Abstract
Background: Sorsby's fundus dystrophy (SFD) and age related macular degeneration (ARMD) are retinal diseases associated with a high level of accumulation of mutant and wild type TIMP-3, respectively, in Bruch's membrane. The pathogenic role of TIMP-3 in these diseases is uncertain, but causative mutations have been identified in the TIMP-3 gene of patients with SFD. Recent reports that TIMP-3 causes apoptosis in certain cell types and not in others prompted the authors to investigate whether TIMP-3 causes apoptosis in cultured retinal pigment epithelium (RPE) cells.
Methods: RPE and MCF-7 cells (as a positive control) were initially infected with replication deficient adenovirus, to overexpress β-galactosidase (RAdLacZ) or TIMP-3 (RAdTIMP-3). TIMP-3 was detected by western blotting and ELISA. Cell viability was defined by cell counts. ISEL was used to investigate the mechanism of cell death.
Results: Cultured RPE cells produced small quantities of endogenous TIMP-3 and remained viable. However, overexpression of TIMP-3 caused a dose related death of RPE cells. The mechanism of cell death was apoptosis.
Conclusion: The previously unreported finding of TIMP-3 induced apoptosis of RPE cells may account for some of the early features seen in SFD and ARMD.
Keywords: gene transfer, retinal pigment epithelium, apoptosis, macular degeneration
Sorsby's fundus dystrophy (SFD)1 is a rare autosomal dominant retinal macular degeneration with onset in the fourth decade of life. The disease is caused by mutations in exon 5 of the gene for TIMP-3 and is characterised by extracellular deposits in Bruch's membrane.2 These deposits are rich in TIMP-3 and in this respect similar to drusen found in Bruch's membrane of patients with age related macular degeneration (ARMD),2–4 one of the most common causes of blindness in developed countries. The similarities between SFD and ARMD extend beyond the deposition of TIMP-3 in Bruch's membrane. Disease pathology appears to follow the same course since, in both situations, Bruch's membrane thickens, the transport of essential metabolites from the choriocapillaris to the retinal pigment epithelium (RPE) is impeded, and the RPE cells become dysfunctional and die.2–5 In addition to these events, new vessels, derived from the choriocapillaris, grow through the thickened Bruch's membrane into the sub-RPE space. Exudation and haemorrhaging from these vessels eventually result in severe macular scarring and irreversible loss of central vision.3,5
Recent studies have localised TIMP-3 mRNA to RPE cells and suggest that the TIMP-3 present in Bruch's membrane originates from RPE cells.6–13 Since TIMP-3 is a matrix metalloproteinase (MMP) inhibitor, it might be expected to decrease extracellular matrix turnover in normal healthy eyes. If this is the case, in SFD and ARMD, where excessive quantities of mutant and wild type TIMP-3, respectively, accumulate in Bruch's membrane, this should favour matrix synthesis over degradation.
In addition to inhibiting MMP activity, TIMP-3 has also been shown to promote apoptosis in various cell lines. These include vascular smooth muscle,14 melanoma,15 human colon carcinoma,16 HeLa, HT1080, and MCF-7 cells.17 Interestingly, COS-7 cells do not undergo TIMP-3 mediated apoptosis.18 This is a property not shared by TIMPs 1 or 2.14,15,17 At the present time it is generally believed that the phenomenon of RPE cell loss in SFD and ARMD is a direct and inevitable consequence of metabolic deprivation. The possibility that RPE cell apoptosis might be directly induced by TIMP-3 and a primary pathological event in these diseases has not been previously considered. We therefore investigated whether RPE cells are susceptible to the apoptotic effects of TIMP-3. To this end we have infected cultured RPE and as a positive control of TIMP-3 induced apoptosis, MCF-7 adenocarcinoma cells with a replication deficient adenovirus vector containing the gene for wild type TIMP-3 (RAdTIMP-3). Following infection, the effects of high level TIMP-3 expression have been investigated.
MATERIALS AND METHODS
Reagents
All chemicals, unless otherwise stated, were obtained from Sigma Chemical Co (UK) and were of the highest grade available. Culture media (MEM and DMEM), fetal calf serum (FCS), and glutamine were obtained from Gibco BRL. A penicillin, streptomycin and amphotericin antibiotic/antimycotic solution, the fluorescent cytokeratin 8 and 18 antibodies, and the horseradish peroxidase conjugated secondary antibodies were all obtained from Sigma Diagnostics (UK). The polyclonal TIMP-3 antibody, raised against loop-1 of human TIMP-3 was obtained from Chemicon (UK).
Experimental tissues and cell lines
Retinal tissue was obtained from cadaver eyes, donated to the Bristol Eye Bank with permission for research. The age of the donors was under 40 years, and the RPE sheets were dissected from the ocular globes within 36 hours of death.
The human adenocarcinoma cell line, MCF-7 was obtained from the American Tissue Culture Collection.
Tissue culture
Human RPE cells
Following a simplified version of previously described methods,19–23 eyes were opened posterior to the ora serrata and the vitreous and retinal tissues were removed. The RPE sheets were dissected and fragmented using sterile forceps and a scalpel in a small quantity of MEM medium supplemented with 10% v/v FCS, glutamine, and the antibiotic/antimycotic mixture in 5 cm diameter petri dishes. The fragmented tissue was then transferred into 25 cm3 culture flasks and left for a minimum period of 1 hour at 36°C in a 5% carbon dioxide incubator to allow the tissue to adhere to the flasks. Additional MEM, with 10% v/v FCS (2 ml), was then added to each flask. After 3 days' incubation under the same conditions, the MEM was removed. As fibroblasts and choroidal cells fail to grow in low serum conditions, to inhibit their propagation, the medium was replaced with MEM containing 2.5% v/v FCS. This was subsequently replenished every 3–4 days, until the cell cultures became established. To propagate the cultures, the cells were washed with Ca2+ free PBS, trypsinised, collected by centrifugation at 1500 rpm for 3 minutes at room temperature, resuspended in fresh MEM medium containing 10% v/v FCS, and reseeded into multiple 75 cm3 culture flasks. For obtaining the cells used experimentally, this passaging procedure was never repeated more than twice. Cells from multiple donors were used for each experiment.
MCF-7 cells
Cryopreserved MCF-7 cells were thawed at 37°C, washed in DMEM supplemented with10% FCS (v/v) and antibiotic/antimycotic solution, and plated out in 75 cm3 culture flasks. All established cell cultures were maintained at 36°C in an atmosphere of 95% air and 5% carbon dioxide (Fig 1). The culture media were changed every 3-4 days.
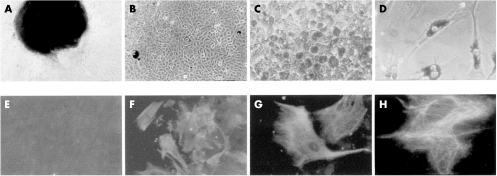
Figure 1.
(A) Retinal explant giving rise to RPE cells (×100). (B) Confluent fusiform RPE cell culture lacking pigment (×100). (C) Pigmented polygonal RPE cell culture (×200). (D) Pigmented fusiform RPE cells (×200). (E) Corneal fibroblasts (negative control) immunostained for cytokeratins 8 and 18 (×200). (F, G, H): RPE cells immunostained for cytokeratins 8 and 18 (×200, ×400, and ×1000, respectively).
Cytokeratin labelling
To confirm that our RPE cell cultures were free from choroidal and fibroblast contamination, immunostaining for cytokeratin 8 and 18 was used.24 Corneal fibroblasts served as a morphologically similar control cell line that lacks these cytoskeletal proteins.
The RPE cells and corneal fibroblasts were washed twice with PBS, fixed with ice cold methanol and ice cold acetone for 5 minutes each, and washed again with PBS. The cells were then treated with block (PBS with 5% FCS) for 30 minutes. Following incubation, at 37°C for 1 hour, with mouse primary anti-cytokeratin 8 and 18 antibody (1:50 with PBS and 5% FCS), the cells were washed in PBS and incubated with a fluorescent anti-mouse secondary antibody (1:50). Cells were visualised microscopically at ×400 magnification using a cobalt blue filter (488 nm).
Recombinant adenoviruses
Recombinant replication deficient adenovirus RAdlacZ (RAd35), which contains the Escherichia coli β-galactosidase (lacZ) gene under the control of cytomegalovirus major immediate early promoter (CMV IE), was kindly donated by Dr Gavin W G Wilkinson (University of Cardiff, Wales).25 The construction and characterisation of the replication deficient adenovirus containing the coding region of the human TIMP-3 (RAdTIMP-3) gene driven by CMV IE promoter has been described in detail previously.14
Adenoviral infection protocol
RPE and MCF7 cells were plated separately into six well plates at densities of 2.5 × 105 cells/well and incubated in complete medium for 24 hours. Immediately before infection, the RPE and MCF cells of three wells were trypsinised and counted with a haemacytometer. Cells in the remaining wells were infected at the required adenoviral titre in 2 ml fresh medium and left for 18 hours. After removing the medium and washing, the cells were incubated in 2 ml fresh complete medium. Viable cell numbers were determined after a further 48 hours.
Determination of infection efficiency of RPE and MCF7 cells
To determine the infection efficiency of RPE and MCF7 cells, plated cells were mixed, in triplicate with 100, 300, and 1000 pfu/cell of RAdlacZ, respectively, and incubated for 18 hours. After washing, these cells were incubated for a further 48 hours in fresh complete medium. Cells were stained with X-gal stain (5-bromo-4-chloro-3indoyl-β-d-galactopyranosidase) (100 mM sodium phosphate pH 7.3 (77 mM Na2HPO4, 23 mM NaH2PO4), 1.3 mM MgCl2, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6, and 1 mg/ml X-gal). Positive (blue) cells were counted in three high power (×400) fields per section from triplicate cultures and the percentage infection efficiency calculated.
Estimation of viable cell density
To estimate the number of viable cells remaining after infection with RAdTIMP-3, cell cultures were trypsinised after 48 hours, mixed with an equal volume of trypan blue (0.2% w/v) in PBS, and counted with a haemacytometer. All counts were performed in triplicate.
Detection of TIMP-3 protein
After RAdTIMP-3 or RAdlacZ infection, the cells and their matrices were harvested in 0.05 M TRIS, pH 7.4 containing 2% w/v SDS (100 μl/well). Conditioned media were collected and 10-fold concentrated using Centricon microconcentrators (Amicon Inc, Stonehouse, UK). The samples were reduced with mercaptoethanol (2%), boiled for 10 minutes, and electrophoretically fractionated on SDS polyacrylamide (10%w/v) gels. The proteins in these gels were then western blotted onto PDVF (Millipore) membranes and immunostained using the polyclonal TIMP-3 primary antibody (1:200 with TRIS buffered saline + 5% FCS) and horseradish peroxidase (HRP) conjugated anti-rabbit IgG secondary antibody (1:1000). Diaminobenzidine (DAB) was used as the HRP substrate, following the manufacturer's instructions.
TIMP-3 ELISA assays
To confirm that normal, healthy, uninfected RPE cells produce TIMP-3, confluent cultures of RPE cells and corneal keratocytes were washed with PBS and harvested in methanol. After homogenisation using a small glass homogeniser, aliquots of suspension were dried in air. Blocking solution (0.05M TRIS-HCl, pH 7.4 containing 150 mM NaCl, 5%v/v FCS, 2 mM mercaptoethanol, and 0.02% NaN3) was added for 2 hours, followed by primary TIMP-3 antibody (1:500 with TRIS buffered saline and 5% FCS) and secondary HRP conjugated rabbit IgG antibody (1:1000) with stringent washing, with TRIS buffered saline and TRIS buffered saline + 0.2% Tween 20, before and after incubation with each antibody. The HRP substrate, TMB (Sigma, UK) was added according to the supplier's instructions and the kinetics of oxidation of TMB were followed at 370 nm at 30°C, using a Molecular Devices Spectromax Spectrophotometer.
In situ end labelling (ISEL) protocol
ISEL was used to determine whether the mechanism of RPE and MCF-7 cell death was apoptosis. The cells were plated out, in triplicate, on sterile glass coverslips and infected with RAdTIMP-3 at 1000 pfu/cell. ISEL was performed according to Baker et al.14 Incubation in cobalt-DAB was used to distinguish positive nicked DNA from negative DNA.
RESULTS
RPE cell cultures
Primary RPE cells isolated from the retinal explants (Fig 1A) were ready for experimentation in 21 days (passage 1 or 2). In concurrence with previous reports,23 the cultured RPE cells adopted several different morphological forms (Fig 1B, C, D). Although cells tended to become fusiform in shape (Fig 1D), pigmented polygonal RPE cells were still present in primary cultures maintained for 12 months (Fig 1C). All cells in the RPE cell cultures were positive for cytokeratin 8 and 18 (Fig 1 F, G, H), indicating no significant contamination by fibroblasts or choroidal cells. The control corneal fibroblasts were negative for these cytoskeletal proteins (Fig 1E).
Determination of transduction efficiency of RPE and MCF7 cells
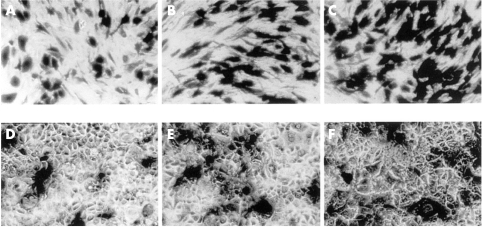
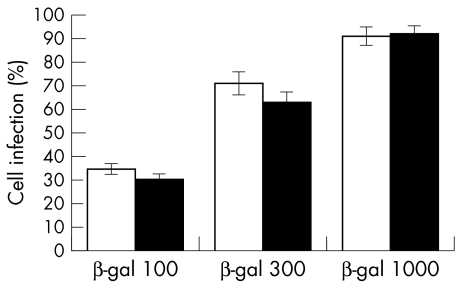
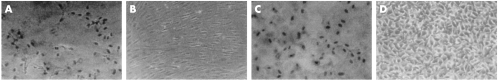
Photographs of RPE and MCF-7 cells infected with RadlacZ and stained with X-gal are shown in Figure 2. Histograms of the estimated numbers of infected cells in these cultures are given in Figure 3. The summated data indicate that 91% (SD 4%) of RPE cells and 92% (4%) of MCF7 cells were infected at 1000 pfu/cell. At 300 pfu/cell, 71% (5%) of RPE cells and 63% (5%) of MCF7 cells were infected and at 100 pfu/cell, 34% (3%) of RPE and 29% (4%) of MCF7 cells were infected. The numbers of RPE and MCF-7 cells infected were not significantly different (p >0.2, Student's t test) and indicate that these cell lines are equally susceptible to infection with RadlacZ.
Figure 2.
RAdlacZ infected RPE cells (A, B, C) and MCF7 cells (D, E, F) at 100, 300, and 1000 pfu/cell, respectively, stained with X-gal (×200).
Figure 3.
A histogram of the numbers of X-gal positive RPE (open bars) and MCF-7 (solid bars) cells, 48 hours after infection with RadlacZ and staining for β-galactosidase activity. Statistical significance by Student's t test compared with corresponding group: p >0.2 in all groups (n=9).
Analysis of transgene production
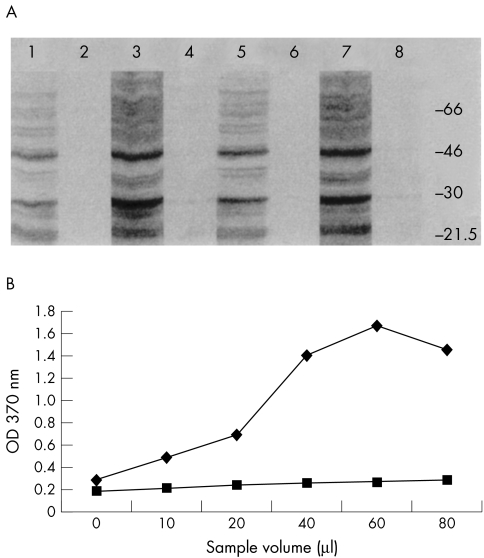
Evidence that TIMP-3 was produced by RAdTIMP-3 infected RPE and MCF-7 cell cultures is given, in Figure 4A, by a representative immunostained western blot of the SDS extracted proteins from cell matrix fractions and from 10-fold concentrated conditioned media. In the cell matrix fractions, the two characteristic TIMP-3 bands of Mr 24 000 and 28 000, corresponding to the unglycosylated and glycosylated forms, respectively, were both present. The glycosylated form of TIMP-3 was predominant (lanes 3 and 7). In addition, a band at Mr 50 000, consistent with dimerised TIMP-3 was seen. Tenfold concentrated media fractions showed similar but lower intensity bands (lanes 1 and 5). Similar extracts of RPE and MCF-7 cell matrices and 10-fold concentrated conditioned media, obtained after infection with RAdlacZ contained little or no detectable TIMP-3 in RPE conditioned media or cell matrix fractions (lanes 2 and 4), or MCF7 fractions (lanes 6 and 8).
Figure 4.
(A) An immunostained western blot of the SDS extracted proteins from RPE and MCF7 cell matrices and 10-fold concentrated media fractions. Lane 1, RPE RAdTIMP-3 media; lane 2, RPE RAdlacZ media; lane 3, RPE RAdTIMP-3 matrix; lane 4, RPE RAdlacZ matrix; lane 5, MCF7 RAdTIMP-3 media; lane 6, MCF7 RAdlacZ media; lane 7, MCF7 RAdTIMP-3 matrix; lane 8, MCF7 RAdTIMP-3 media. (B) A line chart showing the results of ELISA detection of endogenous TIMP-3 in established RPE (upper line) and keratocyte (lower line) cell cultures. The control cells show relatively little endogenous TIMP-3 production compared with RPE cells.
Confirmation that low levels of TIMP-3 are produced by RPE cell cultures
Despite, the failure to reliably detect TIMP-3 in normal RPE cell cultures by western blotting, evidence that cultured RPE cells normally produce TIMP-3, was obtained by ELISA. For comparative purposes, similarly obtained protein samples from corneal fibroblasts and their cell matrices were also assayed. The results obtained are shown in Figure 4B.
Effect of TIMP-3 overexpression on RPE and MCF7 cell survival
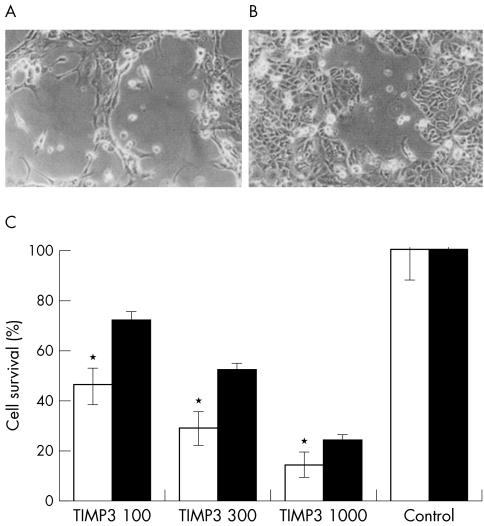
TIMP-3 overexpression resulted in a dose dependent reduction in cell number in both RPE and MCF7 cultures. RPE and MCF7 cells, 48 hours after infection with 300 pfu/cell of RadTIMP-3, are shown in Figure 5A and B, respectively. The percentage survival of RPE and MCF-7 cells at increasing RAdTIMP-3 infection dosage is shown in Figure 5C. The averaged percentage of viable RPE cells remaining after infection with 100, 300, and 1000 pfu/cell RAdTIMP-3 was 46% (7%), 29% (7%), and 14% (5%), respectively. The averaged percentage of viable MCF-7 cells remaining after infection with 100, 300, and 1000 pfu/cell RAdTIMP-3 was 69% (3%), 52% (2%), and 24% (2%), respectively.
Figure 5.
(A, B) Representative photographs (×100) showing RPE (A) and MCF7 (B) cells 48 hours after infection with RAdTIMP-3. Note the cells rounding up and lifting, leaving patches of cell loss, consistent with localised TIMP-3 deposition. (C) A histogram of the numbers of viable RPE (open bars) and MCF7 (solid bars) cells remaining in culture 48 hours after infection with RAdTIMP-3. These cells were trypsinised, stained with trypan blue, and counted with a haemacytometer. Statistical significance, by Student's t test, compared with corresponding MCF7 group: *p <0.001 (n=9). Statistical significance, by Student's t test, compared with corresponding control group p<0.001 (n=9) for all groups.
Given that the RPE and MCF-7 cells were equally susceptible to infection by RadlacZ, it follows that the RPE cells were more readily killed by infection with RAdTIMP-3 than the MCF-7 cells (p values <0.001, Student's t test).
Evidence that TIMP-3 induces RPE cell apoptosis
ISEL staining of the dead or dying RPE and MCF-7 cells in cultures infected with RAdTIMP-3 at 1000 pfu/cell showed dark DAB stained nuclei, providing evidence that the mechanism of TIMP-3 induced RPE and MCF7 cell death was apoptosis. Control cells, infected with RadlacZ, showed no DAB staining. Representative photographs of these cells are given in Figure 6.
Figure 6.
Representative photographs (×100 magnification) of ISEL labelled, RAdTIMP-3 infected RPE and MCF7 cells (A and C, respectively) and RadlacZ infected cells (B and D, respectively). The apoptotic cells were detected with cobalt-DAB and are seen with darkly stained nuclei. The control cells were ISEL negative.
DISCUSSION
The pathological processes that occur in Sorsby's fundus dystrophy and ARMD are similar and involve the excessive accumulation of mutant or wild type TIMP-3 in Bruch's membrane.9–13 The micropathological sequence of events in these conditions, however, is largely unknown.5,10
TIMP-3 is a matrix metalloproteinase inhibitor and as such its accumulation may limit the degradation and promote thickening of tissues rich in collagens, elastin, and basement membrane, such as Bruch's membrane. Diffuse thickening of Bruch's membrane is seen as a late pathological feature in these conditions and is thought to lead to suppression of nutrient delivery to RPE cells, resulting in RPE cell death. Despite this, pathological reports on SFD and ARMD eyes2,4,5,10 describe the presence of localised patches of RPE cell loss very early in the disease process. This is appreciated clinically as early hypopigmented spots in the macular region of these eyes. This sequence of events suggests that RPE death may be a primary component of the pathology.
In addition to MMP inhibition, recent reports have shown that TIMP-3 can induce apoptosis in certain cell types, but not in others.14–18 Our present study confirmed that healthy RPE cell cultures produced small amounts of TIMP-3 and remained viable for long periods. However, infection of RPE cells with recombinant replication deficient adenoviruses resulted in high levels of transgene production. Specifically, high level TIMP-3 overexpression, dose dependently, induced apoptosis in RPE cells. These results were consistent with those of MCF7 cells.
Given that RPE cells are the origin of the majority of TIMP-3 in the eye and that TIMP-3 accumulates in Bruch's membrane,6–12 it is likely that there is a concentration gradient of TIMP-3 between RPE cells and Bruch's membrane. We propose that early, localised accumulation of TIMP-3 around RPE cells accounts for the RPE cell loss seen in early SFD and ARMD. This phenomenon was previously unrecognised. TIMP-3 may subsequently bind to components of Bruch's membrane and interactions within Bruch's membrane and with MMPs are likely to play a part in the late pathology of these diseases. These interactions and the role of the SFD mutant proteins remain to be explored.
Acknowledgments
We thank the Wellcome Trust for financial support (ophthalmology fellowship to M A Majid).
REFERENCES
- 1.Sorsby A, Mason MEJ. A fundus dystrophy with unusual features. Br J Ophthalmol 1949;33:67–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capon MRC, Marshall J, Krafft JI, et al. Sorsby's fundus dystrophy. A light and electron microscopic study. Ophthalmology 1989;96:1769–77 [DOI] [PubMed] [Google Scholar]
- 3.Polkinghorne PJ, Capon MRC, Berninger T, et al. Sorsby's fundus dystrophy. A clinical study. Ophthalmology 1989;96:1763–8. [DOI] [PubMed] [Google Scholar]
- 4.Kamei M, Apte SS, Rayborn ME. TIMP-3 accumulation in Bruch's membrane and Drusen in eyes from normal and age-related macular degeneration donors. In: La Vail MM, Anderson RE, Hollyfield JG, eds. Degenerative diseases of the retina. New York: Plenum Press, 1997.
- 5.Zarbin MA. Age-related macular degeneration: review of pathogenesis. Eur J Ophthalmol 1998;8:199–206. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz A, Brett P, Bok D. TIMP-3 is expressed in the human retinal pigment epithelium. Biochem Biophys Res Commun 1996;226:467–74. [DOI] [PubMed] [Google Scholar]
- 7.Della NG, Campochiaro PA, Zack DJ. Localization of TIMP-3 mRNA expression to the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1996;37:1921–4 [PubMed] [Google Scholar]
- 8.Vranka JA, Johnson E, Zhu X, et al. Discrete expression and distribution pattern of TIMP-3 in the human retina and choroid. Curr Eye Res 1997;16:102–10 [DOI] [PubMed] [Google Scholar]
- 9.Jomary C, Neal MJ, Iwata K, et al. Localisation of tissue inhibitor of metalloproteinases-3 in neurodegenerative retinal disease. Neuroreport 1997;8:2169–72. [DOI] [PubMed] [Google Scholar]
- 10.Fariss RN, Apte SS, Luthert PJ, et al. Accumulation of tissue inhibitor of metalloproteinases-3 in human eyes with Sorsby's fundus dystrophy or retinitis pigmentosa. Br J Ophthalmol 1998;82:1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fariss RN, Apte SS, Olsen BR, et al. Tissue inhibitor of metalloproteinase-3 is a component of Bruch's membrane of the eye. Am J Path 1997;16:102–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Kamei M, Hollyfield JG. TIMP-3 in Bruch's membrane: changes during ageing and in age-related macular degeneration. Invest Ophthalmol Vis Sci 1999;40:2367–75. [PubMed] [Google Scholar]
- 13.Leco KJ, Khoka R, Pavloff N, et al. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 1994;269:9352–60. [PubMed] [Google Scholar]
- 14.Baker AH, Zaltsman AB, George SJ, et al. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 over-expression on rat vascular smooth muscle cell invasion, proliferation and death in vitro. J Clin Invest 1998;101:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahonen M, Baker A, Veli-Matti K. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinase-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 1998;58:2310–5. [PubMed] [Google Scholar]
- 16.Smith MR, Kung H, Durum SK, et al. TIMP-3 induces cell death by stabilising TNF-α receptors on the surface of human colon carcinoma cells. Cytokine 1997;9:770–80. [DOI] [PubMed] [Google Scholar]
- 17.Baker AH, George SJ, Zaltsman AB, et al. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP–3. Br J Cancer 1999;79:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langton KP, Barker MD, McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J Biol Chem 1998;273:16778–81. [DOI] [PubMed] [Google Scholar]
- 19.Albert DM, Tso MOM, Rabson AS. In vitro growth of pure cultures of retinal pigment epithelium. Arch Ophthalmol 1972;88:63–9. [DOI] [PubMed] [Google Scholar]
- 20.Aronson JF. Human retinal pigment cell culture. In vitro 1983;19: 642–50. [DOI] [PubMed] [Google Scholar]
- 21.Del Monte MA, Maumenee IH. In vitro culture of human retinal pigment epithelium for biochemical and metabolic study. Vis Res 1981;21: 137–42. [DOI] [PubMed] [Google Scholar]
- 22.Tezel TH, Del Priore LV, Kaplan HJ. Harvest and storage of adult human retinal pigment epithelial sheets. Curr Eye Res 1997;12:802–09. [DOI] [PubMed] [Google Scholar]
- 23.Burke JM, Skumatz CMB, Irving PE, et al. Phenotypic heterogeneity of retinal pigment epithelial cells in vitro and in situ. Exp Eye Res 1196;62:63–73. [DOI] [PubMed] [Google Scholar]
- 24.Mckhenie NM, Boulton M, Robey HL, et al. The cytoskeletal elements of human retinal pigment epithelium: in vitro and in vivo. J Cell Sci 1988;91:303–12. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson GWG, Akrigg A. Constitutive and enhanced expression of the CMV major IE promoter in a defective adenovirus promoter. Nucleic Acids Res 1992;20:2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]