plug [2]. Finally, the clot is retracted and stabilized by an active process dependent on αIIbβ3 and the cytoskeleton [3].
The Vav family of GEFs (guanine-nucleotide-exchange factors) consists of three members [4–7] which share a common structural arrangement. The N-terminus contains a calponin homology domain and an acidic region, which contains regulatory tyrosine phosphorylation sites. This is followed by Dbl homology, PH (pleckstrin homology) and zinc-finger domains, which form the guanine-nucleotide-exchange region of Vav family proteins. The C-terminal portion contains a short proline-rich region and an SH3-SH2-SH3 (SH is Src homology) region. Vav2 and Vav3 are widely expressed, whereas Vav1 is specifically expressed in haemopoietic cells [5–7]. The guanine-nucleotide-exchange activity of Vav proteins is specific for the Rho family of small G-proteins. The individual Vav proteins have specificity towards different Rho family G-proteins. For example, Vav1 selectively activates Rac1, Rac2 and RhoG, whereas Vav2 and Vav3 show less activity towards Rac1 [6,8]. The guanine-nucleotide-exchange activity of all three Vav proteins is modulated through tyrosine phosphorylation of regulatory tyrosine residues by Src and Syk family kinases [9].
Vav proteins are prominent tyrosine kinase substrates downstream of ITAM (immunoreceptor tyrosine-based activation motif) receptors, including the platelet collagen receptor GPVI, and the T- and B-cell antigen receptors [6,9–12]. The importance of Vav in signalling by ITAM receptors has been demonstrated by studying mice that lack individual or combinations of Vav family proteins [13–21]. In all cases, Vav proteins have been shown to play a critical role in the regulation of PLCγ (phospholipase Cγ) by ITAM receptors. Vav1−/− T-cells have significantly reduced TCR (T-cell receptor) signalling, with a more pronounced phenotype observed in Vav1/3−/− and Vav1/2/3−/− cells. B-cells defi-cient in Vav1 have a mild reduction in B-cell receptor signalling, whereas Vav1/2−/− B-cells have a severe phenotype that is even more pronounced in Vav1/2/3−/− cells [17,18,20]. These observations therefore demonstrate a limited redundancy between the three members of the Vav family.
Vav1 and Vav3 are expressed at significant levels in platelets, whereas there is only residual expression of Vav2 [10,11,22]. In striking contrast with B- and T-cells, however, Vav1 and Vav3 are completely redundant in platelets [11]. Platelets deficient in Vav1 or Vav3 respond normally to GPVI stimulation, whereas cells deficient in both Vav isoforms display a severe block in activation through the collagen receptor [10,11]. Vav2 is not phosphorylated in platelets, and cells deficient in Vav1, Vav2 and Vav3 exhibit a similar defect in response to GPVI agonists as platelets deficient in Vav1 and Vav3 [10,11].
Importantly, Vav family proteins have been demonstrated to play a role in ITAM signalling independent of GEF activity and Rac activation, most notably through regulation of PLCγ isoforms downstream of the antigen receptors [12,21,23–26]. This action has been attributed to the adapter function of Vav isoforms in the formation and stabilization of signalling complexes [21,23,27–29]. There appear to be at least two pathways through which Vav regulates PLCγ downstream of ITAM receptors, namely via regulation of Tec family kinases and through assembly of a LAT–Gads–SLP-76–PLCγ (where LAT is linker for activation of T-cells, Gads is Grb2-related adaptor downstream of Shc, and SLP-76 is SH2 domain-containing leucocyte protein of 76 kDa) signalosome [12,21,23–26,30]. The regulation of Tec family kinases is dependent on PI3K (phosphoinositide 3-kinase) and has been proposed to be regulated through Rac1 [30–32]. On the other hand, the role of Vav in the assembly of the LAT–Gads–SLP-76–PLCγ signalosome is independent of PI3K and, presumably, Rac activation. In addition, Rac has been shown to directly activate PLCγ isoforms in vitro independently of PI3K [33]. Therefore Vav is able to control PLCγ activity via at least two pathways that differ in their dependence on PI3K and Rac, although the relative contribution of these pathways is unclear.
Vav family proteins have been shown to be tyrosine-phosphorylated downstream of β1, β2 and β3 integrins in a number of haemopoietic cells, including platelets and in CHO (Chinese-hamster ovary) cells transfected with αIIbβ3 [22,34–38]. Furthermore, Vav proteins have been shown to be critical for outside-in signalling by β2 integrins in neutrophils and to contribute to their stable adhesion and spreading [39]. Vav proteins have also been shown to bind to tyrosine-phosphorylated β3 in a K562 cell line model of αvβ3 function [40].
In platelets, the integrin αIIbβ3 activates a signalling pathway that uses many of the same proteins as ITAM receptors (reviewed in [41]) including Src kinases [22,42], Syk [22,43], SLP-76 [44] and PLCγ2 [45–47]. Platelets deficient in these proteins do not spread on fibrinogen, demonstrating the critical importance of outside-in signalling by the integrin for the spreading response. Vav1 and Vav3 undergo tyrosine-phosphorylation downstream of αIIbβ3 when platelets spread on fibrinogen [22] and when platelets undergo aggregation in suspension [37]. In addition, Vav proteins interact with several of the membrane-proximal signalling proteins in the αIIbβ3 signalling cascade, including Syk [48] and SLP-76 [49–51].
These observations raise the possibility that Vav family proteins may play a role in the regulation of PLCγ2 by integrin αIIbβ3 in platelets. This hypothesis has been investigated in the present study using platelets from mice deficient in Vav1 and Vav3 to assess the role of Vav family proteins in αIIbβ3 outside-in signalling. We show that Vav1/3−/− platelets have reduced spreading on fibrinogen compared with wild-type platelets, which is associated with defective PLCγ2 phosphorylation and Ca2+ mobilization. Pre-treatment of platelets with thrombin overcomes the spreading defect in Vav1/3−/− platelets. These results demonstrate that Vav family proteins are required for normal regulation of PLCγ2 by αIIbβ3 and for optimal platelet spreading on fibrinogen.
MATERIALS AND METHODS
Antibodies and reagents
Anti-phosphotyrosine monoclonal antibody 4G10 and anti-Rac monoclonal antibody were purchased from Upstate Biotechnology (through TCS Biologicals). The anti-PLCγ2 and anti-Syk polyclonal antibodies were kindly supplied by Dr Mike Tomlinson (DNAX, Palo Alto, CA, U.S.A.). The FITC-conjugated rat anti-(mouse αIIb) (MwReg30), anti-(mouse β3) (Luc.A5) and anti-(mouse αIIbβ3) (Leo.D2) antibodies and rat IgG were purchased from Emfret Analytics. The cDNA for GST (glutathione S-transferase)–PAK (p21-activated kinase) CRIB domain was a gift from Dr Doreen Cantrell (School of Life Sciences, Dundee University, Dundee, U.K.). FITC–phalloidin and Oregon Green BAPTA-1/AM [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester)] were from Molecular Probes. All other reagents were purchased from Sigma or obtained from sources described previously [43,52].
Animals
The generation of mice disrupted in the vav1 gene (Vav1−/−) is described by Turner et al. [53]. The generation of mice disrupted in the vav2 gene (Vav2−/−) is described by Doody et al. [17]. The generation of mice disrupted in the vav3 gene (Vav3−/−) is described by Fujikawa et al. [20]. Compound knockout mice were generated by appropriate crossing of the individual knockout genotypes. Mutant and control mice were age- and background-matched. All animals were maintained using housing and husbandry in accordance with local and national legal regulations.
Preparation of mouse platelets
Blood was taken from a terminally CO2-narcosed mouse by cardiac puncture on the day of the experiment into 1:10 (v/v) ACD (85 mM sodium citrate, 110 mM glucose, 71 mM citric acid). Blood was diluted 1:6 (v/v) in Tyrode's Hepes buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM Hepes, 5 mM glucose and 1 mM MgCl2, pH 7.3) and centrifuged at 200 g for 6 min to obtain platelet-rich plasma. Platelet-rich plasma was centrifuged in the presence of 0.1 μg/ml prostacyclin at 1000 g for 6 min, and the platelet pellet was resuspended in Tyrode's Hepes buffer.
Flow cytometry staining
Washed platelets (1×107/ml) were stained with the indicated FITC-conjugated antibodies for 15 min at room temperature (22 °C). Staining was quenched with 400 μl of Tyrode's Hepes buffer, and samples were analysed using a FACScalibur flow cytometer and CellQuest software (Becton Dickinson).
Static adhesion spreading assay
Glass coverslips were coated with 100 μg/ml fibrinogen solution overnight at 4 °C followed by washing with PBS. Slides were then blocked using 0.5% heat-denatured BSA in PBS for 1 h at room temperature, followed by washing in PBS. Mouse platelets, resuspended at a concentration of 3×107/ml in Tyrode's Hepes containing 2 units/ml apyrase and 10 μM indomethacin, were transferred to the coverslips and incubated at 37 °C for 45 min in a humid atmosphere. Excess platelets were removed, and the adherent platelets were fixed with 3.7% (w/v) paraformaldehyde for 10 min at room temperature. The coverslips were washed in PBS, mounted using HydroMount mounting medium (National Diagnostics) and viewed under DIC (differential interference contrast) microscopy under a 63× oil-immersion lens and Slidebook software (Intelligent Imaging Innovations). Surface areas were calculated using a Java plugin for ImageJ software.
Immunoprecipitation and immunoblotting
Platelets at 2×108/ml were incubated over fibrinogen-coated dishes for 45 min at 37 °C in the presence of 2 units/ml apyrase and 10 μM indomethacin. Non-adherent basal platelets were removed and lysed in an equal volume of 2× lysis buffer {2% Nonidet P40, 300 mM NaCl, 20 mM Tris/HCl, 2 mM EDTA, 2 mM EGTA, 2 mM Na3VO4, 200 μg/ml AEBSF [4-(2-aminoethyl)benzenesulfonyl fluoride] hydrochloride, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 1 μg/ml pepstatin A, pH 7.4}. Adherent platelets were lysed in a final volume of 1 ml of 1× lysis buffer. Insoluble cell debris was removed by centrifugation at 13000 g for 5 min at 4 °C, and cell lysates were pre-cleared using Protein A–Sepharose. Platelet lysates were incubated with the indicated primary antibodies, and resulting protein complexes and immunoprecipitates were resolved by SDS/PAGE (10% polyacrylamide) and transferred on to PVDF membranes. Immunoblotting was performed as described previously [11] with detection by ECL® (enhanced chemiluminescence) (Amersham Biosciences).
Rac activation assay
Rac activity was measured as described previously [10]. The CRIB domain of PAK1 (amino acids 67–150) was expressed as a GST-fusion protein and bound to glutathione–Sepharose beads. Platelet stimulations were stopped with an equal volume of 2× Rac assay lysis buffer [2% (v/v) Nonidet P-40, 1% (w/v) N-octylglucoside, 300 mM NaCl, 20 mM Tris/HCl, 2 mM EDTA, 2mM EGTA, 20 mM MgCl2, 2 mM Na3VO4, 200 μg/ml AEBSF hydrochloride, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 1 μg/ml pepstatin A, pH 7.4]. Insoluble material was removed by centrifugation at 13000 g for 5 min at 4 °C, and freshly prepared GST–PAK1 was added to the samples and incubated for 1 h at 4 °C. The beads were then washed with 1× Rac assay lysis buffer, and the bound protein was taken up into Laemmli buffer. The resulting samples were separated by SDS/PAGE (12% polyacrylamide) and transferred on to PVDF membranes for immunoblotting as described above.
F-actin (filamentous actin) assay
F-actin was measured using a modification of the method of Machesky and Hall [54]. Briefly, platelets at a concentration of 2×108/ml were fixed with an equal volume of 3.7% (w/v) formaldehyde containing FITC–phalloidin (20 mM KH2PO4, 10 mM Pipes, 5 mM EGTA, 2 mM MgCl2, 0.1% Triton X-100, 3.7% formaldehyde and 2 μM FITC–phalloidin) and rotated for 1 h at room temperature. Platelets were then pelleted for 2 min in a microcentrifuge and washed in 0.1% saponin, 20 mM KH2PO4, 10 mM Pipes, 5 mM EGTA and 2 mM MgCl2. Platelets were rotated in methanol for 1 h to extract the FITC–phalloidin. FITC–phalloidin binding was measured by measuring the emission at 520 nm using an excitation wavelength of 488 nm on a spectrofluorimeter.
Ca2+-mobilization assay
Platelets were incubated with 15 μM Oregon Green BAPTA-1/AM in DMSO containing 1.2 mg/ml pluronic acid for 90 min in the dark at room temperature. Excess dye was removed by washing the platelets in Tyrode's Hepes buffer in the presence of 0.1 μg/ml prostacyclin. Platelets were allowed to settle on to fibrinogen-coated coverslips in the presence of 2 units/ml apyrase and 10 μM indomethacin and were imaged in real time using the 63× oil-immersion lens on a Zeiss Axiovert 200M microscope using Slidebook software (Intelligent Imaging Innovations).
Analysis of data
Experiments were performed on at least three occasions and are shown as representative data. Where appropriate, data are shown ±S.E.M. Where statistical significance is indicated, data have been subjected to Student's t test. For comparison of surface areas of platelets in Table 1(a), where multiple comparisons are required, Tukey's test has been used. P<0.01 was selected to represent statistical significance.
Table 1. Comparison of surface areas.
(a) Washed platelets from the genotypes of mice indicated were incubated over fibrinogen-coated coverslips in the presence of 2 units/ml apyrase and 10 μM indomethacin for 45 min at 37 °C. Slides were imaged by DIC microscopy and the surface area of the platelets was calculated with a graticule standard and using ImageJ software. The surface areas of Vav1−/−, Vav3−/−, Vav1/3−/− and Vav1/2/3−/− cells were compared with wild-type surface areas by Tukey's test; *P<0.01 relative to wild-type. Vav1−/−, Vav3−/− and Vav1/2/3−/− surface areas were compared with that of Vav1/3−/− using Tukey's test; †P<0.01 relative to Vav1/3−/−. (b) Platelets were stimulated with 1 unit/ml thrombin before adding to coverslips and imaging as above. Surface areas were compared using Student's t test and were not significantly different (P<0.01).
| (a) | |
|---|---|
| Genotype | Mean surface area (μm2) |
| Wild-type | 12.6±0.22 (n=301) |
| Vav1−/− | 10.2±0.16*† (n=299) |
| Vav3−/− | 11.3±0.16*† (n=289) |
| Vav1/3−/− | 9.3±0.13* (n=319) |
| Vav1/2/3−/− | 9.44±0.13* (n=330) |
| (b) | |
| Genotype and treatment | Mean surface area (μm2) |
| Wild-type+thrombin | 19.3±0.41 (n=300) |
| Vav1/3−/−+thrombin | 17.9±0.41 (n=300) |
RESULTS
Vav1/3−/− platelets exhibit reduced spreading on fibrinogen
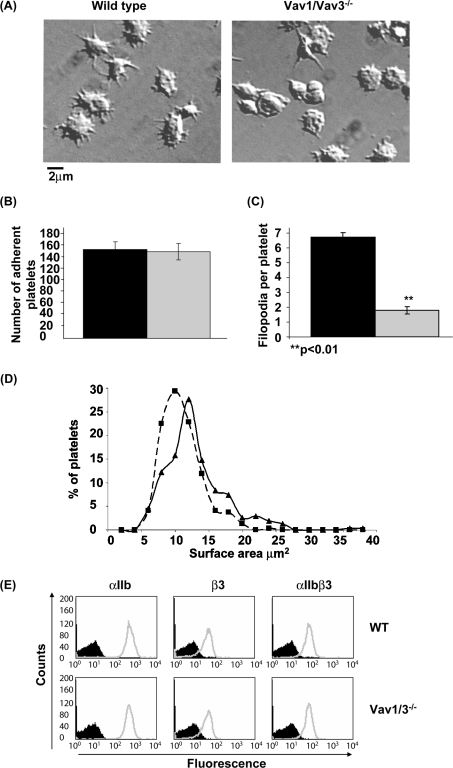
To investigate whether Vav family proteins are involved in outside-in signalling by αIIbβ3, platelets from wild-type and Vav1/3−/− mice were incubated on fibrinogen-coated coverslips for 45 min and imaged by DIC microscopy. These experiments were carried out in the presence of apyrase and indomethacin to block the effects of the secreted G-protein-coupled secondary agonists, ADP and thromboxane A2. Under these conditions, wild-type mouse platelets undergo partial spreading, with formation of filopodia and limited lamellipodia-like structures (Figure 1A). This limited spreading response has previously been shown to be independent of activation of Rac, as shown using Rac1/Rac2−/− platelets [55], but dependent on PLCγ2 [45]. Vav1/3−/− platelets adhere with the same efficiency as wild-type platelets (Figure 1B). However, in contrast with wild-type platelets, Vav1/3−/− platelets do not form lamellipodia and have fewer filopodia than wild-type cells (Figure 1A). The number of filopodia formed by wild-type and Vav1/3−/− platelets were counted and plotted as a histogram. Vav1/3−/− platelets exhibit significantly fewer filopodia than wild-type cells (Figure 1C). The surface area of each set of platelets was measured and plotted as a frequency distribution. The distribution of surface area of Vav1/3−/− platelets is shifted slightly to the left relative to wild-type platelets (Figure 1D), thereby demonstrating a small, but significant, reduction in surface area as shown in Table 1. Platelets deficient in Vav1 or Vav3 alone exhibited an intermediate phenotype, with a slightly lower average surface area than wild-type platelets (Table 1A). The surface area of Vav1/3−/− platelets was reduced significantly relative to the single knockouts, demonstrating a degree of redundancy in the role of these proteins in platelet spreading on fibrinogen (Table 1a). Consistent with a residual expression of Vav2 in platelets, the defect in spreading in Vav1/2/3−/− platelets was not significantly different to that in Vav1/3−/− platelets (Table 1a).
Figure 1. Vav1/3−/− platelets exhibit reduced spreading on fibrinogen.
(A) Washed platelets from wild-type (left-hand panel) or Vav1/3−/− (right-hand panel) mice were incubated over fibrinogen-coated coverslips in the presence of 2 units/ml apyrase and 10 μM indomethacin for 45 min at 37 °C and subsequently imaged by DIC microscopy. A representative field of view is shown. (B) Mean±S.E.M. number of adherent wild-type (black bar) and Vav1/3−/− (grey bar) platelets per 13500 μm2. (C) The mean±S.E.M. number of filopodia per platelet were counted in 50 wild-type (black bar) and Vav1/3−/− (grey bar) platelets chosen at random. (D) The surface area of 301 wild-type (solid line) and 319 Vav1/3−/− (broken line) platelets on fibrinogen was calculated by imaging slides as above with a graticule standard and using ImageJ software to measure surface area. Surface areas are plotted as a frequency distribution. (E) Washed platelets from wild-type (WT) or Vav1/3−/− mice were stained with FITC-conjugated rat IgG (black) or rat anti-mouse αIIb, β3 or αIIbβ3 (grey). Results are representative of three experiments.
A potential explanation for the decreased spreading of Vav1/3−/− platelets on fibrinogen could be due to reduced surface expression of the fibrinogen receptor αIIbβ3 in these cells. To investigate this, we have compared the levels of the integrin on wild-type and Vav1/3−/− platelets by staining with antibodies against each of the subunits of the integrin and to the integrin complex. Expression of αIIb, β3 and αIIbβ3 is identical between wild-type and Vav1/3−/− platelets (Figure 1E). These results demonstrate that the defective spreading of Vav1/3−/− platelets on fibrinogen is not due to a lower expression level of the receptor.
Activation of PLCγ2 by αIIbβ3 is impaired in Vav1/3−/− platelets
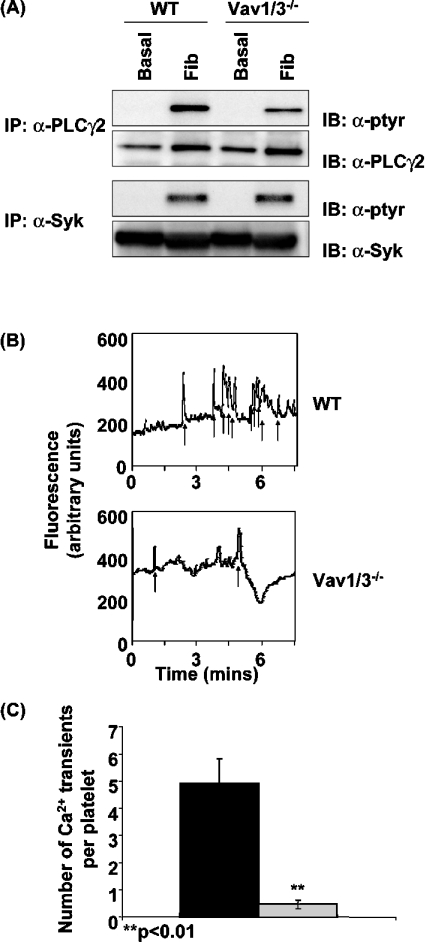
Platelet spreading on fibrinogen has previously been shown to be critically dependent on Ca2+, PKC and PLCγ2 [45,56]. Since Vav family proteins have been shown to be critical for regulation of PLCγ2 by ITAM receptors in platelets and in other cells, we investigated whether a defect in PLCγ2 regulation by the integrin could underlie the reduced spreading on fibrinogen that is observed in the combined absence of Vav1 and Vav3. Wild-type and Vav1/3−/− platelets were incubated over a fibrinogen-coated surface for 45 min, before removal and lysis of basal (non-adherent) and stimulated (adherent) platelets. Following lysis and immunoprecipitation, PLCγ2 phosphorylation was measured by Western blotting using the anti-phosphotyrosine antibody 4G10. Wild-type platelets exhibit a robust increase in tyrosine phosphorylation of PLCγ2 when spread on fibrinogen, which is reduced by approx. 50% in Vav1/3−/− platelets (Figure 2A). In contrast, tyrosine phosphorylation of the upstream kinase Syk is not significantly different in wild-type and Vav1/3−/− platelets (Figure 2A). These results suggest that the same amount of signal is transmitted by the receptor into the cell but that the efficiency of transmitting this signal to PLCγ2 is reduced in the absence of Vav1/3.
Figure 2. Activation of PLCγ2 by αIIbβ3 is impaired in Vav1/3−/− platelets.
(A) Washed wild-type (WT) and Vav1/3−/− platelets were incubated over fibrinogen-coated Petri dishes in the presence of 2 units/ml apyrase and 10 μM indomethacin for 45 min at 37 °C. Basal (non-adherent) and fibrinogen-stimulated (adherent; Fib) cells were lysed in Nonidet P40 lysis buffer, and PLCγ2 and Syk were immunoprecipitated (IP). Immunoprecipitates were separated by SDS/PAGE and were Western-blotted (IB) with anti-phosphotyrosine monoclonal antibody 4G10 (α-ptyr). Membranes were subsequently stripped and reprobed with anti-PLCγ2 (α-PLCγ2) and anti-Syk (α-Syk) antibodies respectively. (B) Washed wild-type (WT) and Vav1/3−/− platelets were labelled with the Ca2+ reporter dye Oregon Green BAPTA-1/AM and incubated over fibrinogen-coated coverslips in the presence of 2 units/ml apyrase and 10 μM indomethacin and imaged in real time using the FITC channel on a fluorescent microscope. The fluorescence intensity of a representative platelet is shown. Spikes in fluorescence indicative of transient cytoplasmic Ca2+ elevation are indicated by arrows. (C) The number of Ca2+ transients in 30 platelets chosen at random from wild-type (black bar) and Vav1/3−/− (grey bar) mice were counted. Results are means±S.E.M. and are representative of three experiments.
In order to investigate whether the defect in platelet spreading is due to the defect in signalling of αIIbβ3 to PLCγ2, we assessed the ability of Vav1/3−/− platelets to mobilize Ca2+ during spreading on fibrinogen. Platelets from wild-type and Vav1/3−/− mice were labelled with the Ca2+ reporter dye Oregon Green BAPTA-1/AM. This dye is a highly sensitive Ca2+ reporter dye that can be used to detect fluctuations in intracellular Ca2+ levels without chelating sufficient Ca2+ to block Ca2+-dependent functional responses ([57,58], and results not shown). Labelled platelets were incubated over fibrinogen-coated coverslips, and spreading was monitored by fluorescence microscopy. Wild-type platelets exhibit a series of oscillations in fluorescence, indicative of transient elevation of cytosolic Ca2+, as shown in Figure 2(B) and Supplementary Video S1(A) (see http://www.BiochemJ.org/bj/401/bj4010753add.htm). In contrast, Vav1/3−/− platelets exhibit significantly less Ca2+ transients over a 7 min recording period as shown by the single-cell records (Figure 2B, and Supplementary Video S1B at http://www.BiochemJ.org/bj/401/bj4010753add.htm) and pooled data (Figure 2C). These results demonstrate that Vav1 and Vav3 are required for αIIbβ3-mediated Ca2+ mobilization, regulated downstream of PLCγ2.
Vav1 and Vav3 do not regulate Rac in fibrinogen- or thrombin-stimulated platelets
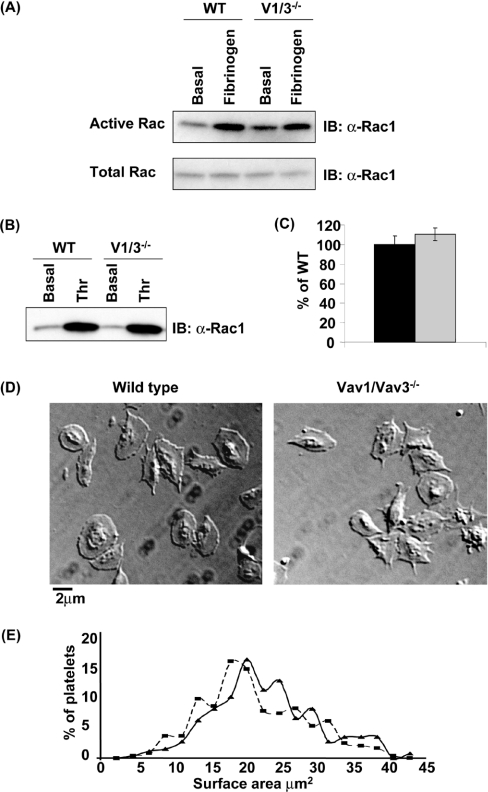
In addition to their role in regulating PLCγ2 downstream of ITAM receptors, Vav family proteins also function as GEFs for Rho family G-proteins. The defect in spreading that is observed in the absence of Vav1 and Vav3 is unlikely to be due to blockade of Rho activity, since platelets are not thought to form stress fibres under these conditions. Furthermore, we have been unable to detect activation of Rho in platelets that have adhered to fibrinogen. However, we have also been unable to detect activation of Cdc42 during adhesion of human or murine platelets to fibrinogen (results not shown). This may reflect that the small G-protein is not activated by the integrin or that it is activated at a level below that required for detection. We have reported previously that spreading of mouse platelets on fibrinogen is independent of activation of Rac, as shown using Rac1/2−/− mice [55]. Furthermore, we were unable to detect activation of Rac1/2 in human and mouse platelets using GST–PAK, which selectively binds to the GTP-bound form of the protein, to precipitate active Rac [55]. In the present study, we have been able to detect weak activation of Rac in murine platelets that have spread on fibrinogen (Figure 3A). In order to investigate whether Rac activation by the integrin is regulated by Vav1/3, Rac activation in Vav1/3−/− platelets was assayed. αIIbβ3-induced Rac activation is normal in Vav1/3−/− platelets (Figure 3A). These results demonstrate that Vav1/3 does not lie upstream of Rac in αIIbβ3 signalling.
Figure 3. Vav1 and Vav3 do not regulate Rac in fibrinogen- or thrombin-stimulated platelets.
(A) Washed wild-type (WT) and Vav1/3−/− (V1/3−/−) platelets were incubated over fibrinogen-coated Petri dishes in the presence of 2 units/ml apyrase and 10 μM indomethacin for 45 min at 37 °C. Basal (non-adherent) and fibrinogen-stimulated (adherent) cells were lysed in Rac assay lysis buffer. An aliquot of lysate was taken into Laemmli buffer for total Rac analysis by Western blotting (IB) with anti-Rac1 (α-Rac1). Remaining lysates were incubated with GST–PAK for 1 h, and active Rac was precipitated. Precipitates were separated by SDS/PAGE and were Western-blotted for Rac. (B) Washed wild-type (WT) and Vav1/3−/− (V1/3−/−) platelets in suspension in the presence of 2 units/ml apyrase and 10 μM indomethacin were stimulated with 1 unit/ml thrombin for 60 s and lysed in Rac assay lysis buffer, and active Rac was precipitated and Western-blotted as above. (C) Platelets were stimulated with 1 unit/ml thrombin for 60 s, fixed and F-actin-stained with FITC–phalloidin. Specifically bound FITC–phalloidin was eluted and quantified in a spectrofluorimeter. The amount of F-actin in wild-type (black bar) and Vav1/3−/− (grey bar) is expressed as the mean±S.E.M. percentage of thrombin induced F-actin formation in wild-type (WT) cells for triplicate samples. (D) Washed platelets from wild-type (left-hand panel) or Vav1/3−/− (right-hand panel) mice were stimulated with 1 unit/ml thrombin and immediately incubated over fibrinogen-coated coverslips for 45 min at 37 °C in the presence of 2 units/ml apyrase and 10 μM indomethacin and were subsequently imaged by DIC microscopy. A representative field of view is shown. (E) The surface area of 300 wild-type (solid line) and 300 Vav1/3−/− (broken line) platelets on fibrinogen was calculated by imaging slides as above with a graticule standard and using ImageJ software to measure surface area. Surface areas are plotted as a frequency distribution. Results are representative of three experiments.
In contrast with signalling by the integrin, thrombin induces robust activation of Rac. In order to investigate whether Vav1/3 lies upstream of Rac in thrombin-stimulated platelets, active Rac was precipitated from thrombin-stimulated platelets as above. Activation of Rac by thrombin is not altered in the absence of Vav1 and Vav3 (Figure 3B). Furthermore, thrombin induced F-actin polymerization is normal in Vav1/3−/− platelets (Figure 3C). These results demonstrate that Vav1/3 does not lie upstream of Rac or actin assembly in thrombin-stimulated platelets. Importantly this is consistent with the observation that thrombin induces full spreading and formation of extensive lamellipodia in both wild-type and Vav1/Vav3−/− platelets (Figure 3D and Table 1), as demonstrated by the indistinguishable frequency distribution curves of platelet surface area (Figure 3E).
DISCUSSION
In the present study, we have investigated the role of Vav family proteins in αIIbβ3-mediated platelet activation and spreading on fibrinogen. Platelets deficient in the major Vav family proteins, Vav1 and Vav3, have reduced spreading on fibrinogen, in association with reduced tyrosine phosphorylation of PLCγ2 and reduced elevation of intracellular Ca2+. These results therefore demonstrate a critical role for Vav1 and Vav3 in the regulation of PLCγ2 by αIIbβ3, as has previously been shown to be the case for regulation of PLCγ2 by the ITAM-coupled collagen receptor, GPVI [11]. The defect in spreading in the Vav1/3−/− platelets is bypassed in the presence of thrombin, a G-protein-coupled receptor agonist, which stimulates a powerful activation of PLCβ isoforms in platelets. Thus the role of Vav1 and Vav3 in mediating spreading on fibrinogen is specifically linked to αIIbβ3-mediated outside-in signalling.
Vav family proteins have been implicated in integrin-dependent responses in a variety of cells [34,39,40]. Vav-deficient neutrophils display defective β2-integrin-mediated spreading and phagocytosis [39], a K562 model of αvβ3-mediated cell adhesion demonstrates recruitment of Vav1 to the phosphorylated β3 subunit in fibronectin-adherent cells [40] and CHO cells overexpressing αIIbβ3, Syk, SLP-76 and Vav1 form extensive lamellipodia on fibrinogen [38]. In the present study, we demonstrated that Vav1 and Vav3 are required for normal platelet spreading on fibrinogen, as a consequence of a reduction in αIIbβ3-mediated activation of PLCγ2 and Ca2+ mobilization. Since β2 integrins in neutrophils also exhibit PLCγ2-dependent Ca2+ mobilization [59], we suspect that Vav proteins are likely to be involved in PLCγ2 regulation by β2 integrins in neutrophils and that this contributes to the defective functional responses in these cells [39]. Interestingly, platelet responses to the G-protein-coupled receptor agonist thrombin are not affected by deficiency of Vav1 and Vav3. Similarly, neutrophil responses to G-protein-coupled chemoattractants are normal in Vav-deficient cells, suggesting that Vav is also dispensable for GPCR signalling in these cells [39].
It is now recognized that integrins use many of the same signalling proteins as ITAM receptors [41]. In platelets, for example, critical roles for Src [22,42] and Syk [22] tyrosine kinases, the adapter protein SLP-76 [44] and PLCγ2 [45–47] have been reported downstream of both GPVI and αIIbβ3. On the basis of the data in the present study, a further similarity in the signalling pathways activated by these two classes of receptors is the requirement of Vav proteins for efficient regulation of PLCγ2 [10,11]. It is noteworthy that Vav proteins appear to be essential for optimal regulation of PLCγ isoforms by all ITAMs. It will therefore be of interest to know whether Vav proteins are required downstream of all integrins for the regulation of PLCγ. Interestingly, we have also recently demonstrated a role for Vav1 and Vav3 in the activation of PLCγ2 by the C-type lectin family receptor, CLEC-2, in platelets which has a single rather than dual YXXL motif in its cytosolic tail [60]. There are, however, significant differences in signalling by these three classes of receptor in platelets. For example, GPVI signalling takes place in lipid rafts and is critically dependent on the transmembrane adaptor protein, LAT [61,62], whereas signalling by αIIbβ3 is independent of these specialized membrane domains and LAT [45,62]. In comparison, signalling by CLEC-2 is partially dependent on the adapters LAT and SLP-76, whereas the latter is essential for responses to GPVI.
Cell spreading involves extensive cytoskeletal re-modifications, which can be regulated by Rho family small G-proteins. The small G-proteins Cdc42, Rac and Rho are implicated in the formation of filopodia, lamellipodia and stress fibres respectively [63–65]. In the present study, we have assayed spreading mediated directly by the integrin, by performing all studies in the presence of apyrase and indomethacin. Under these conditions, mouse platelets undergo a limited spreading response that is dependent on PLCγ2 [45], but independent of Rac [55]. Consistent with this, we have been able to detect only weak activation of Rac by αIIbβ3 in platelets that have undergone spreading on fibrinogen, and this activation is not reduced in Vav1/3−/− platelets. These results demonstrate that the defective regulation of PLCγ2 by αIIbβ3 in platelets is not due to defective regulation of Rac. Interestingly, we have been unable to detect activation of Cdc42 (results not shown) during spreading on fibrinogen, despite robust formation of filopodia. This may represent a low level of activation of the small G-protein that is below the level of detection, or may represent Cdc42-independent formation of filopodia, as has been reported in other cells [66,67]. We cannot therefore rule out the possibility that the defect in spreading on fibrinogen in the absence of Vav1 and Vav3 is, at least partially, due to a direct inhibition of Cdc42 activation or a related G-protein, although it should be noted that a role of Vav family GEFs in the regulation of Cdc42 is controversial [6,8,68–71]. It is also extremely unlikely that the spreading defect of Vav1/3−/− platelets is due to impairment of activation of Rho as there is no evidence of stress fibre formation in platelets spread under these conditions. Under physiological conditions, the limited spreading response initiated by αIIbβ3 is reinforced by G-protein-coupled receptor agonists, such as ADP and thrombin, leading to Rac activation and Rac-dependent full spreading [10,55]. In the present study, we have shown that Vav proteins are not required for G-protein-coupled receptor signalling leading to Rac activation or actin polymerization, suggesting that this process would occur normally in Vav-deficient mice.
It is well established that Vav family proteins have GEF-independent functions thought to be due to their roles as adaptor proteins and stabilizing signalling complexes [21,23,27,28]. Indeed, Vav family proteins have been shown to be required for normal formation of the LAT–Gads–SLP-76–PLCγ2 signalosome downstream of the TCR [23]. It is likely that this function of Vav proteins is responsible for their critical role in the regulation of PLCγ isoforms by ITAM receptors, including GPVI. Demonstration of inhibition of PLCγ2 downstream of αIIbβ3 in the present study provides strong evidence that this mechanism also applies to the regulation of PLCγ isoforms downstream of integrin receptors.
In summary, the data presented here demonstrate an important role for Vav family proteins in signalling by the platelet integrin αIIbβ3 and for normal regulation of PLCγ2, intracellular Ca2+ mobilization and platelet spreading on fibrinogen. These results add to previous studies that demonstrate a pivotal role for Vav family proteins in the regulation of PLCγ2 by distinct classes of surface glycoproteins, including integrins, ITAM and lectin receptors.
Multimedia adjuncts
Acknowledgments
We gratefully acknowledge Helen Reynolds and SABU (Small Animal Barrier Unit) staff for animal care and Majd Protty for computational assistance. This work was supported by the Wellcome Trust, the British Heart Foundation and the BBSRC (Biotechnology and Biological Sciences Research Council). S.P.W. holds a British Heart Foundation Research Chair, and M.T. holds an MRC (Medical Research Council) Senior Fellowship.
References
- 1.Nieswandt B., Watson S. P. Platelet–collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 2.Nesbitt W. S., Giuliano S., Kulkarni S., Dopheide S. M., Harper I. S., Jackson S. P. Intercellular calcium communication regulates platelet aggregation and thrombus growth. J. Cell Biol. 2003;160:1151–1161. doi: 10.1083/jcb.200207119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law D. A., DeGuzman F. R., Heiser P., Ministri-Madrid K., Killeen N., Phillips D. R. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 4.Katzav S., Martin-Zanca D., Barbacid M. Vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuebel K. E., Bustelo X. R., Nielsen D. A., Song B. J., Barbacid M., Goldman D., Lee I. J. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- 6.Movilla N., Bustelo X. R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell. Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henske E. P., Short M. P., Jozwiak S., Bovey C. M., Ramlakhan S., Haines J. L., Kwiatkowski D. J. Identification of VAV2 on 9q34 and its exclusion as the tuberous sclerosis gene TSC1. Ann. Hum. Genet. 1995;59:25–37. doi: 10.1111/j.1469-1809.1995.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 8.Schuebel K. E., Movilla N., Rosa J. L., Bustelo X. R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustelo X. R. Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce A. C., Wilde J. I., Doody G. M., Best D., Inoue O., Vigorito E., Tybulewicz V. L., Turner M., Watson S. P. Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood. 2002;100:3561–3569. doi: 10.1182/blood.V100.10.3561. [DOI] [PubMed] [Google Scholar]
- 11.Pearce A. C., Senis Y. A., Billadeau D. D., Turner M., Watson S. P., Vigorito E. Vav1 and vav3 have critical but redundant roles in mediating platelet activation by collagen. J. Biol. Chem. 2004;279:53955–53962. doi: 10.1074/jbc.M410355200. [DOI] [PubMed] [Google Scholar]
- 12.Turner M., Billadeau D. D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 13.Costello P. S., Walters A. E., Mee P. J., Turner M., Reynolds L. F., Prisco A., Sarner N., Zamoyska R., Tybulewicz V. L. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-κB pathways. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer K. D., Kong Y. Y., Nishina H., Tedford K., Marengere L. E., Kozieradzki I., Sasaki T., Starr M., Chan G., Gardener S., et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 15.Holsinger L. J., Graef I. A., Swat W., Chi T., Bautista D. M., Davidson L., Lewis R. S., Alt F. W., Crabtree G. R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 16.Gulbranson-Judge A., Tybulewicz V. L., Walters A. E., Toellner K. M., MacLennan I. C., Turner M. Defective immunoglobulin class switching in Vav-deficient mice is attributable to compromised T cell help. Eur. J. Immunol. 1999;29:477–487. doi: 10.1002/(SICI)1521-4141(199902)29:02<477::AID-IMMU477>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Doody G. M., Bell S. E., Vigorito E., Clayton E., McAdam S., Tooze R., Fernandez C., Lee I. J., Turner M. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat. Immunol. 2001;2:542–547. doi: 10.1038/88748. [DOI] [PubMed] [Google Scholar]
- 18.Tedford K., Nitschke L., Girkontaite I., Charlesworth A., Chan G., Sakk V., Barbacid M., Fischer K. D. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2001;2:548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- 19.Inabe K., Ishiai M., Scharenberg A. M., Freshney N., Downward J., Kurosaki T. Vav3 modulates B cell receptor responses by regulating phosphoinositide 3-kinase activation. J. Exp. Med. 2002;195:189–200. doi: 10.1084/jem.20011571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujikawa K., Miletic A. V., Alt F. W., Faccio R., Brown T., Hoog J., Fredericks J., Nishi S., Mildiner S., Moores S. L., et al. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manetz T. S., Gonzalez-Espinosa C., Arudchandran R., Xirasagar S., Tybulewicz V., Rivera J. Vav1 regulates phospholipase Cγ activation and calcium responses in mast cells. Mol. Cell. Biol. 2001;21:3763–3774. doi: 10.1128/MCB.21.11.3763-3774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds L. F., Smyth L. A., Norton T., Freshney N., Downward J., Kioussis D., Tybulewicz V. L. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 2002;195:1103–1114. doi: 10.1084/jem.20011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos-Morales F., Druker B. J., Fischer S. Vav binds to several SH2/SH3 containing proteins in activated lymphocytes. Oncogene. 1994;9:1917–1923. [PubMed] [Google Scholar]
- 25.Bertagnolo V., Marchisio M., Volinia S., Caramelli E., Capitani S. Nuclear association of tyrosine-phosphorylated Vav to phospholipase C-γ1 and phosphoinositide 3-kinase during granulocytic differentiation of HL-60 cells. FEBS Lett. 1998;441:480–484. doi: 10.1016/s0014-5793(98)01593-2. [DOI] [PubMed] [Google Scholar]
- 26.Tybulewicz V. L. Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 2005;17:267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y., Janssen E. M., Duncan A. W., Altman A., Billadeau D. D., Abraham R. T. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 2002;21:4809–4819. doi: 10.1093/emboj/cdf499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doody G. M., Billadeau D. D., Clayton E., Hutchings A., Berland R., McAdam S., Leibson P. J., Turner M. Vav-2 controls NFAT-dependent transcription in B- but not T-lymphocytes. EMBO J. 2000;19:6173–6184. doi: 10.1093/emboj/19.22.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billadeau D. D., Mackie S. M., Schoon R. A., Leibson P. J. Specific subdomains of Vav differentially affect T cell and NK cell activation. J. Immunol. 2000;164:3971–3981. doi: 10.4049/jimmunol.164.8.3971. [DOI] [PubMed] [Google Scholar]
- 30.Bokoch G. M., Vlahos C. J., Wang Y., Knaus U. G., Traynor-Kaplan A. E. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem. J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolias K. F., Cantley L. C., Carpenter C. L. Rho family GTPases bind to phosphoinositide kinases. J. Biol. Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 32.Zheng Y., Bagrodia S., Cerione R. A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]
- 33.Piechulek T., Rehlen T., Walliser C., Vatter P., Moepps B., Gierschik P. Isozyme-specific stimulation of phospholipase C-γ2 by Rac GTPases. J. Biol. Chem. 2005;280:38923–38931. doi: 10.1074/jbc.M509396200. [DOI] [PubMed] [Google Scholar]
- 34.Yron I., Deckert M., Reff M. E., Munshi A., Schwartz M. A., Altman A. Integrin-dependent tyrosine phosphorylation and growth regulation by Vav. Cell Adhes. Commun. 1999;7:1–11. doi: 10.3109/15419069909034388. [DOI] [PubMed] [Google Scholar]
- 35.Miranti C. K., Leng L., Maschberger P., Brugge J. S., Shattil S. J. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr. Biol. 1998;8:1289–1299. doi: 10.1016/s0960-9822(07)00559-3. [DOI] [PubMed] [Google Scholar]
- 36.Gotoh A., Takahira H., Geahlen R. L., Broxmeyer H. E. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth. Differ. 1997;8:721–729. [PubMed] [Google Scholar]
- 37.Cichowski K., Brugge J. S., Brass L. F. Thrombin receptor activation and integrin engagement stimulate tyrosine phosphorylation of the proto-oncogene product, p95vav, in platelets. J. Biol. Chem. 1996;271:7544–7550. doi: 10.1074/jbc.271.13.7544. [DOI] [PubMed] [Google Scholar]
- 38.Obergfell A., Judd B. A., del Pozo M. A., Schwartz M. A., Koretzky G. A., Shattil S. J. The molecular adapter SLP-76 relays signals from platelet integrin αIIbβ3 to the actin cytoskeleton. J. Biol. Chem. 2001;276:5916–5923. doi: 10.1074/jbc.M010639200. [DOI] [PubMed] [Google Scholar]
- 39.Gakidis M. A., Cullere X., Olson T., Wilsbacher J. L., Zhang B., Moores S. L., Ley K., Swat W., Mayadas T., Brugge J. S. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J. Cell. Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao C., Schaefer E., Lakkis M., Blystone S. D. β3 tyrosine phosphorylation and αvβ3-mediated adhesion are required for Vav1 association and Rho activation in leukocytes. J. Biol. Chem. 2005;280:15422–15429. doi: 10.1074/jbc.M414457200. [DOI] [PubMed] [Google Scholar]
- 41.Watson S. P., Auger J. M., McCarty O. J., Pearce A. C. GPVI and integrin αIIbβ3 signaling in platelets. J. Thromb. Haemostasis. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 42.Pasquet J. M., Quek L., Stevens C., Bobe R., Huber M., Duronio V., Krystal G., Watson S. P. Phosphatidylinositol 3,4,5-trisphosphate regulates Ca2+ entry via Btk in platelets and megakaryocytes without increasing phospholipase C activity. EMBO J. 2000;19:2793–2802. doi: 10.1093/emboj/19.12.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poole A., Gibbins J. M., Turner M., van Vugt M. J., van de Winkel J. G., Saito T., Tybulewicz V. L., Watson S. P. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Judd B. A., Myung P. S., Obergfell A., Myers E. E., Cheng A. M., Watson S. P., Pear W. S., Allman D., Shattil S. J., Koretzky G. A. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J. Exp. Med. 2002;195:705–717. doi: 10.1084/jem.20011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wonerow P., Pearce A. C., Vaux D. J., Watson S. P. A critical role for phospholipase Cγ2 in αIIbβ3-mediated platelet spreading. J. Biol. Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 46.Goncalves I., Hughan S. C., Schoenwaelder S. M., Yap C. L., Yuan Y., Jackson S. P. Integrin αIIbβ3-dependent calcium signals regulate platelet-fibrinogen interactions under flow: involvement of phospholipase Cγ2. J. Biol. Chem. 2003;278:34812–34822. doi: 10.1074/jbc.M306504200. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki-Inoue K., Inoue O., Frampton J., Watson S. P. Murine GPVI stimulates weak integrin activation in PLCγ2−/− platelets: involvement of PLCγ1 and PI3-kinase. Blood. 2003;102:1367–1373. doi: 10.1182/blood-2003-01-0029. [DOI] [PubMed] [Google Scholar]
- 48.Deckert M., Tartare-Deckert S., Couture C., Mustelin T., Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 49.Tuosto L., Michel F., Acuto O. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J. Exp. Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tartare-Deckert S., Monthouel M. N., Charvet C., Foucault I., Van Obberghen E., Bernard A., Altman A., Deckert M. Vav2 activates c-fos serum response element and CD69 expression but negatively regulates nuclear factor of activated T cells and interleukin-2 gene activation in T lymphocyte. J. Biol. Chem. 2001;276:20849–20857. doi: 10.1074/jbc.M010588200. [DOI] [PubMed] [Google Scholar]
- 51.Fu C., Chan A. C. Identification of two tyrosine phosphoproteins, pp70 and pp68, which interact with phospholipase Cγ, Grb2, and Vav after B cell antigen receptor activation. J. Biol. Chem. 1997;272:27362–27368. doi: 10.1074/jbc.272.43.27362. [DOI] [PubMed] [Google Scholar]
- 52.Pearce A. C., Wonerow P., Marshall S. J., Frampton J., Gartner T. K., Watson S. P. The heptapeptide LSARLAF mediates platelet activation through phospholipase Cγ2 independently of glycoprotein IIb-IIIa. Biochem. J. 2004;378:193–199. doi: 10.1042/BJ20031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner M., Mee P. J., Walters A. E., Quinn M. E., Mellor A. L., Zamoyska R., Tybulewicz V. L. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity. 1997;7:451–460. doi: 10.1016/s1074-7613(00)80367-2. [DOI] [PubMed] [Google Scholar]
- 54.Machesky L. M., Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell. Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarty O. J., Larson M. K., Auger J. M., Kalia N., Atkinson B. T., Pearce A. C., Ruf S., Henderson R. B., Tybulewicz V. L., Machesky L. M., Watson S. P. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J. Biol. Chem. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barkalow K. L., Italiano J. E., Jr, Chou D. E., Matsuoka Y., Bennett V., Hartwig J. H. α-Adducin dissociates from F-actin and spectrin during platelet activation. J. Cell. Biol. 2003;161:557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yap C. L., Hughan S. C., Cranmer S. L., Nesbitt W. S., Rooney M. M., Giuliano S., Kulkarni S., Dopheide S. M., Yuan Y., Salem H. H., Jackson S. P. Synergistic adhesive interactions and signaling mechanisms operating between platelet glycoprotein Ib/IX and integrin αIIbβ3: studies in human platelets and transfected Chinese hamster ovary cells. J. Biol. Chem. 2000;275:41377–41388. doi: 10.1074/jbc.M005590200. [DOI] [PubMed] [Google Scholar]
- 58.Yuan Y., Kulkarni S., Ulsemer P., Cranmer S. L., Yap C. L., Nesbitt W. S., Harper I., Mistry N., Dopheide S. M., Hughan S. C., et al. The von Willebrand factor-glycoprotein Ib/V/IX interaction induces actin polymerization and cytoskeletal reorganization in rolling platelets and glycoprotein Ib/V/IX-transfected cells. J. Biol. Chem. 1999;274:36241–36251. doi: 10.1074/jbc.274.51.36241. [DOI] [PubMed] [Google Scholar]
- 59.Hellberg C., Molony L., Zheng L., Andersson T. Ca2+ signalling mechanisms of the β2 integrin on neutrophils: involvement of phospholipase Cγ2 and Ins(1,4,5)P3. Biochem. J. 1996;317:403–409. doi: 10.1042/bj3170403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki-Inoue K., Fuller G. L., Garcia A., Eble J. A., Pohlmann S., Inoue O., Gartner T. K., Hughan S. C., Pearce A. C., Laing G. D., et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 61.Pasquet J. M., Gross B., Quek L., Asazuma N., Zhang W., Sommers C. L., Schweighoffer E., Tybulewicz V., Judd B., Lee J. R., et al. LAT is required for tyrosine phosphorylation of phospholipase Cγ2 and platelet activation by the collagen receptor GPVI. Mol. Cell. Biol. 1999;19:8326–8334. doi: 10.1128/mcb.19.12.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wonerow P., Obergfell A., Wilde J. I., Bobe R., Asazuma N., Brdicka T., Leo A., Schraven B., Horejsi V., Shattil S. J., Watson S. P. Differential role of glycolipid-enriched membrane domains in glycoprotein VI- and integrin-mediated phospholipase Cγ2 regulation in platelets. Biochem. J. 2002;364:755–765. doi: 10.1042/BJ20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobes C. D., Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 64.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 65.Ridley A. J., Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 66.Czuchra A., Wu X., Meyer H., van Hengel J., Schroeder T., Geffers R., Rottner K., Brakebusch C. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol. Biol. Cell. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pellegrin S., Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr. Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 68.Liu B. P., Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol. Cell. Biol. 2000;20:7160–7169. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marignani P. A., Carpenter C. L. Vav2 is required for cell spreading. J. Cell Biol. 2001;154:177–186. doi: 10.1083/jcb.200103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abe K., Rossman K. L., Liu B., Ritola K. D., Chiang D., Campbell S. L., Burridge K., Der C. J. Vav2 is an activator of Cdc42, Rac1, and RhoA. J. Biol. Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- 71.Movilla N., Dosil M., Zheng Y., Bustelo X. R. How Vav proteins discriminate the GTPases Rac1 and RhoA from Cdc42. Oncogene. 2001;20:8057–8065. doi: 10.1038/sj.onc.1205000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.