Abstract
Aim: To describe the clinical and genetic aspects of a retinal dystrophy that combines central areolar choroidal dystrophy (CACD) and autosomal dominantly inherited drusen.
Methods: The members of three unrelated families who demonstrated the rare combination of CACD and dominant drusen were clinically and angiographically investigated. In addition, DNA samples from the members of these families were screened for the Arg142Trp mutation in the peripherin/retinal degeneration slow (RDS) gene.
Results: The severity of the CACD/dominant drusen maculopathy was age related and the expression of the phenotype varied. All affected individuals carried the Arg142Trp mutation in the peripherin/RDS gene. The clinical spectrum ranged from CACD without noticeable drusen in four individuals to the fully expressed phenotype of CACD with drusen in 14 individuals.
Conclusion: CACD macular dystrophy is associated with dominant drusen in most individuals carrying the Arg142Trp mutation in the peripherin/RDS gene in the three families described. There are no individuals with dominant drusen in the absence of the Arg142Trp mutation, suggesting that the Arg142Trp mutation is one of the factors predisposing to drusen development.
Keywords: choroidal dystrophy, dominantly inherited drusen
Central areolar choroidal dystrophy (CACD) is a macular dystrophy characterised by subtle, mottled depigmentation in the posterior pole in the early stages. In time this geographic depigmentation gradually enlarges until an oval or round area of atrophy of the retinal pigment epithelium (RPE) and choriocapillaris is formed.1 Typically, no flecks or drusen are observed in this type of chorioretinal dystrophy.2 The Arg142Trp mutation in the peripherin/retinal degeneration slow (RDS) gene has been implicated as a cause of autosomal dominant CACD.3 Sporadic cases of CACD have also been described but could not be attributed to mutations in the peripherin/RDS gene.3 Finally, a type of autosomal dominant CACD described in a Northern Irish family has been linked to chromosome 17p13.4,5
A variety of names have been given to the clinical entity of dominantly inherited drusen, including Doyne's honeycomb choroiditis, Holthouse-Batten's superficial chorioretinitis, Hutchinson-Tay choroiditis, malattia leventinese, and crystalline retinal degeneration.6,7 In these disorders drusen are usually recognised at a relatively early age, in general after the age of 20. Stone et al recently identified a single mutation in the EGF containing fibrillin-like extracellular matrix protein 1 (EFEMP1) gene in patients with malattia leventinese and Doyne honeycomb retinal dystrophy.8
There is strong evidence that genetic factors play an important part in the development of age related macular degeneration (AMD).9–12 Heterozygous mutations in the rod photoreceptor specific ATP binding cassette transporter (ABCR) gene were identified in 16% of patients with AMD.13 Recently, a large multicentre study confirmed a significant association between two frequent ABCR mutations and AMD; several other studies, however, failed to demonstrate a significant relation.14–20 Since this disorder accounts for approximately 50% of the registered blindness in the Western world, inherited retinal disorders that display phenotypic overlap with AMD are of great interest.21–23 In this study we present a type of autosomal dominant CACD, which is associated with dominant drusen. We will describe the clinical findings in the affected members of three separate families and we will discuss the results of the genetic analysis of both families.
PATIENTS AND METHODS
Patients
In 1970 Deutman described two families with dominantly inherited drusen of Bruch's membrane.7 Several years later one of the patients of family A (III-10, at that time 40 years of age) visited our clinic with a decrease in visual acuity in both eyes. Besides drusen, funduscopy revealed an irregular atrophy of the RPE surrounding the fovea. Her twin sister demonstrated the same ophthalmoscopic findings when she visited our clinic 3 years later. To our surprise both sisters developed an oval shaped area of chorioretinal atrophy in the posterior pole over the years, resembling CACD. Upon the discovery of the Arg142Trp mutation in the peripherin/RDS gene as being responsible for the development of autosomal dominant CACD in seven families in the south of the Netherlands, both patients tested positive for this mutation. In view of the findings in this family (family A) we were interested to learn whether more patients showed the association of dominantly inherited drusen with CACD. Therefore, the ophthalmic records of all the patients in the seven families with CACD in our clinic were re-examined.
In one family (family B, family Li in the study by Hoyng et al3) three of four CACD patients also demonstrated the combination of CACD and drusen, although this association was not mentioned in the study of Hoyng et al3. Finally, during the preparation of this manuscript another patient presented with the association of CACD and drusen (family C).
Clinical investigations
Members of these families were re-evaluated. After the ophthalmic history was taken, all patients received a standard ophthalmological evaluation. This included assessment of the visual acuity, biomicroscopy of the anterior segment, and fundus examination. In addition, fundus photography and fluorescein angiography were performed. Electrophysiological testing was not a part of this evaluation although some of the patients had undergone these investigations in the past. Electroretinography (ERG) testing was performed as described by Thijssen et al24. The results of the molecular genetic analysis were not available at the time of this evaluation and therefore had no influence on the outcome of the clinical investigations.
Molecular genetic analysis
After informed consent was obtained, blood samples were taken and the nucleotide sequence of the segment spanning codon 142 of the RDS/peripherin gene was determined in one of the affected individuals of family A (individual III-13) by using the BigDye terminator sequencing kit (Amersham) on a 3700 Perkin Elmer sequencer. Subsequently, a diagnostic restriction digest was performed on a segment of the RDS gene containing the Arg142Trp mutation. In short, primers 1302 (5'-GCTCGCTGGAGAACACCCT-3') and 1303 (5'-TCTGACCCCAGGACTGGAAG-3') were employed for a polymerase chain reaction consisting of 32 cycles of 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute. The amplification reaction was performed with 100 ng genomic DNA, 15 pmol of each primer, 200 μM of each dATP, dTTP, dGTP, and dCTP, 1x SuperTaq buffer (10 mM TRIS-HCl pH 9.0, 50 mM KCl, 1.5 mM MgCl2, 0.1%(w/v) Triton X-100, 0.01% (w/v) gelatine), and 1 U SuperTaq (HT Biotechnology Ltd, UK). The reactions were performed in a Perkin Elmer DNA thermal cycler. The amplified fragments were purified by phenol/chloroform extraction and precipitated with 0.1 volume 3M sodium acetate pH 5.2 and 2.5 volume ethanol 96%. The pellet was resuspended in water and the DNA was digested in the applied buffer with 10 U MspI (Life Technologies). The digests were resolved on a 1.5% agarose gel containing 100 mM TRIS-borate, 2 mM EDTA pH 8.3. The wild type PCR fragment (240 bp) is cut by MspI into fragments of 53 and 187 bp; the C>T alteration at nt 423 in patients with CACD destroys the MspI restriction site yielding a 240 bp uncut fragment. To investigate the presence of the EFEMP1 Arg345Trp mutation, we amplified and sequenced exon 10 of the gene as described.8 The intronic primers flanking this mutation in exon 10 are 5'-CTTGCAAACAGAATCTGCCA-3' and 5'-TCCTCACTTTCAAAAGTTCTGATTT-3'.
RESULTS
Family A
All living members of this family underwent ophthalmological examination, except for III-14, III-15, III-16, and IV-14 who did not cooperate. The pedigree of family A is depicted in Figure 1, and the results of our ophthalmic evaluations are summarised in Table 1. Sequence and MspI restriction analyses revealed the Arg142Trp mutation in the individuals indicated in the pedigree. For most of the individuals tested, the restriction analysis is depicted in Figure 2. Only affected and examined family members have been included. Relevant ophthalmic observations reported by Deutman and Jansen in 1970 are described below.7 Our records show that the visual acuity of patient II-3 was diminished to counting fingers. Both eyes showed extensive central chorioretinal atrophy in the last years of his life. In view of his central position in this pedigree and the near certainty that he carried the Arg142Trp mutation his decrease in visual acuity was probably caused by CACD. We have no indication that dominant drusen were present, although Deutman and Jansen considered this patient to be affected.7 Patient II-5 showed some pigmentation and confluent drusen in the posterior poles of both eyes at age 79. Visual acuity at that time was 20/50 in the left eye and 20/40 in the right eye. We could not ascertain whether these drusen were also present at an early age. Both visual acuity and funduscopic aspects do not exclude CACD, although these findings are not typical for this macular dystrophy. Hoyng et al reported drusen in patient III-2 at 59 years of age and a fine granular pigmentation between the drusen.3 At age 35 the monozygotic twin sisters III-9 and III-10 demonstrated a small central scotoma and window defects in the pigment epithelium which were more easily appreciated with fluorescein angiography than with ophthalmoscopy. In addition, subject III-10 complained of mild metamorphopsia in her right eye. At that time their ERGs and electro-oculograms showed no abnormalities. All these findings are compatible with early CACD. Their younger sister (III-13, 33 years of age) demonstrated fine granular pigmentations in the foveal area in addition to some tiny round drusen; the visual acuity was 20/30 in both eyes. The number of drusen in individuals IV-4 and IV-12 have hardly increased in number over the past three decades.
Figure 1.
Pedigrees of families A, B, and C. A bar above an individual indicates individuals who were clinically examined by the author. A plus or minus below an individual indicates the presence or absence of the Arg142Trp mutation in the peripherin/RDS gene. The diamond symbol denotes healthy children of III-4 and III-5 in family A.
Table 1.
Affected members of family A
| Visual acuity | ||||||||
| No | Sex | Age | Age of onset | RE | LE | Funduscopy | Fluorescein angiography | Diagnosis |
| III-9 | F | 65 | 40 | 0.5 | CF | Atrophy of the RPE in the posterior pole. Numerous drusen in this region, centrally the drusen are larger. | Circumscribed hyperfluorescence in the posterior pole and relative hypofluorescence at the foveal region. | CACD with drusen |
| III-10 | F | 65 | 43 | CF | 0.16 | Atrophy of the RPE in the posterior pole. Fewer drusen compared with her twin sister II-10, the more centrally located drusen are larger. | Circumscribed hyperfluorescence in the posterior pole and relative hypofluorescence at the foveal region. | CACD with drusen |
| III-13 | F | 63 | 60 | 0.5 | 0.5 | The area of atrophy of the RPE is smaller compared with II-10 and II-11. Only a few drusen. | Circumscribed hyperfluorescence in the posterior pole and relative hypofluorescence at the foveal region. | CACD with drusen |
| IV-1 | F | 47 | No symptoms | 1.0 | 0.8 | Small yellow grey spots at the macula, one in the right eye and two in the left eye. No drusen. | Small lesions with a hyperfluorescent rim and hypofluorescent centre. These lesions correspond with the spots observed with funduscopy. | Probably early CACD |
| IV-2 | F | 46 | No symptoms | 1.0 | 0.8 | Some drusen, most noticeable in the right eye. | No abnormalities except two lightly hyperfluorescent spots in het macula of the right eye. | Early CACD with few drusen |
| IV-3 | M | 45 | No symptoms | 1.25 | 1.0 | Very discrete perifoveal mottling at the level of the RPE. | Slight hyperfluorescent lesions perifoveal in both eyes, more obvious with fluorescein angiography compared with funduscopy. | Probably early CACD |
| IV-4 | M | 44 | No symptoms | 1.0 | 1.0 | Discrete perifoveal granular pigmentation at the level of the RPE. Some small drusen. | Hyperfluorescent lesions surrounding the fovea. Comparable with III-3 but more extensive. | Early CACD with drusen |
| IV-5 | M | 40 | No symptoms | 1.25 | 1.25 | Very discrete perifoveal mottling at the level of the RPE. | Discrete hyperfluorescent lesions perifoveally. | Probably early CACD |
| IV-6 | M | 37 | No symptoms | 1.0 | 1.0 | No abnormalities. | No abnormalities. | No funduscopic indication of CACD |
| IV-8 | M | 42 | No symptoms | 0.8 | 0.8 | Drusen and irregular atrophy of the RPE in the macular area. | Early and irregular hyperfluorescence in the early phases of the fluorescein angiogram corresponding with the area of atrophy described on funduscopy. No leakage in later phases. | CACD with drusen |
| IV-10 | F | 40 | No symptoms | 1.0 | 1.0 | Irregular atrophy of the RPE in de macular area. Some small drusen. | Early and irregular hyperfluorescence in the early phases of the fluorescein angiogram corresponding with the area of atrophy described on funduscopy. No leakage in later phases. | CACD with drusen |
| IV-11 | M | 39 | 35 | 0.8 | 0.8 | Irregular atrophy of the RPE surrounding the fovea. A few small drusen. | Early and irregular hyperfluorescence in the early phases of the fluorescein angiogram corresponding with the area of atrophy described on funduscopy. No leakage in later phases. | Early CACD with drusen |
| IV-12 | M | 38 | No symptoms | 1.25 | 1.25 | Mild RPE changes in a granular pattern surrounding the fovea. Early stages of III-8, 10, and 11. Some drusen | Very mild focal hyperfluorescence corresponding with the RPE changes seen on funduscopy. | Early CACD with drusen |
Figure 2.
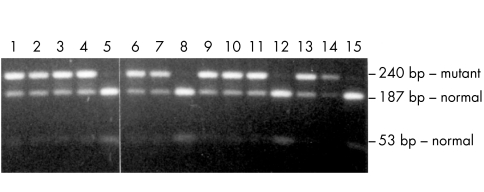
RDS/peripherin Arg142Trp mutation analysis of family A by MspI restriction analysis. Lane 1, IV-3; lane 2, IV-4; lane 3, IV-5; lane 4, IV-6; lane 5, III-6; lane 6, III-9; lane 7, III-10; lane 8, IV-9; lane 9, IV-10; lane 10, IV-11; lane 11, IV-12; lane 12, IV-13; lane 13, III-13; lane 14, unrelated CACD patient with Arg142Trp mutation; lane 15, control normal genomic DNA sample. In the presence of the Arg142Trp mutation, the 240 bp fragment cannot be cut by MspI in the 187 and 53 bp fragments which are derived from the wild type RDS/peripherin gene. The 53 bp fragment is faintly visible in the heterozygotes because of its small length.
At present the retinal disorders in the living members of family A comprise a spectrum ranging from CACD without noticeable drusen (IV-1, IV-3, IV-5) to (early) CACD associated with drusen (III-9, III-10, III-13, IV-2, IV-4, IV-8, IV-10, IV-11, and IV-12). In one individual (family A; IV-6) no symptoms were apparent at the age of 37, despite the presence of the Arg142Trp mutation. Photographs and fluorescein angiograms of the macular dystrophy in individuals III-10, IV-1, and IV-8 are depicted in Figure 3.
Figure 3.
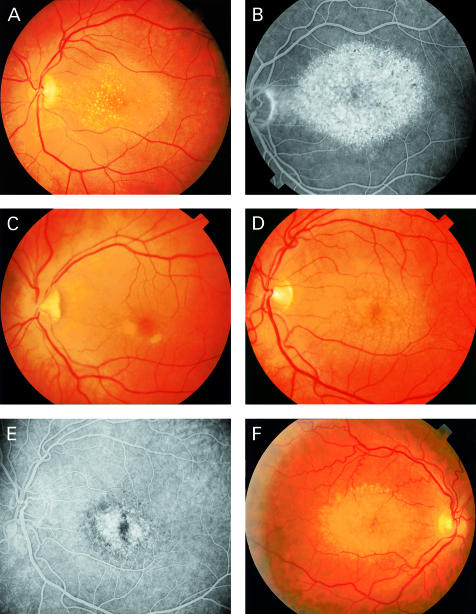
Funduscopic pictures of CACD/dominant drusen patients. Family A: Patient III-10 (A) at age 50 demonstrates drusen with central localisation, the geographic area of atrophy of RPE and choriocapillaris is clearly visible on the fluorescein angiogram (B). Patient IV-1 (C) shows parafoveal lesions; fluorescein angiography (not shown) demonstrated loss of choriocapillaris and RPE typical for early CACD. Patient IV-8 (D) demonstrates a clinical picture at age 42 which resembles that of his mother (III-9) and aunt (III-10), the fluorescein angriogram shows central atrophy (E). Family B: in patient III-8 (F) the drusen are located mainly at the peripheral border of the associated CACD (age 51).
Recently, Stone et al have described the association of a single mutation in the EFEMP1 gene with malattia leventinese and Doyne honeycomb retinal dystrophy (Arg345Trp).8 We sequenced exon 10 containing this sequence alteration in individuals III-10, III-13, and IV-4 of family A but were unable to find this mutation.
Family B
The findings in the affected members of this family are summarised in Table 2, the pedigree is shown in Figure 1. Reportedly, the visual acuity of both patient I-1 and II-2 was severely decreased later in life, unfortunately no ophthalmological diagnosis of these patients could be retrieved. The molecular analysis for individuals III-4, III-6, and III-8 was described previously.3 In addition we found the Arg142Trp mutation to be present in their maternal cousin III-11, but not his unaffected son (IV-7) (data not shown). The presence of drusen in some of the members of this family was not mentioned in the study of Hoyng et al in 1996.3 Nevertheless, drusen were described in patient III-4 at her first visit at our clinic in 1991, and in patient III-8 (Fig 3F) and III-11 drusen have been noted since their first ophthalmological examination at the age of 43 and 38, respectively. Perimetry revealed a relative central scotoma in both eyes of patients III-4 and III-8 at ages 53 and 30, although the visual acuity of III-8 was still 20/20 in both eyes at that time. All the patients carrying the Arg142Trp mutation (III-4, III-6, III-8, and III-11) demonstrated funduscopic changes associated with CACD; in three of those patients drusen were also observed. All patients in generation IV underwent an ophthalmological evaluation; mutational analysis could be performed in IV-2, IV-3, and IV-7 but no mutation was demonstrated in the RDS/peripherin gene in these patients.
Table 2.
Affected members of family B
| Visual acuity | ||||||||
| No | Sex | Age | Age of onset | RE | LE | Funduscopy | Fluorescein angiography | Diagnosis |
| III-4 | F | 61 | 52 | CF | 0.1 | Severe atrophy of the retinal pigment epithelium. A few drusen, most noticeable in the right eye | Hyperfluorescence in the posterior pole. Choroidal vessels are visible in the early phases | CACD with drusen |
| III-6 | M | 56 | 43 | CF | CF | Central atrophy of the retinal pigment epithelium, no drusen | Hyperfluorescence early in the macular area | CACD |
| III-8 | M | 51 | 38 | 0.8 | 0.6 | Atrophy of the pigment epithelium in the posterior pole. Numerous drusen | Central hyperfluorescence. Area of relative hypofluorescence in the early phase of the angiogram in the left eye | CACD with drusen |
| III-11 | F | 56 | 53 | 0.6 | 0.6 | Mild atrophy of the retinal pigment epithelium. Numerous drusen | Marked hyperfluorescence in the posterior pole. Local areas with hypofluorescence | CACD with drusen |
Family C
Patient II-5 was referred to our clinic last year for a second opinion. The presenting symptom in 1989 was a decrease in visual acuity and at that time the referring ophthalmologist noticed drusen and mild atrophy in the macula. Recently, her five brothers could also be included in this study. Although the clinical picture is not as serious as in individual II-5, two of her brothers (II-1 and II-2) also demonstrated small drusen scattered around the fovea; in both patients mild central atrophy was also observed. Patient II-2 had already consulted an eye specialist because of blurred vision. His elder brother (II-1) is currently being treated for normal tension glaucoma; since there is no excavation of his optic disc and in view of his visual fields, which show small parafoveally located scotomas, it is likely that these visual field defects are caused by CACD with drusen. The findings in all members of this family are summarised in Table 3. Molecular analysis revealed the Arg142Trp mutation of the RDS/peripherin gene in all affected patients, but not in their unaffected siblings. The father of this patient (I-1) has been diagnosed with AMD, whether drusen were also present at an early age is unknown. The visual acuity of the sons of patient II-5 is reportedly normal.
Table 3.
Affected members of family C
| Visual acuity | ||||||||
| No | Sex | Age | Age of onset | RE | LE | Funduscopy | Fluorescein angiography | Diagnosis |
| II-1 | M | 55 | 52 | 0.8 | 0.8 | Small drusen located near the fovea, mild chorioretinal atrophy | Not performed | CACD with drusen |
| II-2 | M | 54 | 43 | 0.8 | 0.5 | Small drusen located near the fovea, chorioretinal atrophy is more pronounced than in II-1 | Granular hyperfluorescence in the posterior pole | CACD with drusen |
| II-5 | F | 47 | 32 | 0.1 | 0.05 | Large oval area of chorioretinal atrophy in the posterior pole surrounded by numerous drusen | Marked central hyperfluorescence | CACD with drusen |
DISCUSSION
In this study we have presented three families with CACD associated with dominant drusen. Some of the members of these families demonstrated CACD without noticeable drusen. Since CACD is an autosomal dominant disorder with complete or almost complete penetrance, almost all individuals carrying the Arg142Trp mutation can be considered (future) CACD patients.3 The retinal disorders in the affected individuals comprise a spectrum ranging from minimal changes at the level of the RPE without noticeable drusen to CACD associated with dominant drusen. Sparse reports of similar types of macular dystrophy have appeared in literature over the years. In 1985 Weber et al reported four patients with central chorioretinal dystrophy, dominant drusen, and retinal crystals. The atrophic lesions in these patients, however, were irregularly shaped and did not show the round or oval configuration typical for CACD.25 In Zermatt macular dystrophy, patients in their late teens and twenties exhibited drusen-like deposits, and central pigmentary alterations occurred in adolescent patients. Later, these defects formed focal areas of atrophy, which eventually led to central geographic atrophy with severe visual loss by the fifth decade. Strikingly, the cause of this type of autosomal dominant macular dystrophy was found to be an Arg172Trp mutation of the RDS/peripherin gene.26 Both the Arg142Trp and Arg172Trp mutations are located in the large intradiscal loop D2 of the RDS/peripherin protein. The Arg172Trp mutation was also associated with Stargardt disease-like features in two siblings of an another family and with CACD in their father.27 Another striking example of intrafamilial phenotypic variability associated with an RDS/peripherin mutation was described by Weleber et al28. Different individuals in one family carrying the deletion of codons 153 or 154 showed retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus. Mutations in the peripherin/RDS gene have also been associated with dominant and digenic retinitis pigmentosa, progressive macular degeneration, cone-rod dystrophy, and pattern dystrophy.29,30
The variable expression of the dominant drusen/CACD phenotype can in part be explained by the natural development of CACD. In this type of macular dystrophy visual acuity, visual fields, and ERG only become abnormal in the later stages. Usually, decrease in visual acuity does not start until the patient reaches the third to fifth decade of life.1,2 One of the earliest funduscopic signs is slight hypopigmentation in the parafoveolar area, often only visible on direct ophthalmoscopy or with a 78 D lens. These alterations of the RPE are more easily appreciated with fluorescein angiography.1 The lack of symptoms and subtle findings with ophthalmoscopy and fluorescein angiography in some of the younger affected individuals are therefore not surprising. Drusen are not always present in great numbers, and sometimes not at all. In general, individuals located in the right branch of family A seem to be affected earlier and more seriously compared with their relatives in the left branch. This applies to the dominant drusen as well as to the CACD.
The question remains which factors are involved in the development of drusen in some but not all CACD patients of these families. Most likely, one or several non-genetic factors contribute to the development of drusen in these families which, if acting in an additive way, could explain the significant differences in severity between members of different branches of family A. A less likely possibility is that a modifier gene is physically linked to the peripherin/RDS gene. If the penetrance of the modifier gene is incomplete, it could be situated close to the RDS/peripherin gene; if its penetrance is very high it might not be situated very close in order to explain the fact that only 4/19 CACD patients developed drusen.
The features encountered in the macular dystrophy described in this study, drusen as well as central atrophy of choriocapillaris and RPE, are also characteristic for AMD. Although a report by Shastry et al suggested that the peripherin/RDS gene is not a major factor responsible for AMD, macular dystrophies with overlapping symptoms may prove to be important in unravelling the pathogenesis of AMD.31
Acknowledgments
This study was supported by the Rotterdamse Vereniging Blindenbelangen, the Algemene Nederlandse Vereniging ter Voorkoming van Blindheid, the Stichting Blindenhulp, the Stichting de Drie Lichten, the Gelderse Blindenvereniging, the Landelijke Stichting voor Blinden en Slechtzienden, and the Stichting voor Ooglijders.
REFERENCES
- 1.Hoyng CB, Deutman AF. The development of central areolar choroidal dystrophy. Graefes Arch Clin Exp Ophthalmol 1996;234:87–93. [DOI] [PubMed] [Google Scholar]
- 2.Gass JMD. Stereoscopic atlas of macular diseases. St Louis: CV Mosby, 1987:262.
- 3.Hoyng CB, Heutink P, Testers L, et al. Autosomal dominant central areolar choroidal dystrophy caused by a mutation in codon 142 in the peripherin/RDS gene. Am J Ophthalmol 1996;121:623–9. [DOI] [PubMed] [Google Scholar]
- 4.Lotery AJ, Ennis KT, Silvestri G, et al. Localisation of a gene for central areolar choroidal dystrophy to chromosome 17p. Hum Mol Genet 1996;5:705–8. [DOI] [PubMed] [Google Scholar]
- 5.Hughes AE, Lotery AJ, Silvestri G. Fine localisation of the gene for central areolar choroidal dystrophy on chromosome 17p. J Med Genet 1998;35:770–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piguet B, Haimovici R, Bird AC. Dominantly inherited drusen represent more than one disorder: a historical review. Eye 1995;9:34–41. [DOI] [PubMed] [Google Scholar]
- 7.Deutman AF, Jansen LM. Dominantly inherited drusen of Bruch's membrane. Br J Ophthalmol 1970;54:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone EM, Lotery AJ, Munier FL, et al. A single EFEMP1 mutation associated with both malattia leventinese and Doyne honeycomb retinal dystrophy. Nat Genet 1999;22:199–202. [DOI] [PubMed] [Google Scholar]
- 9.Silvestri G, Johnston PB, Hughes AE. Is genetic predisposition an important risk factor in age-related macular degeneration? Eye 1994;8:564–8. [DOI] [PubMed] [Google Scholar]
- 10.Meyers SM. A twin study on age-related macular degeneration. Trans Am Ophthalmol Soc 1994;92:775–843. [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers SM, Zachary AA. Monozygotic twins with age-related macular degeneration. Arch Ophthalmol 1988;106:651–3. [DOI] [PubMed] [Google Scholar]
- 12.Heiba IM, Elston RC, Klein BE, et al. Sibling correlations and segregation analysis of age-related maculopathy: the Beaver Dam Eye Study [published erratum appears in Genet Epidemiol 1994;11:571]. Genet Epidemiol 1994;11:51–67. [DOI] [PubMed] [Google Scholar]
- 13.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration [see comments]. Science 1997;277:1805–7. [DOI] [PubMed] [Google Scholar]
- 14.Stone EM, Webster AR, Vandenburgh K, et al. Allelic variation in ABCR associated with Stargardt disease but not age-related macular degeneration [letter]. Nat Genet 1998;20:328–9. [DOI] [PubMed] [Google Scholar]
- 15.De La Paz MA, Guy VK, Abou DS, et al. Analysis of the Stargardt disease gene (ABCR) in age-related macular degeneration. Ophthalmology 1999;106:1531–6. [DOI] [PubMed] [Google Scholar]
- 16.Fuse N, Suzuki T, Wada Y, et al. Molecular genetic analysis of ABCR gene in Japanese dry form age-related macular degeneration. Jpn J Ophthalmol 2000;44:245–9. [DOI] [PubMed] [Google Scholar]
- 17.Kuroiwa S, Kojima H, Kikuchi T, et al. ATP binding cassette transporter retina genotypes and age related macular degeneration: an analysis on exudative non-familial Japanese patients. Br J Ophthalmol 1999;83:613–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera A, White K, Stohr H, et al. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet 2000;67:800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souied EH, Ducroq D, Rozet JM, et al. ABCR gene analysis in familial exudative age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41:244–7. [PubMed] [Google Scholar]
- 20.Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Hum Genet 2000;67:487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol 1988;32:375–413. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99:933–43. [DOI] [PubMed] [Google Scholar]
- 23.Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 1995;102:205–10. [DOI] [PubMed] [Google Scholar]
- 24.Thijssen JM, Pinckers AJLG, Otto AJ. A multipurpose system for ophthalmic electrodiagnosis. Ophthalmologica 1974;308–14. [DOI] [PubMed]
- 25.Weber U, Adler K, Hennekes R. Central chorioretinal dystrophy with drusen and retinal crystals. Ophthalmologica 1985;190:134–41. [DOI] [PubMed] [Google Scholar]
- 26.Piguet B, Heon E, Munier FL, et al. Full characterization of the maculopathy associated with an Arg-172-Trp mutation in the RDS/peripherin gene. Ophthalmic Genet 1996;17:175–86. [DOI] [PubMed] [Google Scholar]
- 27.Kohl S, Christ AM, Apfelstedt SE, et al. RDS/peripherin gene mutations are frequent causes of central retinal dystrophies. J Med Genet 1997;34:620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weleber RG, Carr RE, Murphey WH, et al. Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Arch Ophthalmol 1993;111:1531–42. [DOI] [PubMed] [Google Scholar]
- 29.Keen TJ, Inglehearn CF. Mutations and polymorphisms in the human peripherin-RDS gene and their involvement in inherited retinal degeneration. Hum Mutat 1996;8:297–303. [DOI] [PubMed] [Google Scholar]
- 30.Kohl S, Giddings I, Besch D, et al. The role of the peripherin/RDS gene in retinal dystrophies. Acta Anat 1998;162:75–84. [DOI] [PubMed] [Google Scholar]
- 31.Shastry BS, Trese MT. Evaluation of the peripherin/RDS gene as a candidate gene in families with age-related macular degeneration. Ophthalmologica 1999;213:165–70. [DOI] [PubMed] [Google Scholar]