Abstract
Human obesity is a global epidemic, which causes a rapidly increased frequency of diabetes and cardiovascular disease. One reason for obesity is the ready availability of refined food products with high caloric density, an evolutionarily new event, which makes over-consumption of food inevitable. Fat is a food product with high caloric density. The mechanism for regulation of fat intake has therefore been studied to a great extent. Such studies have shown that, as long as fat stays in the intestine, satiety is promoted. This occurs through the fat-released peptide hormones, the best known being CCK (cholecystokinin), which is released by fatty acids. Hence, retarded fat digestion with prolonged time for delivery of fatty acids promotes satiety. Pancreatic lipase, together with its protein cofactor, co-lipase, is the main enzymatic system responsible for intestinal fat digestion. We found that biological membranes, isolated from plants, animals or bacteria, inhibit the lipase/co-lipase-catalysed hydrolysis of triacylglycerols even in the presence of bile salt. We propose that the inhibition is due to binding of lipase/co-lipase to the membranes and adsorption of the membranes to the aqueous/triacylglycerol interface, thereby hindering lipase/co-lipase from acting on its lipid substrate. We also found that chloroplast membranes (thylakoids), when added to refined food, suppressed food intake in rats, lowered blood lipids and raised the satiety hormones, CCK and enterostatin. Consequently, the mechanism for satiety seems to be retardation of fat digestion allowing the fat products to stay longer in the intestine.
Keywords: blood lipid, chloroplast membrane, cholecystokinin (CCK), enterostatin, co-lipase, high-fat food intake, thylakoid
Abbreviations: CCK, cholecystokinin; DGDG, digalactosyldiacylglycerol; LHCII, light harvesting chlorophyll a/b protein complex II; MGDG, monogalactosyldiacylglycerol; NaTDC, sodium taurodeoxicholate
INTRODUCTION
Human obesity is a global epidemic that increases the occurrence of Type 2 diabetes and cardiovascular disease [1]. A likely reason for this obesity is the choice of refined food products with high caloric density, which makes over-consumption of food inevitable [2,3].
Several hormones, neuropeptides and metabolites regulate feeding. There is also a macronutrient-specific appetite regulation, dietary protein being the most satiating and dietary fat the least satiating nutrient [3]. The satiety effects have been explained by various factors released during intake of fat, carbohydrate and protein respectively. Since dietary fat is the least satiating macronutrient, the mechanism for regulation of fat intake has been much studied. The studies have shown that fat-induced satiety is intestinally mediated, i.e. as long as fat stays in the intestine, satiety is promoted [4]. This occurs through the release of fat-released gut hormones, the best known being CCK (cholecystokinin). CCK is released to blood from the intestine by fatty acids formed during digestion. It is widely accepted that CCK causes satiety, both in rats and humans [5]. We therefore hypothesized that retarded fat digestion with prolonged time for delivery of fatty acids without causing steatorrhoea would promote satiety.
The enzyme mainly responsible for intestinal fat digestion is pancreatic lipase and its obligatory cofactor, pancreatic co-lipase. They form a 1:1 molar complex, which is necessary for intestinal hydrolysis [6]. Here, we report that isolated biological membranes inhibit the pancreatic lipase/co-lipase-mediated hydrolysis of dietary fat. The inhibition is inherent in the hydrophobic, transmembrane, α-helices of the intrinsic membrane proteins. Moreover, we show that biological membranes, represented by chloroplast membranes, when added to refined food, suppress food intake in rats, lower blood lipids and increase the release of the satiety-inducing hormone, CCK.
EXPERIMENTAL
Preparation of membranes and membrane proteins
Thylakoids for use in the lipase assay were isolated as described in [7]. For preparing food, the thylakoids were isolated as follows: leaves were homogenized in a blender and filtered through four layers of Monodur polyester mesh (20 μm). The filtrate was centrifuged at 5000 g for 10 min to collect the thylakoids. These were washed by resuspension in water and re-centrifuged as above.
Removal of lipids was carried out as follows. Thylakoid suspension (4 ml; 3.8 mg of chlorophyll/ml) was mixed with 40 ml of chloroform/methanol and incubated for 1 h on ice. After centrifugation at 4000 g for 10 min, the pellet was extracted for a second time and centrifuged as above. The pellet was dried in air and extracted, on ice, with 10 ml of the buffer solution used for thylakoid isolation to remove water-soluble proteins. The mixture was centrifuged at 4000 g for 10 min, and the pellet was collected. The pellet is named ‘membrane protein fraction’ (Figure 1C).
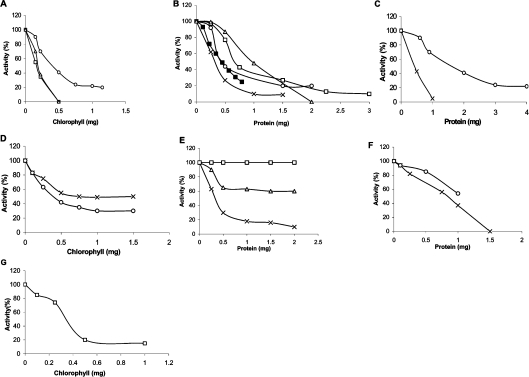
Figure 1. Inhibition of pancreatic lipase by biological membranes and membrane proteins (A–F: in the presence of co-lipase and bile salt).
(A) Thylakoids isolated from spinach (○), clover (□) and Arabidopsis thaliana (△). (B) Mitochondria from potato tuber (■), mitochondria from chicken heart (○), plasma membrane from spinach leaf (□), membranes from Synechocystis (×) and chromatophores of Rhodospirillum rubrum (△). (C) Membrane protein fraction obtained after removing lipids and soluble proteins from thylakoids (×) and untreated thylakoids (○). (D) Thylakoids before (○) and after (×) treatment with trypsin. (E) LHCII, a membrane protein isolated from spinach thylakoids (×), synthetic polypeptide with the same sequence as one of the membrane-spanning α-helices of LHCII, i.e. VIHCRWAMLGALGCVFPELL (△), and BSA (□). (F) Transhydrogenase (×) and cytochrome b6f complex (○). (G) Thylakoids from spinach in the absence of co-lipase and bile salt (□).
Trypsin treatment was carried out by incubating the thylakoids with 300 μg of trypsin (Sigma type III)/mg of chlorophyll, in 20 mM phosphate buffer (pH 7.4), for 45 min at 37 °C. After adding 1 mM PMSF, to inhibit the trypsin, the thylakoids were collected by centrifugation for 10 min at 900 g.
Chlorophyll was determined as described in [8] and protein by the method of Bradford [9]. Mitochondria from potato tubers [10] were a gift from Per Gardeström (Department of Plant Physiology, Umeå University, Umeå, Sweden). Chicken heart mitochondria were prepared by the method of Tang et al. [11] with some modifications: hearts were trimmed of fat, washed and cut into small pieces, on ice, with a pair of scissors and further by a mixer in a medium of 300 mM sucrose, 50 mM phosphate buffer (pH 7.4), 1 mM NaCl, 5 mM MgCl2 and then homogenized with a Potter–Elvehjem-type homogenizer for 1 min and centrifuged for 10 min at 900 g. The supernatant was centrifuged once more at the same time and speed. Then, the supernatant was centrifuged for 15 min at 10000 g. The pellet was washed twice at the same speed and time in the same medium. The pellet was collected.
Plasma membranes from spinach leaves [12] were a gift from Christer Larsson (Department of Biochemistry, Lund University, Lund, Sweden); and membranes from Synechosystis [13] were a gift from Birgitta Norling (Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden). Intracytoplasmic membrane fragments, chromatophores, from Rhodospirillum rubrum [14] were a gift from Agneta Norén (Department of Biochemistry and Biophysics, Stockholm University, Stockholm, Sweden); before use, extrinsic water-soluble proteins were removed by washing with 0.5 M NaCl and 25 mM Tris/HCl (pH 7.8) followed by two washings with the Tris buffer only, as described in [14].
The membrane protein LHCII (light harvesting chlorophyll a/b protein complex II) was prepared as described in [15]. Transhydrogenase [16] from Escherichia coli was a gift from Jan Rydström (Department of Biochemistry and Biophysics, Gothenburg University, Gothenburg, Sweden) and cytochrome b6f from spinach leaves [17] was a gift from Jörgen Ström (Department of Biochemistry, Lund University, Lund, Sweden).
The synthetic polypeptide VIHCRWAMLGALGCVFPELL was obtained from Innovagen AB (Lund, Sweden).
Binding studies
Lipase or lipase/co-lipase (15 ml), at the same concentrations and in the same buffer as used in the lipase assay (see below), were mixed with increasing amounts of thylakoids at room temperature (20 °C) for 1 min. The thylakoids were pelleted by centrifugation at 6800 g for 10 min in the experiments without NaTDC (sodium taurodeoxicholate) and at 8600 g for 10 min when NaTDC was present. The clear chlorophyll-free supernatants were collected; triacylglycerol (0.5 ml) was added and the lipase activity assayed with the pH-stat (see below).
Partition of thylakoids in aqueous/oil two-phase systems
Thylakoids containing 1 mg of chlorophyll were added to a phase system of 9 ml of water and 1 ml of rapeseed oil. After mixing using a homogenizer the phases were allowed to settle for 2 h at room temperature. The upper layer was a stable oil-in-water emulsion containing thylakoids attached to the droplets.
Electron microscopy
The rapeseed oil-in-water emulsion (described in the previous paragraph) was fixed with 2.5% (w/v) glutaraldehyde in 0.15 M cacodylate buffer, embedded in Epon, and stained with 3% (v/v) uranyl acetate and lead citrate.
Calculation of surfaces of triacylglycerol and of thylakoids
Thylakoids consist of 50% protein and 50% lipids. Pigments constitute 26% of the lipids, of which chlorophyll (a+b) account for 85%, i.e. 22% of the lipids. Of the lipids 74%, or 3.36 mg/mg of chlorophyll, are membrane-forming lipids i.e. MGDG (monogalactosyldiacylglycerol) and DGDG (digalactosyldiacylglycerol). With a density of 0.8 for the lipids, their volume will be 4.2 μl/mg of chlorophyll. Calculation of the bilayer surface, assuming a thickness of 40 Å (1 Å=0.1 nm), gives 1 m2/mg of chlorophyll. Together with the membrane proteins the thylakoid surface will be approx. 2 m2/mg of chlorophyll. This is in agreement with the value obtained by Flores et al. [18]. They measured the volume occupied by a known amount of membranes and divided this value by the membrane thickness, estimated to be 60 Å from electron micrographs. This gave a value of 1.85 m2/mol of chlorophyll, i.e. approx. 1.7 m2/mg of chlorophyll, assuming an average molecular mass for chlorophyll to be 900 (chlorophyll a/b ratio=3).
Feeding experiments
Female Sprague–Dawley rats (200 g) from B&K (Sollentuna, Sweden) were housed in a temperature-controlled room (22±1 °C) under a 12 h light (6:00–18:00 h)/12 h dark cycle, given free access to water, and fed ad libitum on a standard chow until the start of the experiment as detailed below. All procedures using animals were approved by the Local Animal Ethics Committee of Lund. For measurement of food consumption, rats were individually housed in cages and given a high-fat diet for one week before the start of the study. The high-fat diet consisted of a diet containing by energy 42.1% fat, 23.9% protein and 34.0% carbohydrate with a caloric density of 4.7 kcal/g (1 kcal≈4.184 kJ) as described in [19]. The high-fat diet containing thylakoids was prepared as for the high-fat diet with the addition of purified thylakoids at a concentration of 2 mg of chlorophyll/g of food. Food intake was measured daily and body weight was measured at the start and end of the feeding period. Cages were carefully monitored for evidence of food spillage.
Blood lipids, glucose and CCK analysis
Blood was drawn from the intra-orbital bulbar plexus under isoflurane anaesthesia and collected in ice-cold tubes. Serum was obtained and stored at −20 °C until analysis. Serum-free fatty acids were measured using a NEFAC kit (Wako Chemicals, Neuss, Germany). Serum triacylglycerols were measured using a GPO-Trinder kit (SIGMA Diagnostics, Steinheim, Germany). Serum glucose was analysed by Infinity™ Glucose Oxidase Liquid Stable Reagent (Thermo AB). Plasma CCK concentrations were measured using a highly specific antiserum (no. 92128) that does not bind any of the homologous gastrin peptides [20].
Pancreatic lipase/co-lipase activity measurement
Co-lipase-dependent lipase activity was determined with pH-stat titration (Mettler Components DK 10, DK 11 and DV11) using 0.2 M NaOH. The substrate was prepared in a vial by adding 0.5 ml of tributyrine to 15 ml of buffer [2 mM Tris maleate (pH 7.0), 4 mM NaTDC, 1 mM CaCl2 and 0.15 M NaCl]. The incubation was performed at 25 °C. Stirring was maintained with a magnetic rod under standardized conditions. Lipase (0.1 mg/ml; 10 μl) was added followed by co-lipase (0.1 mg/ml; 10 μl) as described in [21]. The activity was recorded for a few minutes, thereafter the various membrane and protein solutions were added and the activity recorded.
Western-blot analysis of pancreatic lipase
Pancreases from rats were homogenized and 10 μg of total protein was applied on to an SDS/10% polyacrylamide gel. Proteins were transferred on to nitrocellulose membranes and lipase protein was detected by immunostaining using a polyclonal lipase antiserum diluted 1:2000 (a rabbit anti-porcine lipase that recognizes both rat and mouse pancreatic lipase) and developed with chemiluminescence [22].
Statistics
The StatView software program was used for statistical analysis. The data were analysed using two-way ANOVA followed by post hoc tests for comparison of individual differences or Student's t test (paired comparison). All data are presented as means±S.E.M. The difference was considered significant if P<0.05.
RESULTS AND DISCUSSION
Inhibition of pancreatic lipase
We first assessed if thylakoid membranes – the photosynthetic membranes of chloroplasts – were able to affect lipolysis by pancreatic lipase/co-lipase in vitro. Thylakoid membranes powerfully suppressed the lipolytic activity in a dose-dependent way during in vitro hydrolysis of an emulsion of triacylglycerols dispersed in bile salt (Figure 1A). Other biological membranes, such as mitochondria, plasma membranes and bacterial membranes, also inhibited lipolysis (Figure 1B). This effect, therefore, seems to be a general property of biological membranes.
We next separated the proteins from the lipids in the thylakoid membranes by methanol/chloroform extraction and found that the inhibiting component was contained in the protein fraction (Figure 1C). Membrane proteins are either intrinsic or extrinsic, treatment with trypsin being able to remove most of the extrinsic as well as the exposed loops of the intrinsic proteins. We found that thylakoids treated with trypsin were still able to inhibit lipolysis (Figure 1D), suggesting that the membrane-spanning region of the intrinsic proteins possessed the inhibitory effect.
We therefore isolated one of the major intrinsic membrane proteins of thylakoids, LHCII, which constitutes about half of the protein mass of the thylakoid, and found that this protein alone inhibited lipolysis (Figure 1E). LHCII contains three hydrophobic membrane-spanning α-helices [23] and we hypothesized that the hydrophobicity of these is responsible for the inhibition. If so, a synthetic polypeptide with the same sequence as one of the helices of LHCII should inhibit the lipolysis. Addition of the synthetic peptide inhibited the lipolysis (Figure 1E), although not as powerfully as the three membrane-spanning helices of LHCII, suggesting that the number of membrane-spanning helices, i.e. the length of the protein molecule, was important for the inhibition. Two intrinsic membrane protein complexes, transhydrogenase [16] and cytochrome b6f [24,25], which both have several hydrophobic α-helices, were also found to inhibit lipolysis (Figure 1F). By contrast, water-soluble proteins, like serum albumin, did not affect lipolysis (Figure 1E), in agreement with previous observations using the same assay including NaTDC as used here [26]. Thylakoids were found to inhibit lipase activity also in the absence of co-lipase and NaTDC (Figure 1G).
Mechanism of lipase inhibition by thylakoids
In order to gain information on the mechanism behind the inhibition of thylakoids on lipase/co-lipase activity, we investigated if thylakoids bound lipase/co-lipase directly and if thylakoids bound to the triacylglycerol/water interface.
Binding of lipase to thylakoid membranes
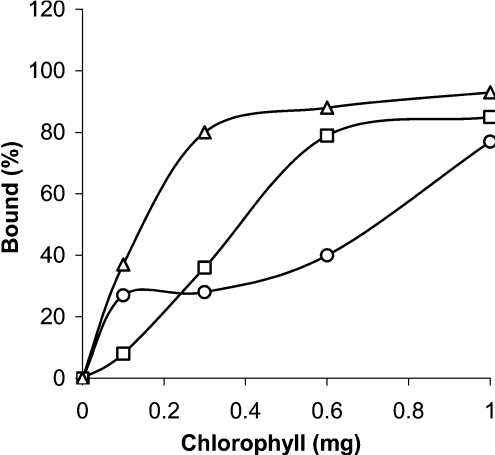
In the absence of bile salt, both lipase and the lipase/co-lipase complex bound to isolated thylakoids, the lipase/co-lipase complex having the strongest affinity for the thylakoids (Figure 2). This is probably due to the larger surface and exposure of more exposed hydrophobic groups of the lipase/co-lipase complex compared with lipase alone. It is known that lipase is a rather hydrophilic protein, while co-lipase is hydrophobic [26–31]. The binding of lipase and the lipase/co-lipase complex to thylakoid probably involves both ionic and hydrophobic interactions. In the presence of bile salt, the lipase/co-lipase complex seems to bind in a weaker manner to the thylakoids than in the absence of bile salt (Figure 2). However, at a concentration of thylakoids equivalent to 1 mg of chlorophyll, approx. 80% of the lipase/co-lipase complex is bound to the thylakoids, both with and without bile salts, suggesting this to be one reason for the observed inhibition of lipase/co-lipase activity by thylakoids shown in Figure 1(A).
Figure 2. Binding of lipase to thylakoids as a function of added thylakoids (expressed as mg of chlorophyll).
Lipase alone (□), lipase/co-lipase in the absence (△) and in the presence (○) of NaTDC. For concentration of lipase, co-lipase and NaTDC, see the Experimental section.
Partitioning of thylakoids in an oil/water two-phase system
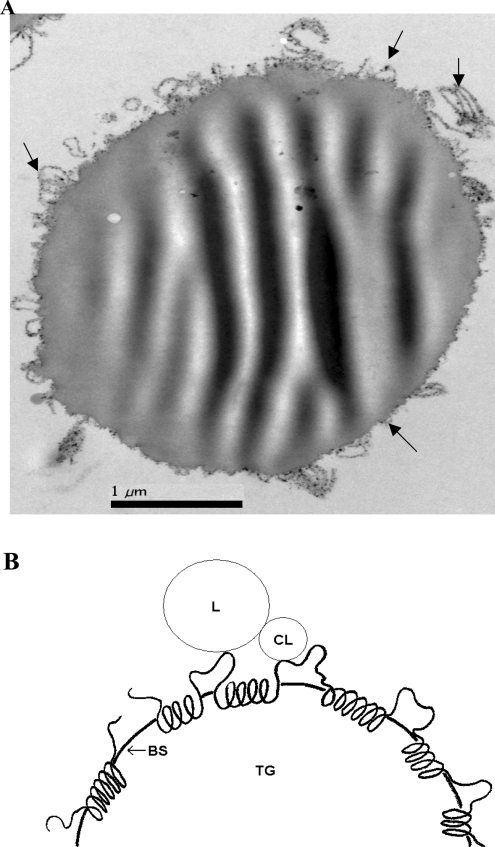
Thylakoid membranes were partitioned in an aqueous/triacylglycerol two-phase system. The thylakoids were all found at the interface. Electron microscopy shows how the thylakoids are firmly bound to the interface of the lipid drops, with no thylakoid membranes being visible in the aqueous phase (Figure 3A), indicating a strong affinity for the oil phase. That cell particles in general have a strong tendency to collect at liquid/liquid interfaces has been described and theoretically explained earlier [32].
Figure 3. Interfacial adsorption of membranes and membrane proteins.
(A) Electron microscopy of thylakoid membranes (arrows) covering the entire interface of an oil droplet in water. No thylakoids were found in the surrounding aqueous phase. (B) Schematic model of how an intrinsic membrane protein chain, adsorbed at the surface of a bile salt (BS)-covered triacylglycerol (TG) droplet, sterically hinders the anchoring of the lipase (L)/co-lipase (CL) complex to its triacylglycerol substrate. The hydrophobic helices are embedded in the lipid–bile salt surface layer, and together with the hydrophilic loops and tails they hinder the contact between lipase/co-lipase complex and the lipid.
A proposal for the mechanism of inhibition
In vitro, triacylglycerols are readily hydrolysed by pancreatic lipase even in the absence of its cofactor co-lipase. During intestinal fat digestion, however, dietary lipids are dispersed in bile salts, and in this situation co-lipase is obligatory for lipolysis by anchoring the lipase/co-lipase complex to the lipid/water interface. This attachment involves hydrophobic as well as ionic interactions [26–31]. For reviews on the catalytic mechanism of lipase/co-lipase, see [30,31] and references therein.
The conventional lipolysis system involves heterogeneous catalysis involving the lipase/co-lipase complex, bile salt and the surface of a lipid or micellar phase. This is in itself a rather complex system. By addition of biological membranes the system becomes even more complex, involving two different surfaces together with bile salt and the lipase/co-lipase complex, which can all interact with each other. Based on our data we propose that the inhibition of lipolysis by biological membranes can be due to two types of mechanism working together.
(i) Binding of the membranes, or the membrane proteins, to the lipase/co-lipase complex thereby blocking its active site and preventing the enzyme complex from coming into in contact with the substrate. The binding involves both ionic and hydrophobic interactions. It is known that the lipase/co-lipase complex exposes on its surface several hydrophobic amino acid residues [28–31]. This type of mechanism is supported by the binding experiments (Figure 2).
Similar arguments can be applied to the inhibitory effect of intrinsic membrane proteins on lipolysis. Due to their hydrophobic α-helices, these can bind to the lipase/co-lipase complex and thereby inhibit its enzyme activity. Intrinsic membrane proteins can be considered as block polymers with strings of hydrophobic, transmembrane α-helices, comprising 20–25 hydrophobic amino acids residues separated by hydrophilic polypeptide stretches. In the flanking region of a transmembrane helix, there is often inserted one or two amino acid residues with charged side chains. This is the case with one of the transmembrane helices of LHCII used in the experiment shown in Figure 1(E). Near the N-terminus you find one arginine residue and near the C-terminus one glutamic acid residue. Thus the long hydrophobic string in the middle of the α-helix together with the charges has a strong potential for binding to the lipase/co-lipase complex, which exposes a large hydrophobic domain on its surface close to charged residues, see [31] and references therein.
(ii) Binding of the membranes or membrane proteins to the triacylglycerol/water interface, thereby covering the substrate surface and blocking the access of the lipase/co-lipase complex to the lipid/water interface. This type of mechanism is supported by the interfacial binding shown in Figure 3(A). A similar mechanism is proposed for intrinsic membrane proteins. The hydrophobic strings are adsorbed on the surface of the lipid–bile salt micelles, while the hydrophilic stretches, loops and tails protrude into the aqueous phase (Figure 3B). Together, the entire membrane protein sterically hinders the anchoring of the lipase/co-lipase complex on to the lipid substrate.
With this mechanism of action an obvious question is whether thylakoids could cover the lipid/water interface. In the lipase assay we use 0.5 ml of triacylglycerol. If we assume that the size of the triacylglycerol droplets in the lipase assay is 1 μm in diameter, then the total interfacial surface area of the droplets is 3 m2. Using two independent estimations (see the Experimental section), a thylakoid surface area was found to be approx. 2 m2/mg of chlorophyll. This means that the surface of the thylakoids and the surface of the oil droplet are of the same order of magnitude. It is therefore reasonable to assume that the thylakoids can inhibit lipase activity by binding to the oil/water interface, thereby preventing the access of lipase/co-lipase to its substrate. When bile salts are added the membranes will be unfolded and the membrane proteins more or less exposed, and these can cover a much larger area than the intact membrane vesicles.
The two mechanisms proposed above do not exclude each other, they rather complement each other and together they can explain the generality of the inhibitory action of biological membrane on lipase/co-lipase.
Effects of thylakoids added to food
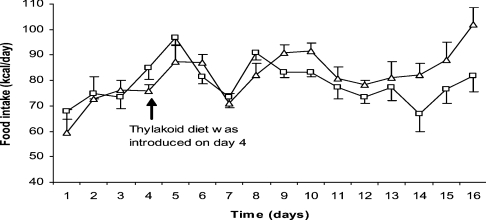
Having found that biological membranes inhibit lipolysis we investigated whether the retarded fat digestion could promote satiety in an animal model for diet-induced obesity using high-fat feeding. We found that thylakoid membranes, added to high-fat food, significantly suppressed food intake in rats (Figure 4) and caused a reduced body weight (Table 1). The reduction of food intake started a few days after the thylakoids had been given in the food. This suggests that thylakoids do not have any aversive effect, which otherwise should have acted immediately. Instead there seems to be a gradual inhibition of food intake, suggesting satiety signalling to be gradually up-regulated. Moreover, circulating levels of triacylglycerols were significantly reduced in the high-fat-fed thylakoid-treated animals compared with high-fat-fed controls (Table 1).
Figure 4. Effect of added thylakoids on Sprague–Dawley rats on a high-fat diet for 13 days.
Food intake with thylakoids (□) and without thylakoids (△). The daily food intake is given as means±S.E.M. from eight animals in each group (n=8). Treatment with thylakoids shows a significant suppression of food intake (two-way ANOVA; P=0.017).
Table 1. Effect of treatment with thylakoids on Sprague–Dawley rats on a high-fat diet for 13 days.
Values are means±S.E.M. *Significant level of P<0.05 between control diet and thylakoid treatment. **Significant level of P<0.01 between control diet and thylakoid treatment.
| Control diet | Thylakoid | |
|---|---|---|
| Body weight gain (g) | 60.5±3.55 | 49.9±3.05* |
| Serum triacylglycerol (mmol/l) | 1.02±0.13 | 0.62±0.04* |
| Lipase/co-lipase activity (units/mg) | 130.5±18.6 | 222.1±37.5* |
| Lipase protein expression (arbitrary units/mm2) | 2269±539 | 5305±809** |
| Plasma CCK (pmol/l) | 0.675±0.08 | 0.0862±0.12* |
We also found the satiety hormone CCK to be significantly elevated in the thylakoid-treated animals (Table 1). The time course for elevation of CCK is not known but may occur coincidentally with the satiety effect observed after day 4. We finally collected intestinal content and found an increased lipase/co-lipase activity (Table 1) in the thylakoid-treated animals compared with control as well as a significantly elevated pancreatic lipase protein expression (Table 1 and Figure 5). Since the animals had been starved for 24 h before the collection of intestinal content there were no thylakoids in the intestinal content, explaining the perhaps counterintuitive observation. The increased lipase activity in the thylakoid-treated animals is rather explained as a consequence of an increased lipase expression in the pancreas, the secreted pancreatic juice hence containing more lipase protein (Figure 5). The increased pancreatic enzyme activity may well be a consequence of elevated CCK levels, the increased CCK concentrations in plasma being due to a prolonged process of fat digestion. Thus the thylakoid-treated animals appear to respond, in a compensatory way, to a thylakoid-induced suppression of lipase/co-lipase activity by raising the endogenous secretion and production of lipase/co-lipase.
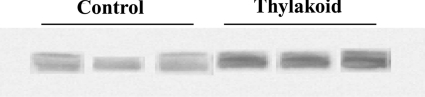
Figure 5. Effect of thylakoids on pancreatic lipase expression.
Representative Western blot of pancreatic lipase expression from pancreatic homogenate. Treatment with thylakoids during high-fat diet resulted in a significant increased expression of lipase in the pancreas compared with control.
It should be emphasized that steatorrhoea was not observed in the thylakoid-treated animals. This indicates that the satiety effects probably are due to a retardation of fat digestion. The membrane proteins added to inhibit lipase activity will be degraded by intestinal proteases and, with time, lose their inhibitory action on lipolysis. The fact that thylakoid membranes promoted the release of CCK is of particular interest since it means that fat digestion was retarded in such a way so as to promote, by a feedback mechanism, a compensatory secretion of lipolytic enzymes. This is a novel mechanism and does not occur with the lipase inhibitor orlistat, which inhibits lipase to 80% but causes undigested fat to leave the intestine through the faeces with no release of CCK [33]. A similar phenomenon was, however, observed by the use of another inhibitor for lipase/co-lipase, namely dimaele, a synthetic positively charged ether that was found to inhibit lipase/co-lipase activity in vitro, suppress feeding in vivo and to increase intestinal CCK secretion [34]. Whether the thylakoids act on a standard diet is not known and needs further studies. That CCK is important for the satiety of duodenal fat has been demonstrated in various animal models, including the Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors, showing a decreased responsiveness to the satiating effect of duodenal fat [35].
The elevated lipase/co-lipase activity induced by thylakoids infers that enterostatin is also elevated. Enterostatin is a pentapeptide produced from pro-co-lipase, in equivalent amounts to co-lipase in the intestine. It promotes satiety for fat and also induces thermogenesis [36,37]. The receptor for enterostatin has been demonstrated to be the F1-ATPase β-subunit, which when targeted causes a transient inhibition of ATP production and an increased thermogenesis [37,38]. The observed body weight loss induced by the thylakoid membranes may thus be both an effect of a suppressed appetite and an increased thermogenesis. It has been demonstrated that obese subjects often have a reduced diet-induced thermogenic response [39]. The addition of compounds into the food that raises thermogenesis is thus a valuable tool for promoting energy balance in obese subjects.
In summary, we have found that thylakoids, the photosynthetic membranes of chloroplasts, isolated from green leaves, suppress appetite in rats during intake of a typical refined food containing 42% fat, a level of fat found in the everyday energy intake in the Western diet. In addition the concentration of circulating triacylglycerols was reduced. We propose that the appetite suppression occurred through the retardation of intestinal fat digestion without causing steatorrhoea. The absence of steatorrhoea is probably due to the fact that the thylakoids will eventually be hydrolysed just like any cell membrane by intra-intestinal proteases, phospholipases and galactolipases. The lipolysis is thus only temporarily blocked.
The inhibitory effect on lipolysis appeared to be a general phenomenon associated with all intrinsic membrane proteins that have hydrophobic α-helices. Biological membranes in general have a favourable nutritional composition. They are composed of ‘functional’ proteins such as enzymes, transport proteins and receptors, and bilayer lipids, i.e. phospholipids, other charged lipids, sterols and glycolipids. The reason that we focused our interest on thylakoids is that their isolation is relatively simple and could easily be scaled up. Thylakoids are the most abundant of all biological membranes on earth. Thylakoids contain more than one hundred different membrane proteins; most of them are found in the major protein complexes [40–42] which, together with their bound pigments chlorophyll and carotenoids [43], account for approx. 70% of the thylakoid mass. Galactolipids with a dominance of ω-3 polyunsaturated fatty acids [44] account for the remaining 30%.
Acknowledgments
This work was funded by the Swedish Research Council (K2006-03X-07904-20C), Royal Physiographic Society (Lund, Sweden) and by Carl Trygger Foundation. We thank Per Gardeström, Christer Larsson, Birgitta Norling, Agneta Norén, Jan Rydström and Jörgen Ström for gifts of membranes and proteins. We thank Rita Wallén (Department of Cell and Organism Biology, Lund University, Lund, Sweden) for taking the electron micrographs.
References
- 1.Schwartz M. W., Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 2.Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic Clin. Pharmacol. Toxicol. 2005;97:61–73. doi: 10.1111/j.1742-7843.2005.pto_179.x. [DOI] [PubMed] [Google Scholar]
- 3.Woods S. C., Seeley R. J., Rushing P. A., D'Alessio D., Tso P. A. A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 2003;313:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 4.Ritter R. C. Gastrointestinal mechanisms of satiation for food. Physiol. Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Beglinger C., Degen L. Fat in the intestine as a regulator of appetite – role of CCK. Physiol. Behav. 2004;83:617–621. doi: 10.1016/j.physbeh.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino D., Cordle R. A., Kullman J., Erlanson-Albertsson C., Muglia L. J., Lowe M. E. Decreased postnatal survival and altered body weight regulation in procolipase-deficient mice. J. Biol. Chem. 2002;277:7170–7177. doi: 10.1074/jbc.M108328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasson E., Svensson P., Weibull C., Albertsson P. Å. Separation and characterization of stroma and grana membranes – evidence for heterogeneity in antenna size of both photosystem I and photosystem II. Biochim. Biophys. Acta. 1988;936:339–350. [Google Scholar]
- 8.Porra R. J., Thompson W. A., Kriedemann P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by absorption spectroscopy. Biochim. Biophys. Acta. 1989;975:384–394. [Google Scholar]
- 9.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Petit P. X., Edman P., Gardeström P., Ericson I. Some properties of mitochondria, mitoplasts and submitochondrial particles of different polarities from plant-tissues. Biochim. Biophys. Acta. 1987;890:377–386. [Google Scholar]
- 11.Tang Z., Iqbal M., Cawthon V., Bottje W. G. Heart and breast muscle mitochondrial dysfunction in pulmonary hypertension syndrome in broilers (Gallus domesticus) Comp. Biochem. Physiol. 2002;132:527–540. doi: 10.1016/s1095-6433(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 12.Larsson C., Sommarin M., Widell S. Isolation of highly purified plant plasma membranes and separation of inside-out and right-side out vesicles. Methods Enzymol. 1994;228:451–469. [Google Scholar]
- 13.Norling B., Zak E., Andersson B., Pakrasi H. 2D-isolation of pure plasma and thylakoid membranes from the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1994;436:189–192. doi: 10.1016/s0014-5793(98)01123-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Franke C. C., Nordlund S., Norén A. Reversible membrane association of dinitrogenase reductase activating glycohydrolase in the regulation of nitrogenase activity in Rhodospirillum rubrum; dependence on GlnJ and AmtB1. FEMS Microbiol. Lett. 2005;253:273–279. doi: 10.1016/j.femsle.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Andersson B., Albertsson P.-Å. Separation of membrane components by partition in dextran-containing polymer phase systems. Isolation of the light harvesting chlorofyll a/b protein. J. Chromatogr. 1981;890:131–141. [Google Scholar]
- 16.Althage M., Bizouarn T., Kindlund B., Mullins J., Ålander J., Rydström J. Cross-linking of transmembrane helices in proton-translocating nicotinamide nucleotide transhydrogenase from Escherichia coli: implications for the structure and function of the membrane domain. Biochim. Biophys. Acta. 2004;1659:73–82. doi: 10.1016/j.bbabio.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Hauska G. Preparations of electrogenic, proton-transporting cytochrome complexes of the b6/f type (chloroplast and cyanobacteria) and bel-type (Rhodopseudomonas sphaerodoides) Methods Enzymol. 1986;126:271–285. [Google Scholar]
- 18.Flores S., Graan T., Ort D. R. Measurement of the permeability of the chloroplast thylakoid membrane to amine buffers. Photochem. Photobiophys. 1983;6:293–304. [Google Scholar]
- 19.Lindqvist A., de la Cour C. D., Stegmark A., Håkansson R., Erlanson-Albertsson C. Overeating of palatable food is associated with blunted leptin and ghrelin responses. Regul. Pept. 2005;130:123–132. doi: 10.1016/j.regpep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Rehfeld J. F. Accurate measurement of cholecystokinin in plasma. Clin. Chem. 1998;44:991–1001. [PubMed] [Google Scholar]
- 21.Erlanson-Albertsson C., Larsson A., Duan R. Secretion of pancreatic lipase and colipase from rat pancreas. Pancreas. 1987;2:531–535. doi: 10.1097/00006676-198709000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Rippe C., Berger K., Mei J., Lowe M. E., Erlanson-Albertsson C. Effect of long-term high-fat feeding on the expression of pancreatic lipases and adipose tissue uncoupling protein in mice. Pancreas. 2003;26:36–42. doi: 10.1097/00006676-200303000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z. F., Yan H. C., Wang K. B., Kuang T. Y., Zhang J. P., Gui L. L., An X. M., Chang W. R. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature. 2004;428:287–292. doi: 10.1038/nature02373. [DOI] [PubMed] [Google Scholar]
- 24.Kurisu G., Zhang H. M., Smith J. L., Cramer W. A. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science. 2003;302:1009–1014. doi: 10.1126/science.1090165. [DOI] [PubMed] [Google Scholar]
- 25.Strobel D. S., Choquet Y., Popot J. L., Picot D. An atypical haem in the cytochrome b6f complex. Nature. 2003;426:413–414. doi: 10.1038/nature02155. [DOI] [PubMed] [Google Scholar]
- 26.Borgström B., Erlanson C. Interactions of serum albumin and other proteins with porcine pancreatic lipase. Gastroenterology. 1978;75:382–386. [Google Scholar]
- 27.Patton J. S., Albertsson P. A., Erlanson C., Borgstöm B. Binding of porcine pancreatic lipase and colipase in the absence of substrate studied by two-phase partition and affinity chromatography. J. Biol. Chem. 1978;253:4195–4202. [PubMed] [Google Scholar]
- 28.van Tilbeurgh H., Egloff M. P., Martinez C., Rugani N., Verger R., Cambillau C. Interfacial activation of the lipase–procolipase complex by mixed micelles revealed by X-ray crystallography. Nature. 1993;362:814–820. doi: 10.1038/362814a0. [DOI] [PubMed] [Google Scholar]
- 29.Chahinian H., Bezzine S., Ferrato F., Ivanova M. G., Perez B., Lowe M. E., Carriére F. The β5′loop of the pancreatic lipase C2-like domain plays a critical role in the lipase–lipid interactions. Biochemistry. 2002;41:13725–13735. doi: 10.1021/bi0257944. [DOI] [PubMed] [Google Scholar]
- 30.Lowe M. E. Molecular mechanism of pancreatic lipase and colipase. In: Mansbach C., Tso P., Kuksis E., editors. Intestinal Lipid Metabolism. New York: Plenum Press; 2001. pp. 37–59. [Google Scholar]
- 31.Brockman H. Pancreatic lipase. In: Mansbach C., Tso P., Kuksis E., editors. Intestinal Lipid Metabolism. New York, and Interscience, New York: Plenum Press; 2001. pp. 61–79. [Google Scholar]
- 32.Albertsson P. A. New York: Wiley; 1986. Partition of Cell Particles and Macromolecules. [Google Scholar]
- 33.Goedecke J. H., Barsdorf M., Beglinger C., Levitt N. S., Lambert E. V. Effects of a lipase inhibitor (Orlistat) on cholecystokinin and appetite in response to a high-fat meal. Int. J. Obes. Relat. Metab. Disord. 2003;27:1479–1485. doi: 10.1038/sj.ijo.0802436. [DOI] [PubMed] [Google Scholar]
- 34.Mei J., Lindqvist A., Krabisch L., Rehfeld J. F., Erlanson-Albertsson C. Appetite suppression through delayed fat digestion. Physiol. Behav. 2006;89:563–568. doi: 10.1016/j.physbeh.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz G. J., Whitney A., Skoglund C., Castonguay T. W., Moran T. H. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am. J. Physiol. 1999;277:R1144–R1151. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- 36.Erlanson-Albertsson C. Enterostatin/procolipase – a peptide system regulating fat intake. In: Mansbach C., Tso P., Kuksis E., editors. Intestinal Lipid Metabolism. New York: Plenum Press; 2001. pp. 105–118. [Google Scholar]
- 37.Berger K., Sivars U., Winzell M. S., Johansson P., Hellman U., Rippe C., Erlanson-Albertsson C. Mitochondrial ATP synthase – a possible target protein in the regulation of energy metabolism in vitro and in vivo. Nutr. Neurosci. 2002;5:201–210. doi: 10.1080/10284150290008604. [DOI] [PubMed] [Google Scholar]
- 38.Park M., Lin L., Thomas S., Braymer H. D., Smith P. M., Harrison D. H. T., York D. A. The F1-ATPase beta-subunit is the putative enterostatin receptor. Peptides. 2004;25:2127–2133. doi: 10.1016/j.peptides.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Jequier E. Thermogenic responses induced by nutrients in man: their importance in energy balance regulation. Experientia Suppl. 1983;44:26–44. doi: 10.1007/978-3-0348-6540-1_3. [DOI] [PubMed] [Google Scholar]
- 40.Albertsson P. Å. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 2001;6:349–358. doi: 10.1016/s1360-1385(01)02021-0. [DOI] [PubMed] [Google Scholar]
- 41.Nelson N., Ben Sham A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004;5:971–982. doi: 10.1038/nrm1525. [DOI] [PubMed] [Google Scholar]
- 42.Nelson N., Yocum C. F. Structure and function of Photosystem I and II. Annu. Rev. Plant Biol. 2006;57:521–565. doi: 10.1146/annurev.arplant.57.032905.105350. [DOI] [PubMed] [Google Scholar]
- 43.Arvidsson P.-O., Carlsson M., Stefansson H., Albertsson P.-Å., Åkerlund H.-E. Violaxanthin accessibility and temperature dependency for de-epoxidation in spinach thylakoid membranes. Photosynth. Res. 1997;52:39–48. [Google Scholar]
- 44.Duchene S., Siegenthaler P. A. Do glycerolipids display lateral heterogeneity in the thylakoid membrane? Lipids. 2000;35:739–744. doi: 10.1007/s11745-000-0580-4. [DOI] [PubMed] [Google Scholar]