Abstract
Mammalian spermatozoa become motile at ejaculation, but before they can fertilize the egg, they must acquire more thrust to penetrate the cumulus and zona pellucida. The forceful asymmetric motion of hyperactivated spermatozoa requires Ca2+ entry into the sperm tail by an alkalinization-activated voltage-sensitive Ca2+-selective current (ICatSper). Hyperactivation requires CatSper1 and CatSper2 putative ion channel genes, but the function of two other related genes (CatSper3 and CatSper4) is not known. Here we show that targeted disruption of murine CatSper3 or CatSper4 also abrogated ICatSper, sperm cell hyperactivated motility and male fertility but did not affect spermatogenesis or initial motility. Direct protein interactions among CatSpers, the sperm specificity of these proteins, and loss of ICatSper in each of the four CatSper−/− mice indicate that CatSpers are highly specialized flagellar proteins.
Keywords: calcium, contraception, flagella
Spermatozoa first acquire the potential for motility in the epididymis. They are capacitated in the female reproductive tract (1), where they acquire hyperactivated motility and other attributes that enable fertilization (2). During hyperactivation, the sperm tail motion changes from symmetric, fast, and low amplitude (sinusoidal) to asymmetric, slow, and large amplitude (whip-like; refs. 3–5). Hyperactivation is required for fertilization, providing the force needed to free the sperm cell from the oviductal reservoir and to penetrate the cumulus and zona pellucida surrounding the egg (1, 6, 7).
Sperm cells become motile and progress directionally once they enter the female reproductive tract. Ca2+-independent flagellar dynein and ATP orchestrate the low-amplitude sinusoidal-activated motility of the tail. As the sperm cells encounter a more alkaline environment in the higher female reproductive tract, they hyperactivate, a process that requires Ca2+ entry (3, 8, 9). Studies with antibodies or nucleotide probes have labeled several Ca2+-permeant channels, including voltage-sensitive Ca2+-selective channels (CatSpers and CaVs), cyclic nucleotide-gated channels, and transient receptor potential channels, in spermatocytes or spermatozoa (10–18). However, recent patch–clamp recordings of mouse epididymal spermatozoa (19) show that the predominant Ca2+-carrying current requires the CatSper1 gene that encodes a six-transmembrane-spanning protein of the voltage-gated ligand ion channel superfamily (20). In both whole-cell and perforated-patch configurations, the Ca2+-selective current (ICatSper) originated from the principal piece of the sperm tail and was absent in spermatozoa from CatSper1−/− mice. CatSper1−/− and CatSper2−/− male mice are infertile (11, 21), and sperm cells from CatSper1−/− and CatSper2−/− mice are unable to hyperactivate (4, 21). ICatSper was dramatically potentiated by a rise in intracellular pH, suggesting that the alkalinization that occurs during sperm capacitation activates ICatSper to increase intracellular [Ca2+] and induce hyperactivated motility (19).
In analogy to other voltage-gated ligand channel family proteins, CatSpers are likely subunits in tetrameric voltage-gated cation channels. Here we show that all four CatSper genes are required for functional ICatSper, hyperactivated motility, and male fertility.
Results
CatSper3 and CatSper4 Are Specifically Expressed in Testis and Sperm.
The CatSper family of ion channels encodes six-transmembrane proteins with homology to known ion channels (Fig. 1a). CatSper3 and CatSper4 have relatively low homology to CatSper1 (20% identity for CatSper3 and 24% for CatSper4) with conserved amino acids primarily in the transmembrane regions; the predicted N-terminal half of CatSper1 is unique among CatSpers [supporting information (SI) Fig. 5].
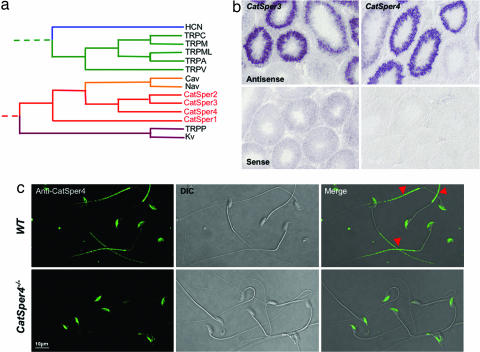
Fig. 1.
CatSper ion channels and CatSper3 and CatSper4 localization. (a) Simplified homology tree of voltage-gated ligand ion channels. One representative member of each channel family was chosen and aligned by using an identity matrix with ClustalW (Ver. 1.4). (b) In situ hybridization using CatSper3- and CatSper4-specific probes shows mRNA in specific stages within the testis. CatSper mRNAs were not detected in some stages of spermatogenic cells along the testis seminiferous epithelium, in contrast to the testis sections stained with sense probes. (c) Immunostaining of mouse epididymal sperm with anti-CatSper4 antibody. Labeling of the sperm head is nonspecific, as shown by comparison with the CatSper4−/− sperm; CatSper4 is specifically labeled only in the principal piece of the tail (arrowheads).
CatSper3 or CatSper4 were detected only in testis as assessed by Northern blot, in agreement with previous work (22). In situ hybridization in mouse testis sections with gene-specific antisense mRNA probes revealed stage-specific expression of both genes (Fig. 1b); early spermatocytes lacked CatSper mRNA. No expression was detected in somatic cells within the testis, suggesting that both CatSper3 and CatSper4 are sperm-specific proteins. Although the anti-CatSper3 antibody worked well for immunoprecipitation and Western blotting, it was not suitable for immunocytochemistry. As shown in Fig. 1c, CatSper4 was localized to the principal piece of the sperm tail. In contrast with the results reported by Jin et al. (22), we concluded that CatSper4 staining in the head was nonspecific, because it persisted in sperm heads from the CatSper4−/− mice (Fig. 1c).
Male Mice Lacking CatSper3 or CatSper4 Are Infertile.
To investigate the in vivo function of the proteins encoded by CatSper3 and CatSper4, we generated individual mouse lines lacking each of these genes. The putative pore region and a portion of the C terminus of CatSper3 and CatSper4 were deleted by homologous recombination (SI Fig. 6 A and B). Southern blotting and PCR screening demonstrated the presence of the mutant CatSper3 and CatSper4 genes in heterozygous and homozygous mice (SI Fig. 6 C–F).
Both CatSper3−/− and CatSper4−/− mutant mice were indistinguishable from their WT littermates in appearance, gross behavior, and survival. CatSper3−/− and CatSper4−/− female mice had normal mating behavior and gave rise to litters comparable to those of WT females when mated with WT males. However, when CatSper3−/− and CatSper4−/− male mice were mated with WT females, no litters were produced (Fig. 2a), indicating that the CatSper3- and CatSper4-null male mice are infertile. In contrast, 14 litters were produced from WT males, yielding an average of 7.8 pups per litter during the same period.
Fig. 2.
CatSper3−/− and CatSper4−/− male mice are infertile, and their sperm fail to hyperactivate. (a) WT, CatSper3−/−, or CatSper4−/− males (four each) were mated to two WT females over 3 months. WT males fathered 14 litters, compared with no litters for CatSper3−/− and CatSper4−/− males. As expected for WT 129Sv mice, an average of approximately eight pups per litter were born. (b) Initially, the percentage of motile sperm from WT and mutant mice was comparable. Over 90 min, 50–70% of isolated sperm cells from WT mice remained motile, whereas ≈80% of spermatozoa from CatSper−/− mice lost their motility. (c) In standard computer-assisted sperm analysis measurements of path velocity (VAP, velocity of the averaged path), linear velocity, and track velocity, WT and CatSper-null sperm cells were initially similar, with significant differences by 90 min. (d) Mature spermatozoa from WT, CatSper3−/−, or CatSper4−/− mice have normal morphology. (e) Measurement of bending angle (inset, α) shows that capacitated WT sperm cells have larger ranges of motion than CatSper3−/− and CatSper4−/− sperm cells. Thus, mutant mice spermatozoa lack this aspect of hyperactivated motility.
Spermatozoa from WT and all four CatSper mutant mice (CatSper1−/−, CatSper2−/−, CatSper3−/−, and CatSper4−/−) were similarly motile within a few minutes after isolation (Fig. 2b). As shown by computer-assisted sperm analysis, all had similar initial velocities irrespective of genotype (Fig. 2c). Linear and track velocity was reduced in mutant sperm after a 90-min incubation in vitro. Path velocities used to measure the directional component of sperm movement were also decreased in mutant sperm cells. Additionally, the percent motility of the CatSper mutant spermatozoa tended to decrease more rapidly than WT. No morphological differences between WT and mutant spermatozoa were observed, suggesting that disruption of either CatSper3 or CatSper4 genes did not affect spermatogenesis (Fig. 2d).
Most strikingly, the large subpopulation of both the CatSper3−/− and CatSper4−/− spermatozoa that remained actively motile failed to develop the hyperactive motility pattern even with extended incubation in capacitating conditions (see SI Movies 1–8), similar to the phenotypes reported for the CatSper1−/− and CatSper2−/− spermatozoa (4, 23). After 90 min in viscous conditions, WT sperm moved more vigorously than initially, whereas CatSper-null sperm lost their motility (compare SI Movies 3 and 4 and Movies 7 and 8). Capacitated sperm cells defective in their ability to hyperactivate motility exhibit decreased bending in the midpiece of the proximal flagellum (4, 23). Here the maximal bending angle of the principal bend in the midpiece, relative to 0° (straight), was only 47° in CatSper3−/− and 49° in CatSper4−/− compared with 92° in WT spermatozoa (Fig. 2e and SI Movies 9–11). Collectively, these results suggest that sperm hyperactivation and fertility require both CatSper3 and CatSper4.
ICatSper Is Not Detectable in CatSper3−/− and CatSper4−/− Sperm.
ICatSper is a Ca2+-selective pH-sensitive sperm current (19). A well known feature of Ca2+-selective channels is their high permeability to monovalent cations when extracellular divalent cations are omitted (24, 25). ICatSper is almost negligible when recorded from WT sperm cells in Hepes-saline (HS) solution (150 mM Na+/2 mM Ca2+) but, as is typical of Ca2+-selective currents, gave rise to a large inward Na+ current (−984 ± 16 pA at −100 mV) in divalent-free (DVF) solution (Fig. 3a and c). Monovalent CatSper current was not detectable (−16 ± 2 pA at −100 mV, indistinguishable from background current; Fig. 3b) in sperm from CatSper1−/− mice (19). As in CatSper1−/− mice, the monovalent current was not detected at −100 mV in sperm cells from CatSper2−/− (−17 ± 2 pA), CatSper3−/− (−17 ± 2 pA), and CatSper4−/− (−15 ± 2 pA) mice (Fig. 3 b and c). There was no measurable difference between WT and CatSper−/− background currents. ICatSper recorded from sNHE−/− (a Na+/H+ exchanger homolog that is important in progressive motility; ref. 26) was not noticeably different from WT (SI Fig. 7). Thus, functional ICatSper requires CatSper1, CatSper2, CatSper3, and CatSper4 putative channel proteins.
Fig. 3.
ICatSper was absent in CatSper−/− sperm. (a) Sperm (WT) whole-cell current evoked by a 1-s ramp from −100 to +100 mV, holding potential (HP) = 0 mV. The Na+-peak current recorded at −100 mV in DVF conditions was −970 pA and decreased to −13 pA in 2 mM Ca2+. (b) Monovalent current through CatSper channels (DVF solution) was absent in CatSper−/− sperm cells. (c) Average of the Na+ current in DVF solution measured from WT and CatSper−/− sperm cells.
Interaction of CatSper Ion Channel Proteins.
To investigate physical interactions among CatSper proteins, epitope-tagged CatSper2, CatSper3, and CatSper4 were first tested for interaction with CatSper1 in a heterologous expression system. Epitope-tagged CatSper2, CatSper3, and CatSper4 cDNAs were transiently expressed in a HEK-293 cell line (MZ8) stably expressing CatSper1; CatSper2, CatSper3, and CatSper4, were detected in Western blots from transfected MZ8 cells (Fig. 4a). Anti-FLAG or -HA antibody immunoprecipitated expressed FLAG-CatSper2, FLAG-CatSper4, or HA-CatSper3, respectively, from MZ8 total cell lysates; CatSper1 was detected in immune complexes with these proteins (Fig. 4a). CatSper1 was not detected in immunoprecipitates from control cells expressing unrelated ion channel proteins (FLAG-GIRK4/Kir3.4 or HA-TRPV6), suggesting that interactions among CatSper1 and the other three CatSper proteins were specific.
Fig. 4.
Interactions between CatSper proteins. (a) Epitope-tagged CatSper2 (FLAG-CS2), CatSper3 (HA-CS3), or CatSper4 (FLAG-CS4) were transfected into an MZ8 cell line stably expressing CatSper1 (CS1). FLAG-CatSper2, HA-CatSper3, and FLAG-CatSper4 were detected in the respective cell lysates (Left). After immunoprecipitation with anti-HA and -FLAG antibodies, immune complexes were probed with anti-CatSper1 antibody (Right). Negative controls were lysates from CatSper1 cells transfected with FLAG-GIRK4 or HA-TRPV6. (b) Proteins solubilized from testis microsomes were immunoprecipitated with specific anti-CatSper antibodies. Anti-CatSper3 and -CatSper4 pulled down CatSper3 and CatSper4 from WT testes but not from homozygous mutant testes (Left). These antibodies also pulled down their respective proteins from CatSper1−/− mice (Center). Anti-CatSper1 coimmunoprecipitated CatSper3 and CatSper4 in the same protein complexes from WT but not from CatSper1−/− testes (Right). Bottom Left shows CatSper1 in preparations used for immunoprecipitations; Bottom Right shows immunoprecipitation input control with anti-Na, K-ATPase, a plasma membrane protein.
Native CatSper interactions were further examined in mouse testis. Immunoprecipitation from testis membrane preparations with anti-CatSper3 and -CatSper4 polyclonal antibodies detected these proteins from WT mice but not from CatSper3−/− and CatSper4−/− mutant mice (Fig. 4b), confirming the specificity of the antibodies. Both proteins were present in CatSper1−/− mice testes (Fig. 4b). CatSper3 and CatSper4 were detected in CatSper1 immunoprecipitates from WT testes (Fig. 4b), demonstrating that both CatSper3 and CatSper4 are in molecular complexes with CatSper1. CatSper3 and CatSper4 were not detected in the same coimmunoprecipitation experiments by using CatSper1−/− mouse testes (Fig. 4b), confirming the specificity of the interaction.
Discussion
We have shown that the channel proteins, CatSper3 and CatSper4, are required for male fertility, late motility, and hyperactivation of motility. Like CatSper1 and CatSper2, they are expressed in sperm and are functional only in the principal piece of the sperm tail. Genetic disruption of any of the four sperm-specific CatSpers results in an identical sperm cell phenotype; all are required for the alkalinization-activated ICatSper necessary for sperm hyperactivation.
We demonstrated that CatSper proteins are also required for sperm motility at longer times (>30 min) after capacitation. Previous work showed that sperm motility fails over time in the absence of external Ca2+ (27–29). Ca2+ is required in many aspects of male germ cells, from spermatogenesis to the acrosome reaction (30–32). Ca2+ is required for hyperactivity in demembranated bull sperm cells (3, 8), but it is not necessary for the activation of progressive motility. As described for CatSper1 and CatSper2 (21) and now for CatSper3 and CatSper4, elimination of any CatSper gene decreases sperm progressive motility over time. Thus, the gradual decrease in motility of the CatSper-null spermatozoa may reflect the contribution of Ca2+ influx through CatSper channels to regulate other signaling pathways and will be addressed in future studies.
As sperm travel from the vagina (pH ≈5) to the cervical mucous (pH ≈8), they undergo intracellular alkalinization. At acidic internal pH and a resting membrane potential of approximately −40 mV, ICatSper of the normal mouse spermatozoan is minimally active, bringing little Ca2+ into the cell. Alkalinization dramatically increases CatSper conductance (it shifts its G-V curve to more negative membrane potentials), thereby potentiating ICatSper-mediated Ca2+ entry (19). Enhanced Ca2+ entry leads to increases in flagellar bending. The mechanism by which intracellular Ca2+ changes the flagellar bend in spermatozoa, as well as somatic cell cilia, is not known.
Why are four distinct CatSper genes of low intersequence identity required for CatSper current? We can only speculate, but it is notable that CatSpers are the only identified flagellum-specific ion channels. The localization of CatSpers to the flagellum may place unique constraints on channel assembly that requires a unique protein component. Also, sperm cells are under high selective pressure, potentially resulting in more fine-tuning of channel composition over evolution (33). Four-fold heterotetramerization would provide more diversity in potential-protein or second-messenger interactions. Finally, CatSper diversity might maximize species-specific differences, perhaps helping to ensure the specificity between male spermatozoa and hyperactivating stimuli in the conspecific female reproductive tract.
Materials and Methods
We have adopted the nomenclature (mCatSper3, NP_084048; and mCatSper4, NP_808534) used by Lobley et al. (34) and Jin et al. (22). Our mCatSper3 is 13 aa shorter than that of ref. 22, probably from alternative mRNA splicing. Gene-targeting vector plasmids created either from a BAC clone (CatSper3) or from PCR amplification (CatSper4) were transfected into J1 ES cells for homologous recombination (SI Table 1). PCR and Southern blots identified clones carrying mutated CatSper3 and CatSper4 genomic sequences at the proper locus. CatSper3 and CatSper4 mutant ES cell clones were expanded and injected into blastocysts isolated from superovulated C57BL/6 female mice and transplanted into the uterus of pseudopregnant foster mothers (see SI Fig. 6 and SI Text).
MZ8 cells stably expressing CatSper1 (a generous gift from D. Ren, University of Pennsylvania, Philadelphia, PA) were transfected with epitope-tagged CatSper cDNAs. Images were acquired by confocal microscopy. Anti-CatSper1 antibody was as described (11). Custom polyclonal anti-CatSper3 or -CatSper4 antibodies were raised in rabbits immunized with CatSper-specific peptides. Rabbit serum was affinity-purified on an immobilized peptide resin (see SI Text).
Spermatozoa were obtained from the cauda epididymis and capacitated in a modified Krebs-Ringer solution containing 25 mM NaHCO3, 1.8 mM CaCl2, and 5 mg/ml fatty acid-free BSA (21). Sperm motility was analyzed with a computer-assisted semen-analysis system; for hyperactivated motility, high-viscosity medium containing 0.75% (wt/vol) long-chain polyacrylamide was used as reported (21). Whole-cell recordings were made on sperm cells from the corpus epididymides from 3- to 8-month-old male mice, as reported (19).
After break-in, access resistance was 25–80 MΩ. The standard pipette solution contained 135 mM Cs methanesulfonate, 5 mM CsCl, 10 mM Hepes, 10 mM EGTA, 5 mM Na2ATP, and 0.5 mM Na2GTP (pH 7.2 with CsOH). Bath solutions were either Hepes–saline solution (135 mM NaCl/5 mM KCl/2 mM CaCl2/l mg of SO4/20 mM hepes/5 mM glucose/10 mM lactic acid/l mM Na pyruvate, pH 7.4) with NaOH or DVF: 150 mM Na gluconate/20 mM Hepes/2 mM Na3HEDTA/2 mM EGTA, pH 7.4, with NaOH. Data are given as mean ± SEM from the indicated number of sperm cells.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of Dr. David L. Garbers for his pioneering work in fertilization, his mentorship, and his friendship. We thank Paul Schmidt for help with ES cell targeting and Urs Berger for in situ hybridization. We thank Dr. Dan Wang (University of Texas Southwestern) for providing sNHE mice and for helping with their characterization. This work was supported by National Institutes of Health Grants U01 45857 and HD045339 (to D.E.C.) and HD36022 (to D. L. Garbers) and by the Howard Hughes Medical Institute (D.E.C. and D. L. Garbers).
Abbreviation
- DVF
divalent free.
Footnotes
Conflict of interest: M.M.M. and J.A.C. are employees of Hydra Biosciences, a company with pending patents related to CatSper protein function. As employees, both have stock options in Hydra Biosciences. D.E.C. also owns stock in Hydra Biosciences. All other authors have no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AAP21831 (mCatSper3, originally called mCatSper4) and NP_808534 (mCapSper4)].
See Commentary on page 1107.
This article contains supporting information online at www.pnas.org/cgi/content/full/ 0610286104/DC1.
References
- 1.Yanagimachi R. Zygote. 1994;2:371–382. doi: 10.1017/s0967199400002240. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbach M, Giojalas LC. Nat Rev Mol Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 3.Ho HC, Granish KA, Suarez SS. Dev Biol. 2002;250:208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 4.Carlson AE, Westenbroek RE, Quill TA, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. Proc Natl Acad Sci USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke HJ, Saunders PT. Nat Rev Genet. 2002;3:790–801. doi: 10.1038/nrg911. [DOI] [PubMed] [Google Scholar]
- 6.Suarez SS, Ho HC. Cell Mol Biol. 2003;49:351–356. [PubMed] [Google Scholar]
- 7.Suarez SS, Pacey AA. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 8.Ho HC, Suarez SS. Reproduction. 2001;122:519–526. doi: 10.1530/rep.0.1220519. [DOI] [PubMed] [Google Scholar]
- 9.Suarez SS, Vincenti L, Ceglia MW. J Exp Zool. 1987;244:331–336. doi: 10.1002/jez.1402440218. [DOI] [PubMed] [Google Scholar]
- 10.Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- 11.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano CJ, Trevino CL, Felix R, Darszon A. FEBS Lett. 1999;462:171–176. doi: 10.1016/s0014-5793(99)01518-5. [DOI] [PubMed] [Google Scholar]
- 13.Westenbroek RE, Babcock DF. Dev Biol. 1999;207:457–469. doi: 10.1006/dbio.1998.9172. [DOI] [PubMed] [Google Scholar]
- 14.Weyand I, Godde M, Frings S, Weiner J, Müller F, Altenhofen W, Hatt H, Kaupp UB. Nature. 1994;368:859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp U, Weyand I. J Cell Biol. 1998;142:473–484. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quill TA, Ren D, Clapham DE, Garbers DL. Proc Natl Acad Sci USA. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darszon A, Lopez-Martinez P, Acevedo JJ, Hernandez-Cruz A, Trevino CL. Cell Calcium. 2006;40:241–252. doi: 10.1016/j.ceca.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Darszon A, Nishigaki T, Wood C, Trevino CL, Felix R, Beltran C. Int Rev Cytol. 2005;243:79–172. doi: 10.1016/S0074-7696(05)43002-8. [DOI] [PubMed] [Google Scholar]
- 19.Kirichok Y, Navarro B, Clapham DE. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 20.Yu FH, Catterall WA. Sci STKE. 2004;253:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 21.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Proc Natl Acad Sci USA. 2003;100:14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin JL, O'Doherty AM, Wang S, Zheng H, Sanders KM, Yan W. Biol Reprod. 2005;73:1235–1242. doi: 10.1095/biolreprod.105.045468. [DOI] [PubMed] [Google Scholar]
- 23.Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF. J Biol Chem. 2005;280:32238–32244. doi: 10.1074/jbc.M501430200. [DOI] [PubMed] [Google Scholar]
- 24.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 25.Sather WA, McCleskey EW. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. Nat Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 27.Chinoy NJ, Verma RJ, Patel KG. Acta Eur Fertil. 1983;14:421–423. [PubMed] [Google Scholar]
- 28.Heffner LJ, Storey BT. J Exp Zool. 1981;218:427–434. doi: 10.1002/jez.1402180314. [DOI] [PubMed] [Google Scholar]
- 29.Morton BE, Sagadraca R, Fraser C. Fertil Steril. 1978;29:695–698. doi: 10.1016/s0015-0282(16)43348-0. [DOI] [PubMed] [Google Scholar]
- 30.Santella L, Lim D, Moccia F. Trends Biochem Sci. 2004;29:400–408. doi: 10.1016/j.tibs.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Wennemuth G, Babcock DF, Hille B. J Gen Physiol. 2003;122:115–128. doi: 10.1085/jgp.200308839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si Y, Olds-Clarke P. Biol Reprod. 2000;62:1231–1239. doi: 10.1095/biolreprod62.5.1231. [DOI] [PubMed] [Google Scholar]
- 33.Podlaha O, Zhang J. Proc Natl Acad Sci USA. 2003;100:12241–12246. doi: 10.1073/pnas.2033555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D. Reprod Biol Endocrinol. 2003;1:53–67. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.