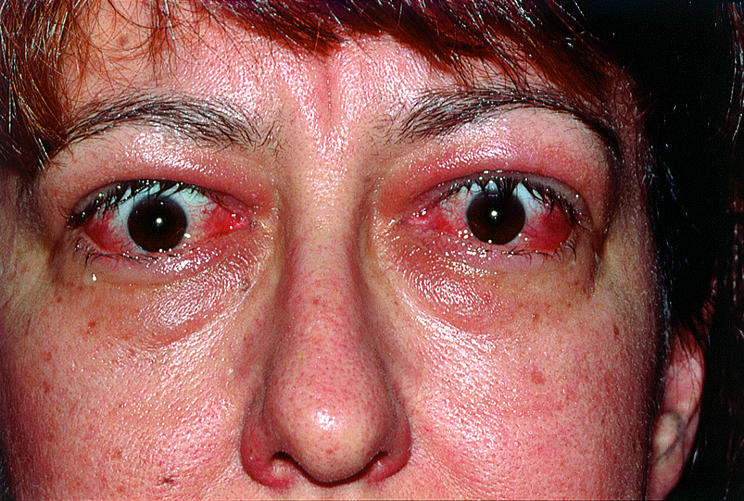
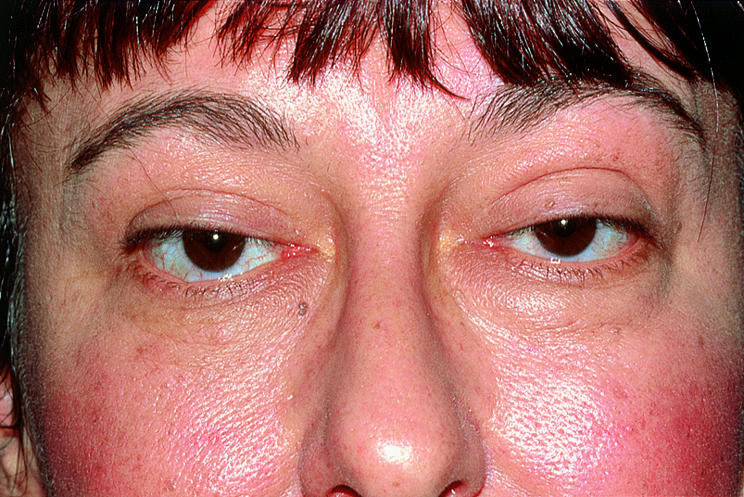
Thyroid orbitopathy (thyroid associated orbitopathy or Graves' ophthalmopathy) is a common cause of proptosis, eyelid retraction, orbital congestion, and motility disturbances in adults. Each patient experiences a unique combination of symptoms and signs for an unpredictable duration and with varying severity. Although orbital manifestations typically improve within 2–5 years, these years feel like a century to the extremely uncomfortable and discontented patients. In addition, visual loss due to optic nerve compression does occur. Radiotherapy has a key role in the management of moderate to severe inflammatory symptoms and is effective treatment for optic nerve compromise.
Our understanding of the link between systemic thyroid disease and orbitopathy has expanded greatly over the past 20 years, although the precise pathogenesis remains uncertain. An unknown mechanism allows thyroid antigen to stimulate the immune system and produce antibodies to the thyroid stimulating hormone (TSH) receptor and other antigens that alter the release of thyroid hormones. Shared orbit thyroid antigens result in the production of activated T lymphocytes that invade the orbital connective tissue.1,2 Orbital fibroblasts proliferate, resulting in increased synthesis and release of glycosaminoglycans. Locally produced cytokines amplify the inflammatory response. This combined cell mediated and humoral response results in inflammatory cell migration and production of oedema in the orbit. The result is thickening of extraocular muscle and an increase in orbital fat volume. External beam radiation can intervene in this process by arresting the fibroblast proliferation and diminishing the orbital inflammation permanently.
Early signs and symptoms
Symptoms and subtle signs of thyroid orbitopathy (TO) are often present for many years before diagnosis. Common and non-specific symptoms include tearing, irritation, aching, and photophobia. Early signs include conjunctival injection, periorbital puffiness, abnormal tear break up time, superficial punctate keratitis, and elevation of intraocular pressure. Eyelid retraction is a common early feature of hyperthyroidism, but can occur in euthyroid or hypothyroid patients with orbitopathy. The classic stare of bilateral, symmetric upper, and lower lid retraction may abate after thyroid function stabilises.
After identification of early symptoms and signs of TO, observation and patient education are indicated. Reassurance and description of the natural course of the disease are helpful. Lubricating drops, cool compresses, and sunglasses will improve symptoms during the day. Elevating the head of the bed and taping the eyelids will minimise periorbital oedema and irritation due to nocturnal lagophthalmos. Stable, significant eyelid retraction can be improved with appropriate surgical therapy. Corticosteroids and external beam radiation are never indicated for these early mild TO symptoms and signs.3
Dysmotility
Transient diplopia is very common and can progress to constant diplopia. Difficulties with fusion are usually worse in the morning because of fluid accumulation in the muscles that occurs with recumbency. Infiltration with inflammatory cells and fluid is typically followed by fibrosis which may create a permanent motility restriction. The inferior rectus and medial rectus are most commonly involved resulting in non-comitant esotropia or hypotropia, respectively. Forced duction testing is usually positive as a result of fibrotic contraction. Computed tomography (CT) imaging reveals bilateral, typically asymmetric, enlargement of extraocular muscles. Radiation can improve motility during the active inflammatory phase, but is ineffective against fibrotic extraocular muscle restriction.4–8
Most chronic motility disturbances caused by fibrosis are not radiosensitive. Radiation may improve motility in actively inflamed patients but rarely resolves the double vision entirely. Restrictive dysmotility is one of the reasons for the high rate of surgery (25–90%) previously noted following radiotherapy.4–6,8 In the recent study by Mourits, et al improvement in motility was the only difference between the group receiving radiation and the sham irradiation group. Twenty five per cent of patients receiving radiation were spared strabismus surgery, but 11/17 had an improvement in the field of binocular vision.8
For the vast majority of patients with dysmotility, the patient should be observed for at least 6 months and then offered strabismus surgery when inflammation has subsided and the restrictive component has stabilised.
Proptosis
Proptosis, the classic feature of thyroid orbitopathy, is actually less prevalent than eyelid retraction. It is caused by infiltration of the eye muscles with inflammatory cells and/or an increase in fat volume, resulting in forward displacement of the globe. Proptosis is usually axial and associated with increased resistance to retropulsion of the globe. Proptosis does not respond to external beam radiation; improvements of only 1–2 mm are typical in our experience. In several published series proptosis improved in 23–51% of patients4–6; in other studies, no significant change in proptosis occurred.7,8
We have used external beam radiation to quieten inflammation so that orbital decompression can be performed in a more timely fashion. No studies have addressed the effectiveness of this combination technique to minimise the duration of the active phase in patients with moderate to severe inflammation. Radiation has no role in the treatment of chronic proptosis.9
Orbital inflammation and congestion
Some patients with TO experience more significant inflammatory symptoms and signs. Rather than mild conjunctival injection over the recti muscle insertions, interpalpebral chemosis and severe periorbital oedema with erythema develop. Increased orbital volume secondary to inflammation is believed to impede venous outflow, which further aggravates congestion and the resulting proptosis. So, although inflammation and congestion are two distinct processes, they are intimately related.
Acute, severe orbital inflammation is effectively treated with corticosteroids combined with radiotherapy.4–6,10,11 We do not routinely use pulse intravenous steroids but they have been effectively used by others.12,13 We prescribe a starting dose of 80 mg of prednisone. The high dose is maintained for at least 1 week and then tapered slowly over 3 months. Care must be taken to tailor dosing to the individual's clinical response. Many patients cannot tolerate the side effects of high dose prednisone and/or are unable to be tapered off the medication without recurrence of symptoms and signs. Low dose radiation (20 Gy) is indicated in these patients to counteract inflammation. Prednisone should be continued during the radiation therapy. This combination of corticosteroids and radiation is only effective during the active phase of inflammation so appropriate patient selection is essential.14–16 Patients also should be counselled that further surgery might be necessary if restrictive myopathy or significant proptosis is present (Table 1).
Table 1 .
Benefits of external beam radiation for thyroid orbitopathy
| Directly treats the inflammation of thyroid orbitopathy |
| Can be utilised in the active phase of disease when surgery is not a good option |
| Minimises congestion, ache, injection and periorbital oedema |
| Decreases proptosis and dysmotility caused by acute inflammation |
| Alleviates visual dysfunction caused by acute optic nerve compression |
| The risks of general anaesthesia are eliminated |
| No bruising, swelling, or discomfort |
| Hospitalisation not required |
| Safe with minimal side effects in non-diabetic adults |
A recent study by Mourits and colleagues disputes the effectiveness of external beam irradiation for soft tissue inflammation.8 Thirty patients received radiation and 29 patients were treated with sham irradiation. There was no significant difference in soft tissue inflammation between the two groups at 24 weeks. This study did not treat patients with oral prednisone as we do and did not provide sufficient follow up information. External beam irradiation initially causes exacerbation of inflammation in TO. Since concomitant prednisone was not utilised, side effects of the radiation such as short term increased inflammation, lid swelling, chemosis, and proptosis would obscure any improvement in soft tissue inflammation. A prospective study comparing placebo and sham irradiation with prednisone and external beam radiation, evaluated for a minimum of 6 months, is needed.
Optic neuropathy
The optic nerve has a serpiginous course that allows for several millimetres of proptosis before compromise of optic nerve function. Proptosis, then, serves a protective function by expanding the total orbital volume. The orbital apex, conversely, has no room for expansion. Muscle enlargement posteriorly results in compression of the optic nerve just anterior to the optic canal. Muscle enlargement in the absence of significant proptosis is most likely to promote optic nerve compression. It is essential to check for signs of afferent dysfunction (for example, decreased visual acuity, abnormal colour vision, afferent pupillary defect, and abnormal visual fields) even in apparently asymptomatic patients. The process is often bilateral, so visual field testing remains essential even in the absence of an afferent pupillary defect. The most common clinical presentation is gradual onset of central visual loss, a central scotoma, and a normal optic nerve head appearance in a patient with mild to moderate proptosis.
If the clinical examination reveals afferent dysfunction, an orbital CT scan (without contrast) should be obtained in a timely fashion. Both axial and direct coronal views should be obtained to clarify the optic nerve position with respect to the extraocular muscles. Crowding of the optic nerve at the orbital apex is diagnostic.
If optic nerve compression is identified, corticosteroids are used in a temporising fashion until radiation or surgical decompression is performed. High dose steroids (at least 80 mg prednisone) should be initiated immediately. In our experience, if the orbit is acutely inflamed, steroids will result in marked visual improvement within 48 hours. Unfortunately steroids are usually only temporarily effective in treating optic neuropathy and significant side effects preclude long term use.
External beam radiation (20 Gy) is the definitive intervention in the actively inflamed orbit with visual loss as a result of optic nerve compression. If the visual loss has been precipitous the patient should be treated with intravenous corticosteroids and radiation initiated immediately to prevent permanent visual dysfunction. External beam is also effective in patients who have undergone orbital decompression but have not achieved complete resolution of the optic nerve impairment. 3,12–16
Radiation is contraindicated in diabetics and children owing to the increased risk of radiation retinopathy. External beam radiation causes temporary ocular irritation and exacerbates soft tissue inflammation from approximately 2 weeks following therapy and continuing for at least 24–36 weeks. Oral corticosteroids should, therefore, be continued during the course of radiation and tapered slowly over 3 months. If vision deteriorates despite corticosteroids and radiation treatment, surgical decompression is warranted. Conversely, if visual status is only temporarily improved with surgical decompression, external beam radiation should be performed.
Conclusions
External beam radiation is a safe and effective intervention in non-diabetic adults with acute congestive dysthyroid orbitopathy. Appropriate patient selection is essential. Orbital inflammatory signs and symptoms are eliminated within weeks of the therapy when radiation is combined with oral corticosteroids. Optic nerve compression is alleviated within 1 month. Only corticosteroids, with their significant side effects, provide a similar direct intervention in the acute disease process.
Clinical studies have consistently demonstrated that radiation, especially in combination with oral prednisone, is effective at decreasing soft tissue signs and symptoms due to inflammation. Radiation is also widely used for treatment of visual loss in patients with actively inflamed orbits. Proptosis and dysmotility are inconsistently improved with radiotherapy; external beam radiation should not be used for these indications where chronic fibrosis is probable.
A large multicentre trial is needed to confirm the clinical impression that radiation can minimise the active inflammatory phase in moderate to severely affected patients with TO. We are currently evaluating the long term outcome of thyroid associated optic nerve compression treated with radiation versus orbital decompression.
REFERENCES
- 1.Barsouk A, Peele KA, Kiljanski J, et al. Antibody-dependent cell-mediated cytotoxicity against orbital target cells in thyroid-associated ophthalmopathy and related disorders. J Endocrin Invest 1996;19:334–41. [DOI] [PubMed] [Google Scholar]
- 2.Wall JR, Barsouk A, Stolarski C, et al. Serum muscle antibodies predicted the development of ophthalmopathy in a euthyroid subject with a family history of autoimmunity. Thyroid 1996;6:353–8. [DOI] [PubMed] [Google Scholar]
- 3.Peele-Cockerham KA, Kennerdell JS. Thyroid-associated orbitopathy. Focal Points 1997;XV(1): 1–14. [Google Scholar]
- 4.Bartalena L, Marcocci C, Chiovato L, et al. Orbital cobalt irradiation combined with systemic corticosteroids for Graves' ophthalmopathy: comparison with systemic corticosteroids alone. J Clin Endocrinol Metab 1983;56:1139–44. [DOI] [PubMed] [Google Scholar]
- 5.Hurbli T, Char DH, Harris J, et al. Radiation therapy for thyroid eye diseases. Am J Ophthalmol 1985;99:633–7. [DOI] [PubMed] [Google Scholar]
- 6.Peterson IA, Kriss JP, McDougall IR, et al. Prognostic factors in the radiotherapy of Graves' ophthalmopathy Int J Radiat Oncol Biol Phys 1990;19:159–64. [DOI] [PubMed] [Google Scholar]
- 7.Prummel MF, Mourtis MP, Blank L, et al. Randomized double-blind trial of prednisone versus radiotherapy in Graves' ophthalmopathy. Lancet 1993;342:949–5. [DOI] [PubMed] [Google Scholar]
- 8.Mourits MPh, Van Kempen-Harteveld ML, Garcia Garcia MB, et al. Radiotherapy for Graves' orbitopathy: randomized placebo-controlled study. Lancet 2000;355:1501–9. [DOI] [PubMed] [Google Scholar]
- 9.Kennerdell JS, Maroon JC, Buerger GF. Comprehensive surgical management of proptosis in dysthyroid orbitopathy. Orbit 1987;6:153–79. [Google Scholar]
- 10.Sakata K, Hareyama M, Oouchi A, et al. Radiotherapy in the management of Graves' ophthalmopathy. Jap J Clin Oncol 1998;28:364–7. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson SS, Bagshaw MA, Kriss JP. Supervoltage radiotherapy for Graves' ophthalmopathy. J Clin Endocrinol Metab 1973;37:276–85. [DOI] [PubMed] [Google Scholar]
- 12.Tagami T, Tanaka K, Nakamura H, et al. High-dose intravenous steroid pulse therapy in thyroid-associated ophthalmopathy. Endocrine J 1996;43:689–99. [DOI] [PubMed] [Google Scholar]
- 13.Matejka G, Verges B, Vaillant G, et al. Intravenous methylprednisolone pulse therapy in the treatment of Graves' ophthalmopathy. Hormone Metab Res 1998;30:93–8. [DOI] [PubMed] [Google Scholar]
- 14.Beckendorf V, Maalouf T, George J-L, et al. Place of radiotherapy in the treatment of Graves'orbitopathy. Int J Radiation Oncology Biol Phys 1999;43:805–15. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WB, Prochoda M. Radiotherapy for thyroid orbitopathy. Arch Ophthalmol 1995;113:1420–5. [DOI] [PubMed] [Google Scholar]
- 16.Kao SCS, Kendler DL, Nugent RA, et al. Radiotherapy in the management of thyroid orbitopathy. Arch Ophthalmol 1993;111:819–23.... [DOI] [PubMed] [Google Scholar]