Approximately 20% of patients with primary central nervous system lymphoma will have eye involvement, which often precedes diagnosis by a number of months.1–4 The diagnosis of intraocular and/or CNS lymphoma depends on histological evidence in tissue obtained from a CNS biopsy or cytological demonstration of malignant cells in the vitreous or cerebrospinal fluid (CSF).4
Cytological differentiation of reactive lymphoid cells from well differentiated lymphoma using morphological characteristics depends on observer skill and the preservation of adequate numbers of cells.5 Immunocytochemical staining of cell surface antigens assists in this differentiation and can detect cell population monoclonality, a common feature of large B cell lymphomas.5 Cytofluorography or flow cytometry is a semiautomated method of immunocytochemistry which has some advantages over slide based immunocytochemistry including objective and quantitative data on cell surface markers.6,7
We present a patient with ataxia and vision loss who was diagnosed with primary CNS lymphoma using cytofluorographic analysis of a vitreous biopsy specimen.
CASE REPORT
A 53 year old woman was diagnosed with bilateral panuveitis. Best corrected visual acuity (BCVA) was 20/30 right eye and 24/40 left eye. Bilateral mild anterior uveitis and vitritis was present. Diagnostic tests for uveitis were negative. The patient was treated with topical (1%) and oral prednisolone (35 mg) over 16 weeks resulting in a BCVA of 20/30 right eye and 20/25 left eye, resolution of the anterior uveitis, and marked reduction in the vitreous cells bilaterally.
Three months later, the patient presented to the Beth Israel Deaconess Medical Center emergency room with ataxia and vision loss. She had recurrence of her bilateral panuveitis, but no chorioretinal lesions were noted on ophthalmoscopy. A magnetic resonance image (MRI) with contrast demonstrated multiple well defined, enhancing lesions of varying size located at the grey-white matter junction consistent with either metastatic neoplasia or lymphoma (Fig 1). Pars plana vitrectomy was performed and an undiluted and diluted vitreous specimen was sent for cytology. The patient recovered a BCVA of 20/16 right eye and 20/40 left eye.
Figure 1.
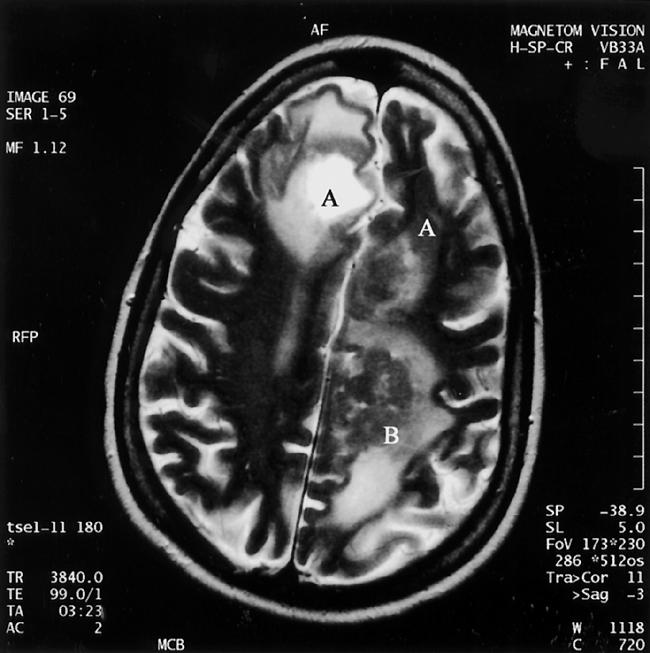
Photograph of MRI with contrast demonstrating multiple well defined, enhancing lesions of varying size located at the grey-white matter junction region involving both frontal lobes (A), and the left parietal lobe (B).
The diluted vitreous was analysed by flow cytometry using three colour gating (light scatter versus CD45) to optimise lymphoid yield. Approximately 58% of the total gated events were composed of abnormal B cells as evidenced by expression of surface antigen CD 19, a pan-B cell marker (Fig 2). The cells showed monoclonal lambda light chain restriction and did not express CD20, CD5, CD10, or CD23 surface antigens (Fig 2). These cytofluorographic findings combined with cytological features of large cell size, moderately abundant amphophilic cytoplasm, and large irregular nuclei with coarse, clumped chromatin and conspicuous nucleoli seen in the undiluted vitreous were consistent with a high grade B cell non-Hodgkin's lymphoma.
Figure 2.
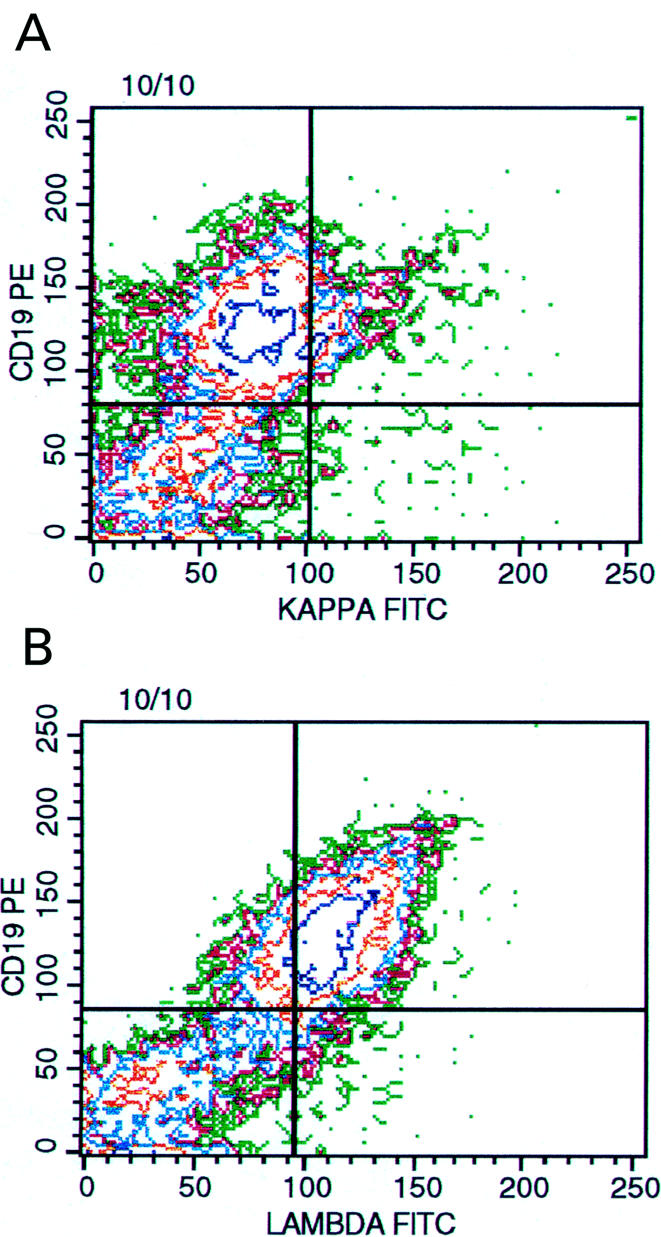
Multivariable, three colour flow cytometric histogram of vitreous lymphocytes showing a cluster of cells (upper right quadrant, 20% of the lymphocyte gate) coexpressing CD 19 and kappa light chain (A). However 58% of the B lymphocytes express surface lambda light chains (B). The pattern of dot plots indicates the presence of a monoclonal, lambda light chain restricted B cell population.
The patient declined chemotherapy and underwent whole brain external beam radiotherapy consisting of a total of 30 Gy administered over 4 weeks and adjunctive intravenous dexamethasone, 1.5 mg twice daily tapered off over 3 weeks. Treatment resulted in a reduction in the size of the cerebral lesions on follow up MRI.
Comment
This case highlights typical features of intraocular lymphoma including uveitis of unknown aetiology presenting before evidence of CNS-NHL, bilateral involvement, and an initial response to steroids.1–4 Decisions regarding the method of diagnosis weigh the lower morbidity and mortality of vitrectomy and lumbar puncture compared to CNS biopsy, against a lower diagnostic yield using cytology.4–6 Flow cytometry is a semiautomated method of immunocytochemistry that can rapidly identify abnormal B and T lymphocyte subsets. Other benefits include easier cell preparation and multiparameter analyses of specimens.7 Earlier shortcomings including blood contamination, errors introduced by non-viable cells, difficulty in identifying monoclonality, and slow, single cell suspension analysis, have been overcome. However, correlation of flow cytometry analysis with morphological examination of the tissue section or cytology preparation is still required.7 Although two early reports did not find flow cytometry helpful in diagnosing intraocular lymphoma,8,9 three recent cytofluorography studies detected malignancy in 100% of 13 cases 3,10 and 70% of 10 cases6 usually with one vitrectomy.3,6,10
While primary CNS-NHL is initially extremely sensitive to radiotherapy or corticosteroids, tumours will recur in 90% of patients within 1 year.4 Combined treatment with radiotherapy, corticosteroid and chemotherapy may double median survival time, but the 5 year survival rate is only 4% to 30%.4 In the minority of patients with this rare disease who have eye involvement, early vitrectomy using cytology and flow cytometry can provide a prompt diagnosis.
Acknowledgments
Dr Arroyo is a recipient of an NIH K-23 physician training award.
None of the authors has any proprietary interest in any of the products in this paper.
REFERENCES
- 1.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology 1993;100:1399–406. [DOI] [PubMed] [Google Scholar]
- 2.Verbraeken HE, Hanssens M, Priem H, et al. Ocular non-Hodgkin's lymphoma: a clinical study of nine cases. Br J Ophthalmol 1997;81:31–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akpek EK, Ahmed I, Hochberg FH, et al. Intraocular-central nervous system lymphoma. Clinical features, diagnosis and outcomes. Ophthalmology 1999;106:1805–10. [DOI] [PubMed] [Google Scholar]
- 4.Blay JY, Ongolo-Zogo P, Sebban C, et al. Primary cerebral lymphomas: unsolved issues regarding first-line treatment, follow-up, late neurological toxicity and treatment of relapses. The FNCLCC. French Federation Nationale des Centres de Lutte contre le Cancer. Ann Oncol 2000;11(Suppl l1):39–44. [PubMed] [Google Scholar]
- 5.Davis JL, Solomon D, Nussenblatt RB, et al. Immunocytochemical staining of vitreous cells. Indications, techniques, and results. Ophthalmology 1992;99:250–6. [DOI] [PubMed] [Google Scholar]
- 6.Davis JL, Viciana AL, Ruiz P. Diagnosis of intraocular lymphoma by flow cytometry. Am J Ophthalmol 1997;124:362–72. [DOI] [PubMed] [Google Scholar]
- 7.McCoy Jr JP. Basic principles of clinical flow cytometry. In: Keren D F, Hanson CA, Hurtubise PE, eds. Flow cytometry and clinical diagnosis. 1st ed. Chicago: ASCP Press, 1994:26–55.
- 8.Ljung BM, Char DM, Miller TR, et al. Intraocular lymphoma: cytologic diagnosis and the role immunologic markers. Acta Cytol 1987;2:840–7. [PubMed] [Google Scholar]
- 9.Char DH, Ljung BM, Deschenes J, et al. Intraocular lymphoma: immunological and cytological analysis. Br J Ophthalmol 1988;72:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson DJ, Braziel R, Rosenbaum JT. Intraocular lymphoma. Immunopathologic analysis of vitreous biopsy specimens. Arch Ophthalmol 1992;110:1455–8. [DOI] [PubMed] [Google Scholar]


