Abstract
Endometrial stromal sarcomas account for 0.25% of all uterine malignancies. These tumours were originally divided into low grade and high grade stromal sarcomas, but the recent World Health Organisation classification (2003) recognises low grade stromal sarcoma and undifferentiated endometrial sarcoma. Low grade sarcomas may exhibit other forms of differentiation, including smooth muscle and sex cord differentiation. In the latter form, the tumour contains epithelial-like or sex cord-like elements often with epithelioid appearance, arranged in nests, cords, trabeculae, solid, or tubular structures. If this element predominates, the tumour is considered to be a uterine tumour resembling ovarian sex cord tumour, and may cause diagnostic difficulties. This case report describes the histological and immunohistochemical features of a uterine stromal sarcoma showing exclusively a pattern reminiscent of ovarian sex cord tumour.
Keywords: uterus, endometrial stromal sarcoma, sex cord differentiation
Endometrial stromal sarcomas, which represent 0.25% of all uterine malignancies, were originally divided into low grade and high grade, but the 2003 World Health Organisation classification recognises low grade stromal sarcoma and undifferentiated endometrial sarcoma.1
Other forms of differentiation may be present in low grade sarcomas, including smooth muscle and sex cord differentiation,2–4 in which the tumour contains epithelial-like or sex cord-like elements. If this element predominates the tumour is considered to be a uterine tumour resembling ovarian sex cord tumour (UTROSCT), and may cause diagnostic difficulties.2,3
We describe the histological and immunohistochemical features of a uterine stromal sarcoma with an exclusive pattern reminiscent of ovarian sex cord tumour.
CASE REPORT
Clinical presentation
A 74 year old woman presented with recurrent postmenopausal bleeding. Initially, atypical hyperplasia of the endometrium was diagnosed and she was treated with progestogens. However, her bleeding recurred and further endometrial sampling revealed a poorly differentiated carcinoma. A subsequent total abdominal hysterectomy with bilateral salpingoophorectomy was performed.
MATERIALS AND METHODS
The surgical specimen was fixed in 10% neutral buffered formalin. Sections for histological examination were routinely processed in paraffin wax and stained by the avidin–biotin complex method using a Labvision (Freemont, California, USA) autostainer. The following antibodies were used: Cam5.2 (Becton-Dickinson, Oxford, UK), AE1/AE3 (Dako, Ely, Cambridgeshire, UK), 34β E12 (Novocastra, Newcastle on Tyne, UK), anti-CK18 (Novocastra), anti-vimentin (Dako), anti-S100 (Dako), anti-desmin (Novocastra), anti-actin (Novocastra), anti-inhibin (Dako), anti-CD10 (Novocastra), anti-oestrogen (clone 6F11; Novocastra), anti-CD99 (Dako), anti-calretinin (Novocastra), and anti-WT1 (Santa-Cruz Biotechnology, Santa Cruz, California, USA).
Immunostaining was carried out using appropriate positive controls.
Macroscopic findings
The surgical specimen was a total hysterectomy specimen consisting of the uterine body (65 × 65 × 45 mm) and a separate cervix (30 × 35 × 20 mm). There were also separate bilateral adnexa: one ovary (25 × 15 × 20 mm) with attached tube (40 × 5 × 5 mm) and the other ovary (25 × 25 × 15 mm) with attached tube (40 × 5 × 5 mm).
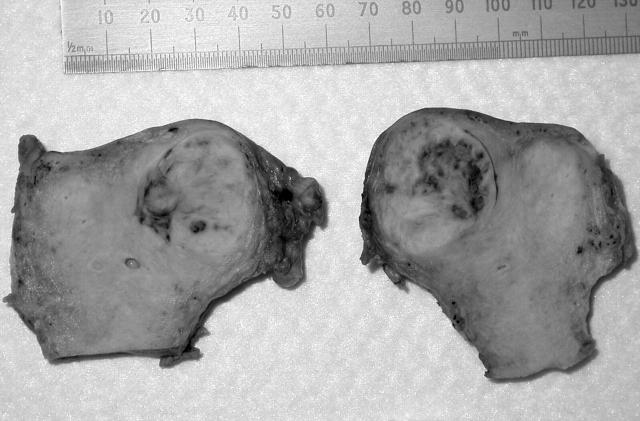
In the right uterine cornu there was a fairly well circumscribed tumour mass, 22 mm in diameter, deeply invading the myometrium and protruding into the uterine cavity. The tumour cut surface was soft, white, and partly haemorrhagic (fig 1).
Figure 1.
Macroscopically, the tumour was well circumscribed with a white, soft cut surface.
Both adnexa were unremarkable. Representative sections from the tumour were taken and were examined microscopically.
Microscopic findings
The luminal aspect of the tumour was lined by attenuated endometrial epithelium.
The tumour mass displayed fairly well circumscribed borders, and the entire tumour showed a pattern reminiscent of an ovarian sex cord stromal tumour. The tumour cells were arranged into sheets with the formation of cords, trabeculae, rosettes, and pseudotubular structures (fig 2).
Figure 2.
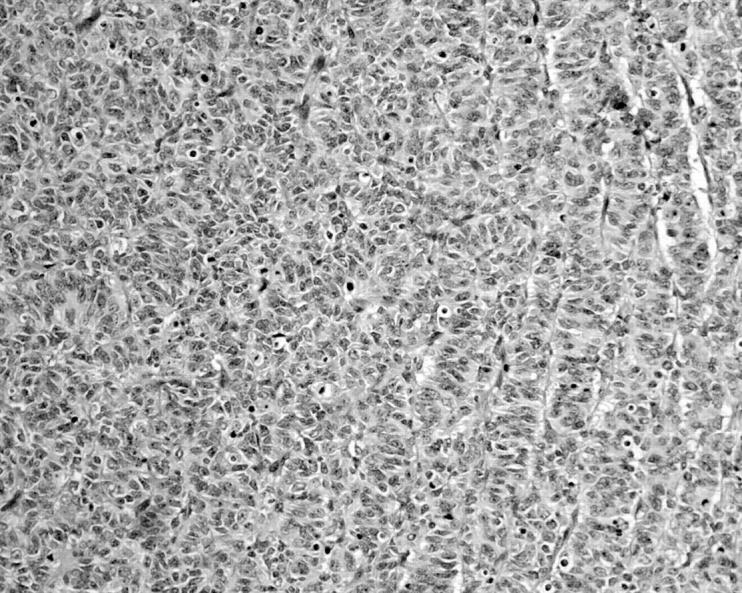
The tumour cells were arranged into cords, trabeculae, and rosettes highly resembling an ovarian sex cord tumour (haematoxylin and eosin stain; original magnification, ×200).
The cells showed epithelial-like features with vesicular nuclei and prominent nucleoli. There was no necrosis within the tumour. The mitotic activity was moderate: seven mitoses/10 high power fields. Extensive intratumorous lymphovascular invasion was noted.
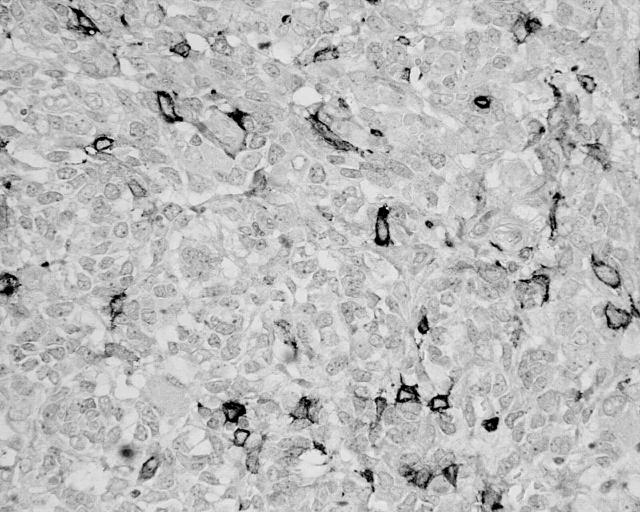
Immunohistochemistry revealed that the tumour cells were negative for epithelial markers—Cam5.2, CK18, 34β E12, and AE1/AE3—and also for S100 and smooth muscle markers—desmin and smooth muscle actin. Staining for inhibin, progesterone, and calretinin was also negative. The tumour showed widespread positivity for vimentin, focal nuclear positivity for WT-1, and focal membrane positivity for CD99 and CD10 (fig 3).
Figure 3.
Tumour cells showing focal positivity for CD10 (CD10 immunostaining; original magnification, ×400).
DISCUSSION
UTROSCT was first described by Clement and Scully in 1976. They described 14 endometrial stromal sarcomas with epithelial-like differentiation of a different pattern.2
These tumours are thought to be a rare and unique variant of uterine stromal sarcoma and pose problems in diagnosis and nomenclature. Histologically, they closely resemble ovarian sex cord tumours. The neoplastic cells grow predominantly in an epithelial-like pattern and form anastomosing trabeculae and cords. Some of these tumours can be accompanied by focal rhabdoid5 and smooth muscle4,6 differentiation. They were originally divided into two groups, namely: group I, made up of tumours with predominant endometrial stromal differentiation and only focal differentiation into sex cord-like elements; and group II, composed predominantly or exclusively of a pattern reminiscent of ovarian sex cord tumour. Recently, the designation UTROSCT has been reserved for group II tumours only.7
UTROSCTs are generally round, well circumscribed masses, up to 10 cm in diameter, that may be submucosal, intramural, or subserosal. The cut surface is soft yellow or tan. Clinically, these tumours have a low malignant potential.2,3,8
In their original article, Clement and Scully found that on follow up eight patients with tumours composed predominantly or exclusively of sex cord-like elements were clinically free of disease four months to seven years postoperatively.2 However, because there are few reported cases and little or limited follow up in most cases, other authors think that UTROSCT should be regarded as unpredictable, with a potential for recurrence or possibly even metastasis, particularly if they are invasive, until longer follow up with larger numbers of cases becomes available.3
The pathological features of the present tumour were similar to those of previously reported cases of UTROSCT, so that the tumour was diagnosed as such. However, the immunohistochemical features of the tumour were somewhat inconsistent with the previously described cases. It was negative for inhibin, progesterone, calretinin, and smooth muscle actin, which have been described previously as positive in these tumours.3,5,9 The tumour showed strong diffuse vimentin positivity, and focal CD10, CD99, and WT1 positivity.
“Focal positivity for CD10, CD99, and WT-1 is well described in these tumours but none of these markers is specific”
The differential diagnosis included epithelioid leiomyoma, epithelioid leiomyosarcoma, epithelial neoplasm, mixed müllerian tumour, and metastatic ovarian sex cord tumour. These tumours could be ruled out mostly on morphology, with the help of immunohistochemistry. The tumour cells showed no evidence of smooth muscle differentiation microscopically—they did not have abundant eosinophilic cytoplasm and the tumour cells were also negative for smooth muscle markers. Epithelial and mixed müllerian tumours were ruled out because of the complete lack of glandular differentiation, and the absence of biphasic epithelial mesenchymal differentiation, which is characteristic of müllerian tumours. The tumour cells were uniform and showed no evidence of pleomorphism or atypia. In addition, the cells showed no immunoreactivity for epithelial markers.
Both ovaries were unremarkable and did not contain “primary” ovarian sex cord tumour.
The hysterectomy was performed in September 2003, and to date (15 months later) our patient has been clinically free of disease.
The diagnosis of endometrial stromal tumour resembling ovarian sex cord tumour is largely based on the highly characteristic microscopic appearances described above. Focal positivity for CD10, CD99, and WT-1 is well described in these tumours but none of these markers is specific.10
Familiarity with this tumour by gynaecologists and pathologists is essential to avoid misdiagnosis, because the correct diagnosis of this neoplasm is important in patient management.
Take home messages.
This case report describes a 74 year old woman who was diagnosed with uterine sarcoma resembling ovarian sex cord tumour
The morphological and histological features of the present tumour were similar to those of previously reported cases, although the immunohistochemical features were somewhat inconsistent with previously described cases
Gynaecologists and pathologists should be aware of this tumour because the correct diagnosis is important in patient management
Abbreviations
UTROSCT, uterine tumour resembling an ovarian sex cord tumour
REFERENCES
- 1.Fattaneh A, Tavassoli, Devlee P. Pathology and genetics of tumours of the breast and female genital organs. WHO classification of tumours. Lyon: WHO, 2003.
- 2.Clement PB, Scully RE. Uterine tumours resembling ovarian sex-cord tumours: a clinicopathological analysis of fourteen cases. Am J Clin Pathol 1976;66:512–25. [DOI] [PubMed] [Google Scholar]
- 3.Fukugana M, Miyazawa Y, Ushigome S. Endometrial low grade stromal sarcoma with ovarian sex cord-like differentiation: report of two cases with immunohistochemical and flow cytometric study. Pathol Int 1997;47:412–15. [DOI] [PubMed] [Google Scholar]
- 4.Oliva E, Clement B, Young RH. Endometrial stromal tumours: an update on a group of tumors with protean phenotype. Adv Anat Pathol 2000;7:257–81. [DOI] [PubMed] [Google Scholar]
- 5.McCluggage WG, Date A, Bharucha H, et al. Endometrial stromal sarcoma with sex cord-like areas and focal rhabdoid differentiation. Histopathology 1996;29:367–74. [DOI] [PubMed] [Google Scholar]
- 6.Zamecnik M, Michal M. Endometrial stromal nodule with retiform sex-cord differentiation. Pathol Res Pract 1998;194:449–53. [DOI] [PubMed] [Google Scholar]
- 7.Olive E, Young RH, Amin MB, et al. An immunohistochemical analysis of endometrial stromal and smooth muscle tumours of the uterus. A study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol 2002;26:403–12. [DOI] [PubMed] [Google Scholar]
- 8.Dionigi A, Oliva E, Clement PB, et al. Endometrial stromal nodules and endometrial stromal tumours with limited infiltration. Am J Surg Pathol 2002;26:567–81. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Blakey GL, Zhang L, et al. Uterine tumour resembling ovarian sex cord tumour: report of a case with t(X;6)(p22.3;q23.1) and t(4;18)(q21.1;q23.1). Diagn Mol Pathol 2003;12:174–80. [DOI] [PubMed] [Google Scholar]
- 10.McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology 2001;39:273–8. [DOI] [PubMed] [Google Scholar]