Abstract
Aim: To investigate the correlation between clinical, high frequency ultrasound biomicroscopy (UBM) and, where possible, histological findings in cases of congenital corneal opacification presenting to the departments of ophthalmology, Great Ormond Street Hospital for Children, London, and the Hospital for Sick Children, Toronto, Canada.
Method: 22 eyes of 13 children (age range 3–225 days) with congenitally opaque corneas were examined. UBM was performed using the ultrasound biomicroscope (Allergan-Humphrey). All eyes underwent penetrating keratoplasties (PKP) except five. The host corneas were all sent for histological examination.
Results: The final diagnosis in our series was Peters' anomaly in nine cases (70%), corneal dystrophy in two cases (15%), and sclerocornea in two cases (15%). The UBM findings changed the clinical diagnosis in five cases (38%). In these five cases histology was available in four and confirmed the UBM diagnosis in each case. In no case of the 13 where histology was available did it contradict the UBM findings. In two cases a hypoechoic region in the anterior stroma was seen on UBM which correlated histologically with absent Bowman's layer and oedema. In two cases UBM revealed aniridia and in one, congenital aphakia, which was not apparent clinically.
Conclusion: UBM examination is not only very useful in evaluating the clinical diagnosis in congenital corneal opacification, it also acts as a preoperative guide in cases undergoing PKP by detecting keratolenticular and iridocorneal adhesions and other ocular abnormalities such as aniridia and congenital aphakia. In all cases where PKP was performed the UBM diagnosis was confirmed histologically. The clinical diagnosis was incorrect in five cases. This has important implications in studies of phenotype/genotype correlation of congenital corneal opacification.
Keywords: congenital corneal opacification, ultrasound biomicroscopy
High frequency ultrasound biomicroscopy (UBM) is well established as a useful tool for the examination of the anterior segment, especially in eyes with opaque corneas.1–3 The prevalence of congenital corneal opacity is approximately 3/100 000 newborns and this figure increases to 6/100 000 newborns if congenital glaucoma is included.4 To date there have been several single case reports of the use of UBM in the evaluation of corneal opacification.5–15 Few have been about congenital corneal opacification6–12 and the only one that included both UBM and histology of congenital corneal opacification8 was in an adult.
We describe 13 cases of congenital corneal opacification which presented to the departments of ophthalmology, the Hospital for Sick Children, Toronto, and Great Ormond Street Hospital for Children, London, in whom clinical correlation was made with UBM findings and where possible histology. To our knowledge this is the largest such series reported to date.
METHOD
The notes were reviewed of all cases of congenital corneal opacification presenting for the first time between December 1997 and October 1999. All cases had undergone a full clinical evaluation with or without examination under anaesthetic including anterior segment photography, high frequency UBM, and relevant serology and, in those cases where penetrating keratoplasty was performed, histopathology. In all cases a clinical diagnosis had been made before UBM was performed and then again after UBM was performed. The decision to proceed to penetrating keratoplasty was made by the principal surgeon in each centre (DSR, Toronto and KKN, London). The technique of penetrating keratoplasty was the same as that described by Ehrlich and colleagues in 1991.16 UBM was performed using the ultrasound biomicroscope (Allergan-Humphrey, San Leandro, CA, USA). Scans were performed in all cases except three, during examination under anaesthetic. A lid speculum was used to keep the eyelids open and Teargel or Viscotears (Ciba Vision) were used as the coupling agent between the transducer head and the patient's cornea. Scans were performed by two of the authors (LDM and KKN) using the same protocol. This protocol consisted of a minimum of four scans radial to the limbus and four scans parallel to the limbus at positions 12, 3, 6, and 9 o'clock. At least one scan axial to the estimated position of the pupil was also performed. Scan images were saved onto hard disc and hard copies were also made.
RESULTS
In total, 13 cases were seen with a mean postnatal age of 32.1 days (range 3–225 days). Nine cases presented to the Hospital for Sick Children, Toronto, between December 1997 and March 1999, while four presented to Great Ormond Street Hospital for Children, London, between March 1999 and September 1999.
The clinical cases and results are summarised in Tables 1, 2, and 3.
Table 1.
Clinical features
| Case | Sex | Age at presentation | Clinical features | B/L or U/L | Systemic features | Clinical diagnosis |
| 1 (KL) | F | 3 days | Diffuse panstromal corneal clouding (see Fig 1) | B/L | Nil | Congenital glaucoma |
| 2 (CC) | M | 5 days | RE complete opaque cornea (see Fig 5) | U/L | CHARGE syndrome | Sclerocornea |
| Bilateral choanal atresia ASD, VSD, coarctation of aorta, micropenis | ||||||
| 3 (CC) | 20 days | RE opaque enlarged cornea with stromal vascularisation | U/L | Nil | Peters' anomaly | |
| ? Keratolenticular adhesion | ||||||
| 4 (KP) | F | 4 days | B/L dense central corneal opacities with peripheral irido-corneal adhesions | B/L | Orofacial digital syndrome | Peters' anomaly |
| Cleft lip and palate, brachycephaly, short femurs, brachydactyly, clinodactyly | ||||||
| 5 (MG) | M | 4 days | B/L corneal opacification with a central area of relative ectasia posterior embryotoxon (see Fig 3) | B/L | Nil | Corneal ectasia |
| 6 (SH) | F | 7 days | LE central corneal opacity with irido-corneal touch | U/L | Nil | Peters' anomaly |
| 7 (VCN) | M | 7 days | B/L scleralised corneas temporally | B/L | Nil | Sclerocornea |
| 8 (EF) | M | 5 days | Opaque corneas HCD 11 mm R and 11.5 mm L | B/L | Nil | Congenital glaucoma |
| 9 (NS) | M | 7.5 months | B/L opaque corneas with evidence of scleralisation | B/L | Nil | Sclerocornea |
| 10 (ET) | M | 6 weeks | B/L diffuse opaque corneas with complete scleralisation RE and some sparing inferiorly LE | B/L | Nil | Sclerocornea |
| 11 (TH) | M | 5 months | B/L diffuse opaque corneas with microcornea and scleralisation | B/L | Intrauterine growth retardation, failure to thrive | Sclerocornea |
| 12 (RM) | M | 7 days | B/L corneal ring opacities with thinning centrally | B/L | Peters' plus syndrome | Peters' anomaly |
| B/L cleft lip and palate, rhizomelia, clinodactyly, long philtrum | ||||||
| 13 (MR) | F | 7 days | RE central corneal opacity | U/L | Nil | Peters' anomaly |
| LE Axenfeld anomaly but clear cornea (see Fig 4) |
HCD = horizontal corneal diameter, RE = right eye, LE = left eye, ASD = atrial septal defect, VSD = ventricular septal defect, U/L = unilateral, B/L = bilateral.
Table 2.
UBM and histology features
| Case | UBM method | Clinical features | UBM features | UBM Dx | PKP | Histological features | Histology Dx |
| 1 (KL) | EUA | Normal IOP under GA | Normal anterior segments, irregular corneal thickness with abnormal echogenecity from DM and endothelium. At its greatest corneal thickness was 2.3 mm RE and 2.2 mm LE | Corneal dystrophy | B/L | BE: vacuolation of the basal epithelium with intact Bowman's layer. Focal absence of DM with multilayering of the endothelium confirmed on EM. Immunostain positive for cytokeratin in endothelium | Posterior polymorphous dystrophy |
| 2 (CC) | Awake | Shallow anterior chamber with keratolenticular adhesion and abnormal thick zonules | Peters' anomaly | N/A | N/A | N/A | |
| 3 (CC) | EUA | Normal IOP | Shallow anterior chamber with keratolenticular adhesion with cataract and aniridia. Zonules enmeshed with stretched or elongated ciliary processes | Peters' anomaly | N/A | N/A | N/A |
| 4 (KP) | EUA | No evidence of glaucoma | Iridocorneal adhesions with a central posterior corneal defect. Hypoechoic region in the anterior stroma, not able to be explained at time of UBM. Features common to both eyes | Peters' anomaly | B/L | LE: variable epithelial thickness with complete absence of Bowman's layer with anterior stromal oedema; complete absence of DM and endothelium | B/L Peters' anomaly |
| RE: Vacuolation of basal epithelium with variable thickness and irregular arrangement of the stromal lamellae. Centrally deficient DM with endothelial attenuation. | |||||||
| 5 (MG) | EUA | RE perforated and had emergency PKP | Only LE had UBM. Aniridia and central large posterior corneal defect in the region of which the hyperreflectivity of DM seen on UBM was absent. Ciliary processes appeared stretched. Hypoechoic region in anterior stroma similar to case 4. | Peters' anomaly | B/L | LE: oedema of the basal epithelium, absent Bowman's layer, and irregular alignment of the stromal lamellae. Centrally marked thinning of stroma with absence of DM and endothelium | Peters' anomaly – most likely B/L but right host tissue only showed necrosis as it had perforated awaiting PKP |
| 6 (SH) | EUA | No evidence of glaucoma | Central iridocorneal adhesion. Normal hyperreflectivity of DM and endothelium not seen centrally but present peripherally | Peters' anomaly | U/L | LE: variable epithelial thickness, thickened Bowman's layer with focal absence centrally, marked stromal disorganisation and irregular stromal thickness with a central mound of tissue in posterior cornea, over which DM extremely attenuated and focally absent | Peters' anomaly |
| 7 (VCN) | EUA | Iridocorneal adhesions mainly central but also some peripheral with shallow AC. Hyperreflectivity normally seen at level of DM/endothelium not seen centrally. Hypoechogenic area in anterior stroma similar to that seen in cases 4 and 5. | B/L Peters' anomaly | B/L | RE: oedema of the basal epithelium with absence of Bowman's layer. Disorganised layering of the stroma and absence of endothelium and DM | B/L Peters' anomaly | |
| LE: epithelial oedema, absent Bowman's layer, disorganised stromal layering and attenuation of endothelium with absence of DM centrally | |||||||
| 8 (EF) | EUA | IOP: Tonopen | No evidence of iridocorneal or keratolenticular adhesions or posterior corneal defects. Cornea thickened at 2.1 mm RE and 2.3 mm LE centrally. Normal hyperreflectivity of DM/endothelium stippled with discrete discontinuations | Corneal dystrophy | B/L | BE: oedema of basal epithelium and subepithelial bullae. Marked stromal scarring with irregular layering and severe endothelial attenuation with almost complete absence. Irregular fibrous thickening of Descemet's membrane, especially posterior part but complete in some areas. | B/L congenital hereditary |
| R=37 mm Hg | Endothelial dystrophy | ||||||
| L=65 mm Hg | |||||||
| IOP: Perkins | |||||||
| R=10 mm Hg | |||||||
| L=10 mm Hg | |||||||
| 9 (NS) | EUA | No evidence of glaucoma | Formed anterior chambers with odd fragments of iris and aphakia. No evidence of posterior corneal defect | Aphakia | B/L | RE: flattened and attenuated epithelium with absent Bowman's layer. Stroma abnormally organised, thin and vascularised. DM and endothelium could not be identified due to presence of adherent iris | Primary aphakia |
| Disorganised ant. segment | |||||||
| ? Sclerocornea | |||||||
| LE: in addition to features above subepithelial calcification | |||||||
| 10 (ET) | EUA | No evidence of glaucoma | Shallow ACs with posterior corneal defects with no keratolenticular adhesion but some irdiocorneal adhesions seen, centrally especially in right eye. | Peters' anomaly | LE: autorotational keratoplasty (elsewhere) | N/A | N/A |
| 11 (TH) | EUA | No evidence of glaucoma | Formed ACs with no corneal defect or iridocorneal or keratolenticular adhesion | Sclero-cornea | N/A | N/A | |
| 12 (RM) | Awake | Central thinning of the posterior cornea with keratolenticular and iridocorneal adhesions | Peters' anomaly | B/L | BE: absence of DM, endothelium and posterior stroma centrally with absent Bowman's layer | Peters' anomaly | |
| 13 (MR) | Awake | IOP: Perkins while feeding | Peripheral iridocorneal adhesions. Centrally mild posterior corneal defect with corresponding defect in anterior capsule of lens | Peters' anomaly | U/L | RE: centrally focal absence of DM and endothelium | Peters' anomaly |
| R=26 mm Hg | |||||||
| L=16 mm Hg |
Table 3.
Cases where diagnosis changed
| Cases | Eyes | Clinical diagnosis | UBM diagnosis | Histological diagnosis |
| 1 (KL) | B/L | Congenital glaucoma | Corneal dystrophy | Posterior polymorphous dystrophy |
| 2 (CC) | U/L | Sclerocornea | Peters' anomaly | N/A |
| 5 (MG) | B/L | Corneal ectasia | Peters' anomaly with aniridia | Peters' anomaly |
| 7 (VCN) | B/L | Sclerocornea | Peters' anomaly | Peters' anomaly |
| 8 (EF) | B/L | Congenital glaucoma | Corneal dystrophy | Congenital hereditary endothelial dystrophy |
DISCUSSION
There have been four isolated reports of the UBM findings in sclerocornea and Peters' anomaly7,8,10,11 and while one8 had histology, it was in an adult. We have reported the UBM findings in 13 cases (22 eyes) of congenital corneal opacification, 12 of which presented within 3 weeks of birth. More importantly, we have correlated the UBM findings with the clinical features in all cases and the histological findings in nine cases. The UBM findings changed the clinical diagnosis in five cases (see Table 3). Two cases of suspected congenital glaucoma (1 and 8) were thought to be cases of corneal dystrophies (posterior polymorphous dystrophy and congenital hereditary endothelial dystrophy) while two cases of suspected sclerocornea and the one case of corneal ectasia were found to be Peters' anomaly after UBM examination. The UBM findings in posterior polymorphous dystrophy and in congenital hereditary endothelial dystrophy have not previously been reported (see Table 2 and Fig 1). The final diagnosis in our series was Peters' anomaly in nine cases (70%), corneal dystrophy in two cases (15%), and sclerocornea in two cases (15%).
Figure 1.
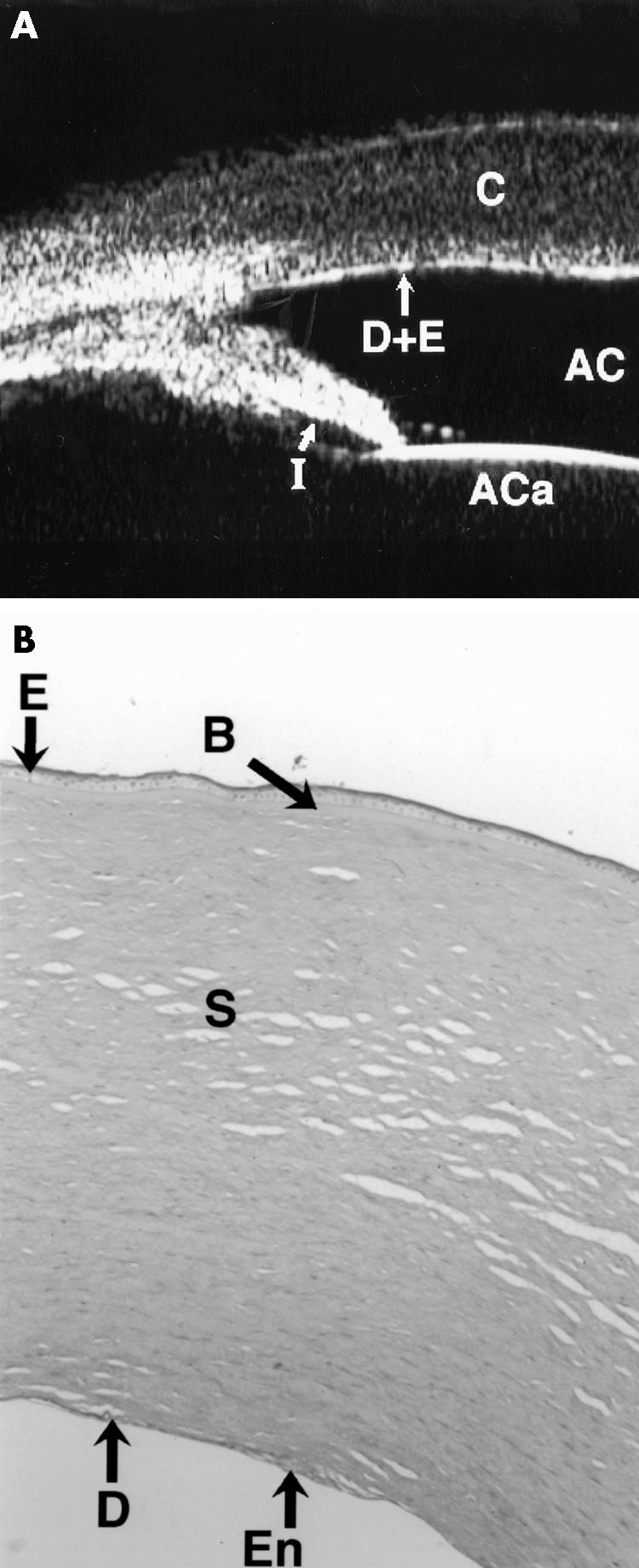
(A) The UBM of case 1 shows irregular thickness of the cornea (C) and focal anomalies in the Descemet's membrane and endothelial echo (D+E) (compare with Fig 2). The pupil is dilated, and so the iris appears shorter than normal (I). The anterior chamber (AC) and the anterior capsule of the lens are also seen. The corneal thickness for the right eye was 2.3 mm centrally and 2.2 mm for the left eye centrally. A diagnosis of corneal dystrophy was made, which was confirmed histologically as being posterior polymorphous dystrophy. (B) Full thickness (×10) cornea section is shown with periodic acid Schiff (PAS) stain. There is normal epithelium (E) and Bowman's layer (B) but the Descemet's membrane (DM) is abnormally thin with multilayering of the endothelium seen (En).
Ultrasound biomicroscopy (UBM) allows visualisation of the anterior segment to a depth of 5 mm with a resolution of approximately 50 μm 1–3 and gives excellent differentiation of ocular tissues because different tissues produce different amounts of beam backscatter.1 Using an 80 MHz transducer Pavlin has demonstrated that UBM will differentiate corneal epithelium, Bowman's membrane and stroma but not between Descemet's membrane and the endothelial layer.1,2 In our study, using a 50 MHz transducer which is the commonest commercially available UBM, the corneal epithelium and Bowman's layer appear as a single hyperechoic layer. In order to record corneal epithelium and Bowman's layer as two separate layers the transducer must be exactly perpendicular to the corneal surface and the gain must be low. Since the intracorneal structures were not the focus of our examination we did not stringently try to meet these criteria which may explain the discrepancy between our findings and those of Pavlin.1,2 Beneath the corneal epithelium/Bowman complex we found the relatively hypoechoic but heterogeneous stroma and finally Descemet's membrane (DM) and endothelial layer again as a single hyperechoic layer (see Fig 2).
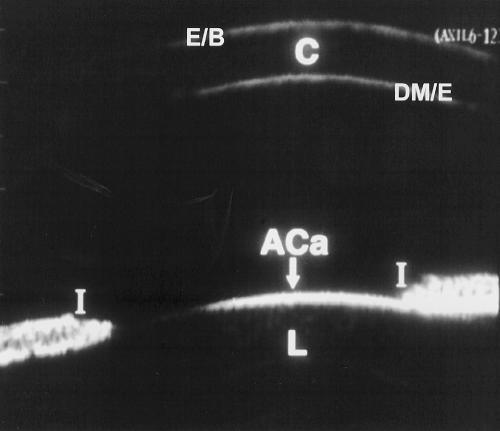
Figure 2.
UBM of a normal age matched eye. The echogenicity from the epithelial/Bowman's layer complex (E/B), cornea (C), Descemet's membrane/endothelial complex (DM/E), anterior capsule of the lens (ACa), lens (L), and iris (I) is shown.
In cases 1 and 8 the usually contiguous reflectivity of the DM/endothelium layer seen on UBM1 was found to be irregular (Fig 1A) which was confirmed on histology to be due to focal absences of Descemet's membrane with multilayering of the endothelium case 1 (Fig 1B) and absences of endothelium in case 8; a feature not previously reported to our knowledge.
In seven cases (4–7, 10, 12, 13) where the normal hyperreflectivity of the DM/endothelium was not seen in association with a central posterior corneal defect (Fig 3B, 4A), absence of DM and endothelium was confirmed histologically where available (Fig 3C, 4B).
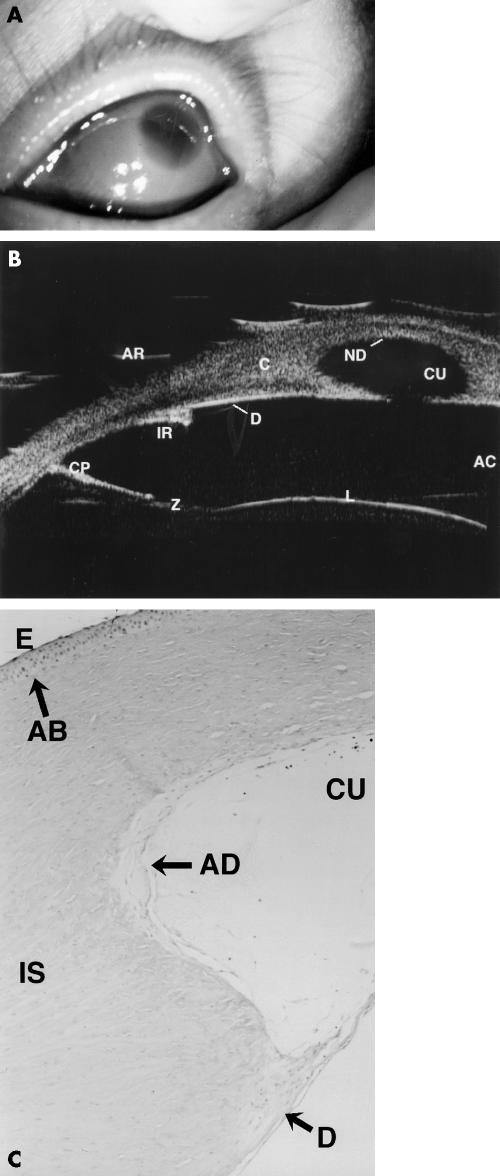
Figure 3.
Clinical, UBM, and histological findings in case 5. (A) Shows the corneal opacity with relative clearing centrally. (B) A composite of two UBM scans showing aniridia with an iris stump (IR), stretched ciliary processes (CP), zonules (Z), the lens (L), intact Descemet's membrane/endothelial echo (D), central defect in posterior cornea (CU), and loss of the Descemet's membrane/endothelial echo within the defect (ND). AR = artefact. C = cornea. AC = anterior chamber. (C) (×10) Periodic acid Schiff (PAS) stain section of cornea is shown. This demonstrates the central defect (CU) with absent Descemet's membrane and endothelium (AD), but a thin Descemet's membrane is seen peripherally (D). Bowman's layer is absent (AB) and stromal lamellae are irregular (IS). E = epithelium. The relative clarity centrally seen in (A) is due to the gross central defect.
Figure 4.
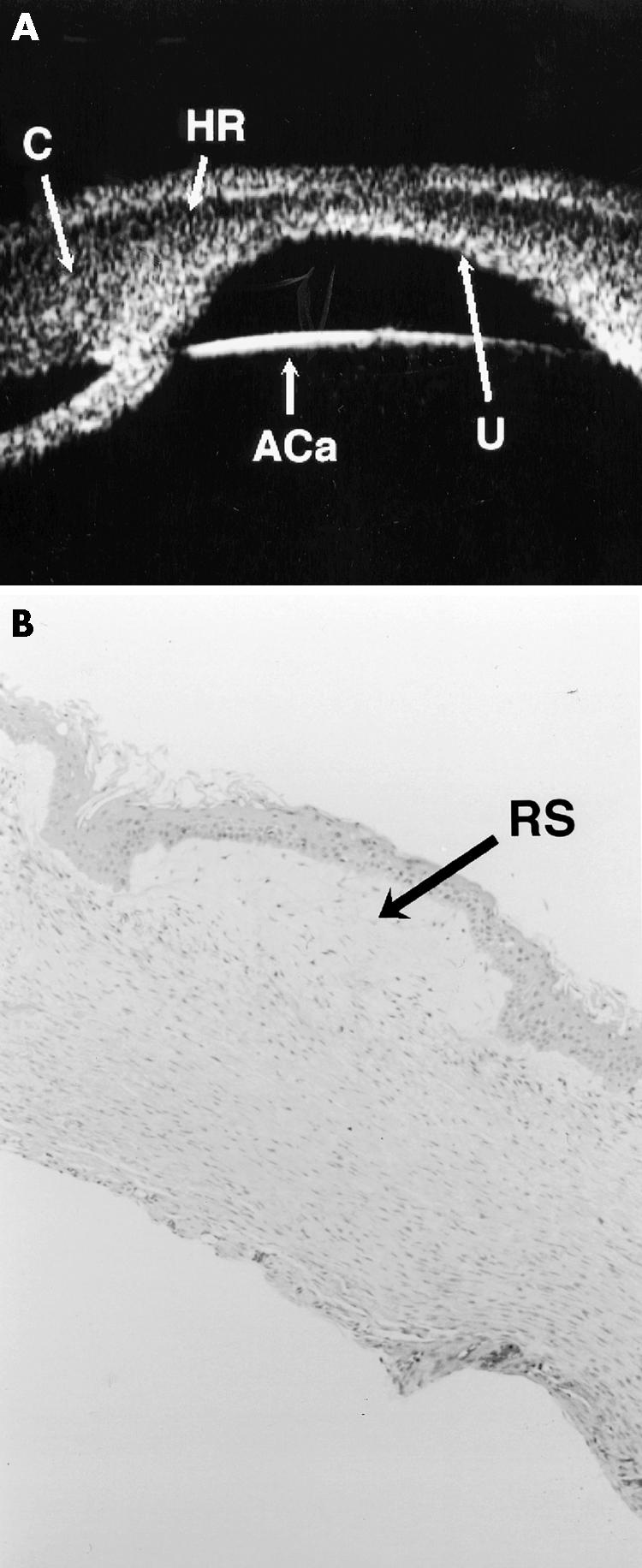
UBM scans and histology of case 4. (A) Shows the cornea (C), with a hypoechoic region (HR) in the anterior stroma with a central defect in the posterior aspect of the cornea (U). The anterior capsule of the lens is seen (ACa) as is a central iridocorneal adhesion. (B) Full thickness corneal section (haematoxylin and eosin stain) shows absence of Bowman's layer with marked rarefaction of the subepithelial stroma (RS), which corresponds to the hypoechoic layer seen on UBM. There is absence of Descemet's membrane and endothelium. All these histological features are consistent with a diagnosis of Peters' anomaly.
In four cases (4, 5, 7, 12) an unusual hypoechoic region was seen in the anterior stroma on UBM examination (Fig 3B, 4A). In all these four cases (eight eyes) histology revealed an absence of Bowman's layer with oedema in the region adjacent to where Bowman's layer should have been, together with absence of Descemet's membrane (Fig 3C, 4B). In case 9 Bowman's layer was also found to be histologically absent but no hypoechoic region was seen in the anterior stroma. One possible explanation may be that there was also vascularisation of the stroma and this case had presented after 7 months while all the other cases had presented within 2 weeks of birth.
In case 6 histology revealed a thickened Bowman's layer but there was no obvious UBM correlate. However, hyperplasia or thickened Bowman's layer has previously been described.17–19
In the only clinicopathological correlation to date of clinically diagnosed sclerocornea,8 the authors described a flattened cornea, diffuse scleralisation of the cornea indicated by hyperreflectivity, abnormal Bowman's layer, thickening of the peripheral cornea, with central posterior excavations involving the posterior stroma, Descemet's membrane and endothelium. Histologically all the UBM findings were confirmed and in addition Bowman's layer was noted to be absent and replaced by a few irregular patches of hyaline material. It is noteworthy that despite the presence of a central posterior corneal defect both on UBM and histologically, a feature consistent with Peters' anomaly,17,20–29 the authors failed to comment on this. Additionally no comment was made about the state of the anterior stroma on UBM8 but if the UBM figure is perused in the report there is a clear area of hypoechogenicity in the anterior stroma; this is identical to our findings on UBM in cases 4, 5, 7, and 12. In this report Bowman's layer was reported to be absent also histologically.
We suggest that the presence of a hypoechoic layer in the anterior stroma just below the epithelial hyperechoic layer may be indicative of absent Bowman's layer with concomitant oedema as evidenced by the histology of our cases and that of the only other clinicopathological report.8 To our knowledge, this has never been previously reported.
Avitabile et al5 have studied acquired corneal oedemas using UBM; however, all of their studies were at least 30 days after the initial insult, at which stage the opacity of the cornea seen was most probably related to scarring rather than true acute corneal oedema. This would explain why they describe increased hyperreflectivity within the stroma.
The description of UBM in Peters' anomaly has been reported in three papers 10,11,13 but none had any correlation with histology. Azuara-Blanco et al13 described three eyes of two patients who had had a clinical diagnosis of Peters' anomaly made without histological confirmation. Their UBM findings were similar to ours with the central posterior corneal defect described as an excavation. We agree with their description of central keratolenticular and iridocorneal adhesions as seen in cases 2, 3, 4, 6, 7, and 12 (Fig 5B). Although case 13 showed iridocorneal adhesions, these were peripheral.
Figure 5.
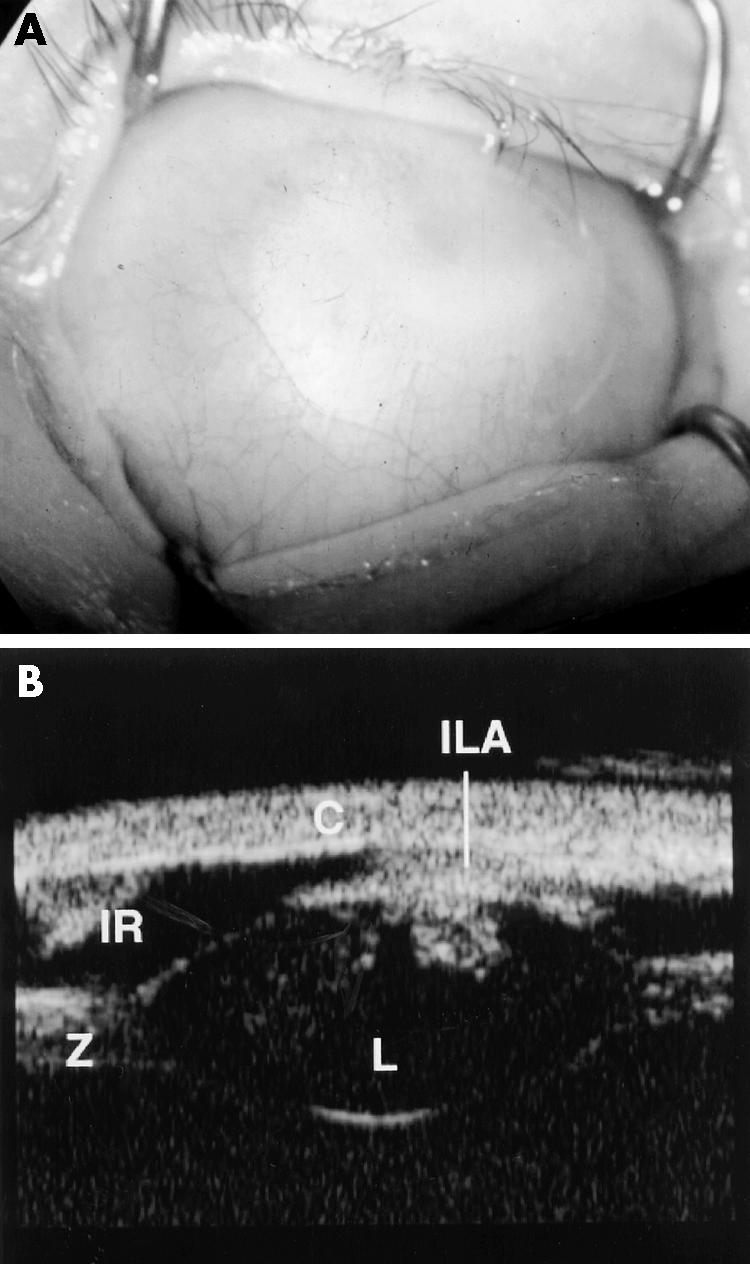
Clinical and UBM findings in case 2. (A) Shows complete corneal opacification thought clinically to be sclerocornea. (B) The UBM of the same case shows keratolenticular adhesion (ILA), aniridia with only an iris stump detected (IR), a small lens (L), and thickened looking zonules (Z). The cornea is also seen (C). The diagnosis post UBM was thought to be Peters' anomaly given the keratolenticular adhesion. Case 2 did not have penetrating keratoplasty performed.
As early as 186730 the clinical condition of defect in Descemet's membrane giving rise to a central corneal opacification was attributed to defective separation of the lens from surface ectoderm. Peters31 in 1906 emphasised this aetiology and in so doing gave the condition its eponymous name. There is a substantial volume of literature regarding the histology of Peters' anomaly20–29,32,33 and less so for sclerocornea.26,34 Regardless of the author, the hallmark of Peters' anomaly histologically is the central deficiency of the posterior stroma, Descemet's membrane, and endothelium with or without keratolenticular and/or iridocorneal adhesions17,20–29 with a corresponding central corneal opacity clinically. Interestingly, absence of Bowman's membrane is also alluded to in a number of reports21,22,34–37 but some of these reports clinically describe sclerocornea with a rudimentary presence of DM.34,36
In sclerocornea there is extension of opaque scleral tissue and fine vascular conjunctival and episcleral tissue into the peripheral cornea obscuring the limbus.26 The severity of scleralisation varies from mild to complete but is usually bilateral in 90% of cases.26,38–40 Histologically the corneal epithelium shows secondary changes with Bowman's layer absent in the affected areas26,34 with interstitial vascularisation without inflammation and the stromal collagen fibrils are comparable to scleral collagen in size and organisation. There may be irregular absence of both endothelium and Descemet's membrane or an abnormally thinned Descemet's membrane composed of multilaminar basement membrane.26,34
It appears that Peters' anomaly and sclerocornea are most likely conditions in the same spectrum of anterior segment dysgenesis.
UBM was useful in evaluating both the cornea itself as shown above and very useful in revealing associated ocular anomalies as demonstrated most clearly by cases 5, 9, and 13.
In case 9 no evidence of a lens could be found either on UBM or posterior segment ultrasound and we feel confident that this is bilateral primary congenital aphakia. Congenital aphakia is extremely rare41–44 and when associated with Peters' anomaly even rarer.41,42 Controversy exists as to whether primary aphakia (failure of any lens formation as opposed to secondary type where lens forms but subsequently is resorbed) can occur with an otherwise normal anterior segment or not.43–46 Clinically, sclerocornea precluded a thorough examination of the anterior segment, which could only be done using the UBM and this revealed the absence of a lens and aniridia bilaterally (Table 2). This is the first reported case of congenital aphakia in association with clinically diagnosed sclerocornea to our knowledge.
Case 13 demonstrated the presence of Axenfeld-Rieger anomaly with Peters' anomaly. The scarcity of previous reports may be due to the fact that young infants are difficult to examine and gonioscopy to look for the iridocorneal adhesions is particularly difficult if the only such findings are in the affected eye with the corneal opacification.17,42,47–49 The use of UBM demonstrated the peripheral iridocorneal adhesions very clearly in our case although the fellow eye also demonstrated Axenfeld-Rieger anomaly very clearly.
Both cases 5 and 9 demonstrated the presence of aniridia which was not readily obvious prior to UBM because of the corneal opacity. Cases of aniridia and Peter's anomaly have been previously been reported35,50 and so has one case of Peters' anomaly with Wilm's tumour.51 The iris in this latter report was described as being very hypoplastic.51
At least three developmental genes, PAX 6, REIG 1, and PITX 3, are involved in the development of the anterior segment of the eye.52,53 There has been much investigation into the genetics of Peters' anomaly48,52,54–56 with controversy over the role of PAX 6.52,54,56,57 PAX 6 (OMIM 106210) is a homeobox gene responsible for the control of ocular embryogenesis.52,53,58 Mutations in PAX 6 are responsible for human aniridia and it has been suggested that no locus other than chromosome 11p13 has been implicated in aniridia and that PAX 6 may be the only gene responsible.57
Doward et al have reported a case of Peters' anomaly in which a mutation of REIG 1 gene was found.48 Mutations in the REIG 1 homeobox gene (OMIM 180500) on chromosome 4q25 have been reported in association with Rieger syndrome.48 A mutation in the PITX 3 gene (OMIM 602669) on chromosome 10 has been associated with autosomal dominant Peters' anomaly, congenital cataract and other anterior segment malformations.59
We believe that the accurate description of the phenotype of congenital corneal opacification is crucial in the evolution of phenotype-genotype correlation. In our series three of five cases clinically diagnosed as sclerocornea were found on UBM and, in some cases, histologically to have Peters' anomaly. This suggests that the clinical definition of phenotype in such cases is unreliable and the water is further muddied by the fact that sclerocornea and Peters' anomaly appear to be conditions whose histological features overlap suggesting they are part of the same spectrum of disease.17,20–29,32–36 In a series of articles in 1974 Townsend et al17,20,24 tried to move away from the eponymous designations for developmental congenital corneal opacifications classifying them histologically according to the position and presence of defects in DM.
Whether the absence of Bowman's layer in cases of sclerocornea and Peters' anomaly21,22,34–37 is a primary event or secondary to an absent Descemet's membrane and endothelium, is unclear. If it were a primary event then elucidating a genetic association would be significant. The embryogenesis of Bowman's layer occurs late (4–5 months). It is thought to be produced by both the epithelium and the anterior stroma.18 If the lens is removed in the chick embryo on day 3 of gestation there is a resultant failure of the corneal stroma, DM and endothelium to develop and a greatly decreased density of Bowman's layer.18 Other authors have suggested that in Peters' anomaly the epithelium may be abnormal with an absent Bowman's layer.22 The central posterior defect of the cornea seen in Peters' anomaly may be as a result of failure of lens separation or due to apposition of the lens to the cornea.17,20,24,26 Townsend has suggested that the posterior defect could be a passive effect of pressure by a forwardly displaced lens against the cornea at a time in development when the DM was absent or still a delicate structure.17 This suggests that the central corneal opacity of Peters' anomaly could be the final pathway for a number of varied pathologies, much like pulmonary fibrosis is the final pathway for conditions as varied as sarcoid, TB and cystic fibrosis.
Under these circumstances any phenotype-genotype correlation must be undertaken only with the most accurate phenotypic description available. We suggest that in the absence of histological diagnosis, the use of high frequency ultrasound should be mandatory in the description of phenotype where the anterior segment cannot be visualised. It is reasonable to suggest that the presence of Peters' anomaly with aniridia is most likely associated with a PAX 6 mutation according to Prosser and van Heyningen56 while Peters' anomaly with Axenfeld-Rieger anomaly may be associated with RIEG 1 mutations.
We performed penetrating keratoplasty in nine cases (16 eyes) and one case had autologous rotational keratoplasty elsewhere. Penetrating keratoplasty for such cases is well described,16,37,60–69 while there are fewer reports of optical iridectomy and rotational keratoplasty.70,71 It is noteworthy that one group of authors37 named absence of Bowman's layer and, separately, absence of DM histologically as poor prognosticators; our UBM findings suggest that both these features could be determined preoperatively, thus giving the parents more information before consenting to surgical intervention. Furthermore, other authors65 make the point that in most cases of Peters' anomaly the clinician has difficulty detecting keratolenticular adhesions hidden behind the dense corneal opacity and that for proper graft centration and wound entry site retroillumination must be employed.60 By using UBM all surgical planning can be done before the eye is opened.
In summary then we have described the first series of clinico-ultrasonico-pathological descriptions of congenital corneal opacification. We have demonstrated that the clinical description of phenotype may be unreliable, by showing that the clinical diagnosis was changed in five out of 13 cases (38%) by the UBM findings and that in every case but one the UBM finding was confirmed histologically
In so doing we have described a new sign in high frequency ultrasound of hypoechogenecity of the anterior stroma (subepithelium) which has been shown histologically to be due to absent Bowman's layer with associated oedema. It is necessary to emphasise that sclerocornea and Peters' anomaly are part of the same spectrum of pathology. The importance of preoperative assessment and diagnosis in cases of corneal opacity cannot be overstated and is easily undertaken with UBM even in the awake infant.
Finally, in the present climate of increasing emphasis on studies of phenotype-genotype correlation we feel we have shown that UBM examination is an invaluable adjunct in accurately defining the phenotype.
REFERENCES
- 1.Pavlin CJ, Sherar MD, Foster S. Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology 1990;97:244–50. [DOI] [PubMed] [Google Scholar]
- 2.Pavlin CJ, Harasiewicz K, Sherar MD, et al. Clinical use of ultrasound biomicroscopy. Ophthalmology 1991;98:287–95. [DOI] [PubMed] [Google Scholar]
- 3.Pavlin CJ. Interpreting technology; practical application of ultrasound biomicroscopy. Canad J Ophthalmol 1995;30:225–9. [PubMed] [Google Scholar]
- 4.Bermejo E, Martinez-Frias ML. Congenital eye malformations: clinical epidemiological analysis of 1,124,654 consecutive births in Spain. Am J Med Genet 1998;75:497–504. [PubMed] [Google Scholar]
- 5.Avitabile T, Russo V, Ghirlanda R, et al. Corneal oedemas: diagnosis and surgical planning with ultrasound biomicroscopy. Ophthalmologica 1998;212(Suppl 1):13–16. [DOI] [PubMed] [Google Scholar]
- 6.Kiryu J, Park M, Kobayashi H, et al. Ultrasound biomicroscopy of the anterior segment of the eyes of infants. J Pediatr Ophthalmol Strabismus 1998;35:320–2. [DOI] [PubMed] [Google Scholar]
- 7.Park M, Kiryu J, Kurimoto Y, et al. Ultrasound biomicroscopic observation of the anterior eye segment in a sclerocornea and a microcornea. J Jap Ophthalmol Soc 1997;101:69–73. [PubMed] [Google Scholar]
- 8.Kim T, Cohen EJ, Schnall BM, et al. Ultrasound biomicroscopy and histopathology of sclerocornea. Cornea 1998;17:443–5. [DOI] [PubMed] [Google Scholar]
- 9.Funaki H, Shirakashi M, Fukuchi T, et al. A case of Peters' anomaly complicated by Axenfeld's anomaly. Folia Ophthalmol Jap 1996;47:322–5. [Google Scholar]
- 10.Haddad AM, Greenfield DS, Stegman Z, et al. Peters' anomaly: Diagnosis by ultrasound biomicroscopy. Ophthalmic Surg Lasers 1997;28:311–12. [PubMed] [Google Scholar]
- 11.Bessho K, Sawada A, Yamamoto T, et al. A case of Peters' anomaly observed by ultrasound biomicroscopy. Jap J Clin Ophthalmol1998;52:215–18. [Google Scholar]
- 12.Katsushima H, Maruyama I, Endo H, et al. Ultrasound biomicroscopy in a case of congenital anterior staphyloma. Folia Ophthalmol Jap 1997;48:366–70. [Google Scholar]
- 13.Burger JJ, Katz LJ, Calhoun JH, et al. Ultrasound biomicroscopy in infantile glaucoma. Ophthalmology 1997;104:1116–19. [DOI] [PubMed] [Google Scholar]
- 14.Fries U, Muller HM, Heider W. Imaging (resolution and quantification) of corneal findings with ultrasound biomicroscopy. Ophthalmologe 1996;93:257–61. [PubMed] [Google Scholar]
- 15.Kobayashi H, Kiryu J, Kobayashi K, et al. Ultrasound biomicroscopic measurement of anterior chamber angle in premature infants. Br J Ophthalmol 1997;81:460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erlich CM, Rootman DS, Morin JD. Corneal transplantation in infants, children and young adults: experience of the Toronto Hospital for Sick Children, 1979–88. Canad J Ophthalmol 1991;26:206–10. [PubMed] [Google Scholar]
- 17.Townsend WM, Font RL, Zimmerman LE. Congenital corneal leukomas.2. Histopathologic findings in 19 eyes with central defect in Descemet's membrane. Am J Ophthalmol 1974;77:192–206. [PubMed] [Google Scholar]
- 18.Apple DJ, Olson RJ, Jones GR, et al. Congenital corneal opacification secondary to Bowman's layer dysgenesis. Am J Ophthalmol 1984;98:320–8. [DOI] [PubMed] [Google Scholar]
- 19.Ohrloff C, Olson R, Apple D, et al. Congenital corneal opacity caused by hyperplasia of Bowman's membrane. Fortschr Ophthalmol 1984;81:429–32. [PubMed] [Google Scholar]
- 20.Townsend WM . Congenital corneal leukomas. I Central defect in Descemet's membrane. Am J Ophthalmol 1974;77:80–6. [PubMed] [Google Scholar]
- 21.Stone DL, Kenyon KR, Green WR, et al. Congenital central corneal leukoma (Peters' anomaly). Am J Ophthalmol 1976;81:173–93. [DOI] [PubMed] [Google Scholar]
- 22.Polack FM, Graue EL. Scanning electron microscopy of congenital corneal leukomas ( Peters' anomaly). Am J Ophthalmol 1979;88:169–78. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi I, Brown SI. The histopathology and ultrastructure of congenital, central corneal opacity. Am J Ophthalmol 1971;72:801–12. [DOI] [PubMed] [Google Scholar]
- 24.Townsend WM, Font RL, Zimmerman LE. Congenital corneal leukomas. 3. Histopathologic findings in 13 eyes with noncentral defect in Desecemet's membrane. Am J Ophthalmol 1974;77:400–12. [PubMed] [Google Scholar]
- 25.Kupfer C, Kuwabara T, Stark WJ. The histopathology of Peters' anomaly. Am J Ophthalmol 1975;80:653–60 . [DOI] [PubMed] [Google Scholar]
- 26.Kenyon KR. Mesenchymal dysgenesis in Peters' anomaly, sclerocornea and congenital endothelial dystrophy. Exp Eye Res 1975;21:125–42 . [DOI] [PubMed] [Google Scholar]
- 27.Myles WM, Flanders ME, Chitayat D, et al. Peters' anomaly: a clinicopathologic study. J Pediatr Ophthalmol Strabismus 1992;29:374–81. [DOI] [PubMed] [Google Scholar]
- 28.Eggink CA, Mooy CM, Pinckers A. Peters' anomaly: an unusual case. Ophthalmic Paediatr Genet 1991;12:19–22. [DOI] [PubMed] [Google Scholar]
- 29.Offret H, Saraux H, Pouliquen Y, et al. Congenital leukomas with anomalies of the lens migration. J Fr Ophtalmol 1978;1:517–27. [PubMed] [Google Scholar]
- 30.Steffan P. Beitrag zur Erklaerung angeborener anomalien der Hornhaut. Klin Monatsbl Augenheilkd 1867;5:209. [Google Scholar]
- 31.Peters A. Ueber angeborene Defektbildung der Descemetschen Membran. Klin Monatsbl Augenheilkd 1906;44:27–105. [Google Scholar]
- 32.Wertelecki W, Dev VG, Superneau DW. Abnormal centromere-chromatid apposition (ACCA) and Peters' anomaly. Ophthalmic Paediatr Genet 1985;6:247–55. [PubMed] [Google Scholar]
- 33.Johnson BL, Brown SI. Congenital epithelialisation of the posterior cornea. Am J Ophthalmol 1976;82:83–9. [DOI] [PubMed] [Google Scholar]
- 34.Petroutsos G, Patey A, Savoldelli M, et al. Ultrastructural and morphologic study. J Fr Ophtalmol 1983;6:769–75. [PubMed] [Google Scholar]
- 35.Zaidman GW, Juechter K. Peters' anomaly associated with protruding corneal pseudo staphyloma. Cornea 1998;17:163–8. [DOI] [PubMed] [Google Scholar]
- 36.Redbrake C, Salla S, Becker J, et al. A rare case of bilateral congenital corneal malformations. Acta Ophthalmol 1993;71:256–61. [DOI] [PubMed] [Google Scholar]
- 37.Kampik A, Lund OE, Halbig W. Penetrating keratoplasty in congenital corneal opacities. Klin Monatsbl Augenheilkd 1986;188:188–92. [DOI] [PubMed] [Google Scholar]
- 38.Stanley JA. Congenital anomalies of the peripheral cornea. Int Ophthalmol Clin 1986;26:15–28. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson AW, Lehmann W, Forsius J. Congenital cornea plana in Finland. Clin Genet 1973;4:301–10. [DOI] [PubMed] [Google Scholar]
- 40.Bloch N. The different types of sclerocornea, their hereditary modes and concomitant congenital malformations. J Genet Hum 1965;14:133–72. [PubMed] [Google Scholar]
- 41.Trabucchi G, Piantanida A, Bandello F, et al. Congenital aphakia in Peters' anomaly syndrome; a case report. Acta Ophthalmol Scand 1997;75:595–7. [DOI] [PubMed] [Google Scholar]
- 42.Harris R, Brownstein S, Little JM. Peters' anomaly with congenital aphakia. Canad J Ophthalmol 1980;15:91–4. [PubMed] [Google Scholar]
- 43.Johnson BL, Cheng KP. Congenital aphakia: a clinicopathologic report of three cases. J Pediatr Ophthalmol Strabismus 1997;34:35–9. [DOI] [PubMed] [Google Scholar]
- 44.Manschot WA. Primary congenital aphakia. Arch Ophthalmol 1963;69:71–7. [Google Scholar]
- 45.Vermeij-Keers C. Primary congenital aphakia and the rubella syndrome. Teratology 1975;11:257–66. [DOI] [PubMed] [Google Scholar]
- 46.Hartwig NG, Vermeij-Keers C, Versteeg J. The anterior eye segment in virus induced primary congenital aphakia. Acta Morphol Neerl-Scand September1988;26:283–92. [PubMed] [Google Scholar]
- 47.Saitoh A, Ohira A, Amemiya T. Peters' anomaly in an infant with a mild degree of Axenfeld's anomaly. Br J Ophthalmol 1995;79:862–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doward W, Perveen R, Lloyd IC, et al. A mutation in the RIEG1 gene associated with Peters' anomaly. J Med Genet 1999;36:152–5. [PMC free article] [PubMed] [Google Scholar]
- 49.Awan KJ. Peters-Rieger's syndrome. J Pediatr Ophthalmol 1977;14:112–6. [PubMed] [Google Scholar]
- 50.Koster R, van Balen AT. Congenital corneal opacity (Peters' anomaly) combined with buphthalmos and aniridia. Ophthalmic Paediatr Genet 1985;6:241–6. [PubMed] [Google Scholar]
- 51.Eiferman RA. Association of Wilm's tumour with Peters' anomaly. Ann Ophthalmol 1984;933–4. [PubMed]
- 52.Withers SJ, Gole GA, Summers KM. Autosomal dominant cataracts and Peters anomaly in a large Australian family. Clin Genet 1999;55:240–7. [DOI] [PubMed] [Google Scholar]
- 53.Oliver G, Gruss P. Current views on eye development. Trends Neurosci 1997;20:415–21. [DOI] [PubMed] [Google Scholar]
- 54.Azuma N, Yamada M. Missense mutation at the C terminus of the PAX 6 gene in ocular anterior segment anomalies. Invest Ophthalmol Vis Sci 1998;59:828–30. [PubMed] [Google Scholar]
- 55.Churchill AJ, Booth AP, Anwar R, et al. PAX 6 is normal in most cases of Peters' anomaly. Eye 1998;12(Pt 2):299–303. [DOI] [PubMed] [Google Scholar]
- 56.Prosser J, van Heyningen V. PAX6 mutations reviewed. Hum Mut 1998;11:93–108. [DOI] [PubMed] [Google Scholar]
- 57.Churchill A, Booth A.Genetics of aniridia and anterior segment dysgenesis. Br J Ophthalmol 1996;80:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanson IM, Fletcher JM, Jordan T, et al. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet 1994;6:168–73. [DOI] [PubMed] [Google Scholar]
- 59.Semina EV, Ferrell RE, Mintz-Hittner HA, et al. A novel Homeobox gene PITX3 is mutated in families with autosomal dominant cataracts and ASMD. Nat Genet 1998;9:167–70. [DOI] [PubMed] [Google Scholar]
- 60.Frueh BE, Brown SI. Transplantation of congenitally opaque corneas.Br J Ophthalmol 1997;81:1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dana MR, Moyes AL, Gomes JA, et al. The indications for and outcome in pediatric keratoplasty. A multicenter study. Ophthalmology 1995;102:1129–38. [DOI] [PubMed] [Google Scholar]
- 62.Cowden JW. Penetrating keratoplasty in infants and children. Ophthalmology 1990;97:324–9. [DOI] [PubMed] [Google Scholar]
- 63.Frucht-Pery J, Chayet AS, Feldman ST, et al. The effect of corneal grafting on vision in bilateral amblyopia. Acta Ophthalmol (Suppl) 1989;192:20–3. [DOI] [PubMed] [Google Scholar]
- 64.Brown SI, Salamon SM. Wound healing of grafts in congenitally opaque infant corneas. Am J Ophthalmol 1983;95:641–4. [DOI] [PubMed] [Google Scholar]
- 65.Waring GO III, Parks MM. Successful lens removal in congenital cornneolenticular adhesion (Peters' anomaly). Am J Ophthalmol 1977;83:526–9. [DOI] [PubMed] [Google Scholar]
- 66.Waring GO 3d, Laibson PR. Keratoplasty in infants and children. Trans Am Acad Ophthalmol Otolaryngol 1977;83:283–96. [PubMed] [Google Scholar]
- 67.Hertle RW. Orlin SE. Successful visual rehabilitation after neonatal penetrating keratoplasty. Br J Ophthalmol 1997;81:644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cameron JA. Good visual result following early penetrating keratoplasty for Peters'anomaly. J Pediatr Ophthalmol Strabismus 1993;30:109–12. [DOI] [PubMed] [Google Scholar]
- 69.Joseph A, Fernandez ST, Ittyerah TP, et al. Keratoplasty in congenital corneal opacity. Ind J Ophthalmol 1980;28:79–80. [PubMed] [Google Scholar]
- 70.Zaidman GW, Rabinowitz Y, Forstot SL. Optical iridectomy for corneal opacities in Peters' anomaly. J Cataract Refract Surg 1998;5:719–22. [DOI] [PubMed] [Google Scholar]
- 71.Naumann GO, Volcker HE, Gackle D. Ipsilateral rotational autokeratoplasty. Klin Monatsbl Augenheilkd 1977;170:488–93. [PubMed] [Google Scholar]