Abstract
Aims: To determine if mild hyperoxia induces senescence in retinal pigment epithelium (RPE) cells in vitro.
Methods: RPE340 cells and WI38 cells were grown in 20% oxygen and 40% oxygen until proliferative exhaustion. A combined senescence associated β galactosidase (SABG) and 5-bromo-2`-deoxyuridine (BrdU) double labelling technique was performed at different times and labelled cells were counted.
Results: Cells grown in 40% oxygen stopped proliferating at an earlier population doubling level than when grown in 20% oxygen. An increase in SABG positive cells and decrease of BrdU positive cells in 40% oxygen developed at an earlier time than when grown in 20% oxygen.
Conclusion: Mild hyperoxia induces premature senescence in a specific RPE cell line.
Oxidative stress causes a number of changes to cellular components, which can alter the cell's phenotype. To investigate the mechanism by which oxidative stress changes cellular function, several in vitro models using chemical oxidants or hyperoxia have been established.1–4 Each oxidant, however, has different acute effects and, thus, the relevance to cells in vivo is open to speculation.1 We have used mild hyperoxia to introduce low and chronic oxidative stress in retinal pigment epithelial (RPE) cells in vitro in an effort to simulate the environment of the RPE in vivo during ageing. We have found that 40% oxygen generates detectable oxidative stress and induces a modest change in the RPE phenotype with altered gene expression and genomic DNA damage.2,5 In this study, we examined the induction of premature senescence in RPE cells with mild hyperoxia using senescent associated β galactosidase (SABG), a recognised biomarker of senescent cells in vitro6–10 and in vivo.11,12
MATERIALS AND METHODS
Cell culture
For this study, the RPE340 cell line which has been used in a number of oxidative stress and ageing related work, was used for all experiments.2,9,10,13–15 The WI38 fibroblast cell line, whose senescent characteristics has been previously studied, was used as a reference cell line to make direct comparisons possible.3,6 Both cell lines were maintained as previously described.2 The oxygen tension was regulated at 20% or 40% oxygen in a three gas incubator (NuAire, Plymouth, MN, USA), and the oxygen concentration was verified as previously described.2 Forty per cent oxygen was used to induce chronic, mild oxidative stress since this tension generates significant oxidative stress in both cell lines without inducing cell death.2 For experiments, cells were grown in 75 cm2 flasks at an initial seeding density of 10 000/cm2 unless stated. Cells were passaged before reaching confluence to avoid contact inhibition. At each passage, cell number was counted using a Coulter counter Z1 (Coulter, Miami, FL, USA) and population doubling level (PDL) was determined as current PDL = last PDL + log2 (collected cell number/seeded cell number).
Combined senescence associated β galactosidase and 5-bromo-2`-deoxyuridine labelling assay
The combined SABG and 5-bromo-2`-deoxyuridine (BrdU) labelling assay was performed as previously published to determine the proportion of SABG labelled and replicating cells.10 Briefly, cells were seeded on eight well chamber slides at 20 000 cells/cm2 and incubated under identical conditions as described above. Twenty four hours after seeding, cells were treated with 10 mM BrdU (Boehringer Mannheim, Indianapolis, IN, USA) and grown for 72 hours. Cells were fixed in 3% paraformaldehyde, stained with pH 6 X-gal solution (Promega, Madison, WI, USA) in 40 mM citric acid for 4 hours. Cells were fixed again with 70% ethanol in 50 mM glycine buffer (pH2), and labelled with BrdU monoclonal antibody according to the manufacturer's instructions.
Cells were imaged with a light microscope (BH-2; Olympus Optical Co, Ltd, Tokyo, Japan) interfaced with a charge coupled device camera (ProgRes 3012; Kontron Electronik GmbH, Eching, Germany) and a computer using graphic software (Photoshop 5.0; Adobe, Mountain View, CA, USA). The percentage of positive staining cells was determined compared with the total number of cells in the same field under phase contrast microscopy. For each determination, approximately 200 cells were observed.
RESULTS
The proliferation of RPE340 and WI38 cells with PDL
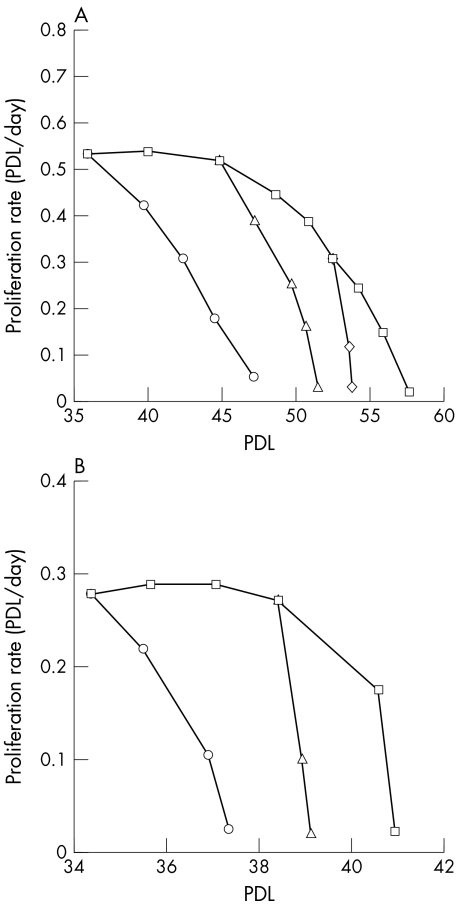
RPE340 cells cultured in 20% oxygen proliferated at a constant rate (0.53 PDL/day) until PDL 45, and gradually stopped dividing by PDL 58 (Fig 1A). RPE340 cells (starting PDL 35, 45, and 52) displayed significantly shortened replicative lifespans and cell proliferation was inhibited by PDL 48, 51, and 53 respectively, when grown in 40% oxygen. This growth arrest was irreversible even after cells were returned to 20% oxygen incubation. For comparison, WI38 cells grown in 20% oxygen had constant proliferation (0.29 PDL/day) until PDL 38, and stopped proliferating by PDL 41 (Fig 1B). Forty per cent of oxygen rapidly inhibited the proliferation of WI38 cells by PDL 37 and 39 when the treatment started at PDL 34 and 38, respectively.
Figure 1.
The proliferation rate of RPE340 and WI38 cells. (A) Proliferation rate of RPE340 cells. Cells were grown in 20% oxygen (squares), or 40% oxygen starting at PDL 35 (circles), 45 (triangles), and 52 (diamonds). (B) Proliferation rate of WI38 cells. Cells were grown in 20% oxygen (squares), or 40% oxygen starting at PDL 34 (circles), and 38 (triangles).
SABG activity and BRDU incorporation in early PDL and late PDL cells
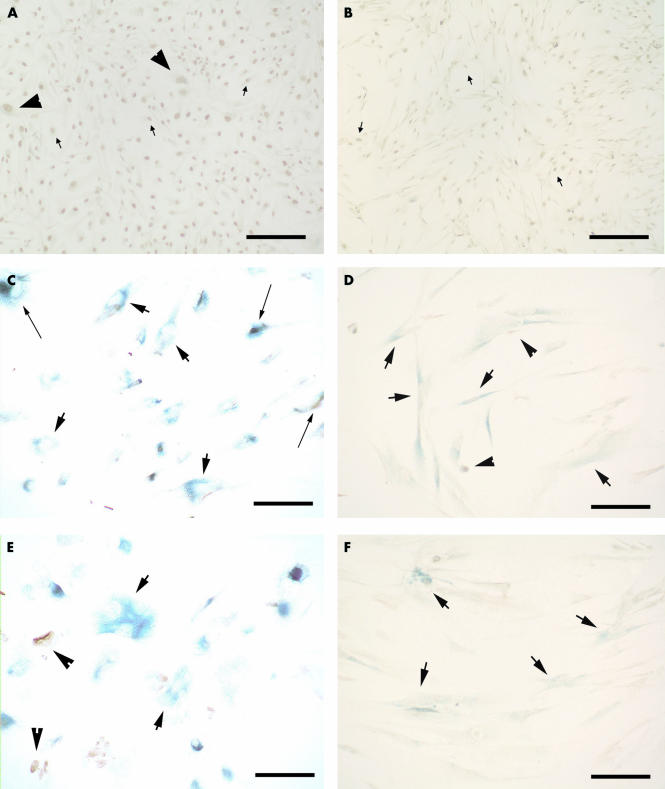
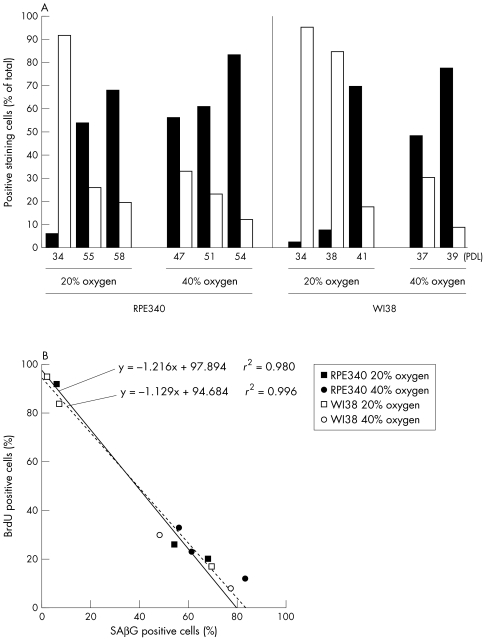
RPE340 and WI38 cells at early PDL typically had small cell bodies, displayed strong BrdU immunoreactivity, and very few cells expressed SABG activity (Fig 2A, B). Cells that stopped proliferating developed flat, large cell bodies with long processes and strong SABG activity (Fig 2C, D). When cultured in 40% oxygen, both RPE340 and WI38 cells grown to replicative exhaustion showed SABG activity (Fig 2E, F) with morphological features of post-mitotic cells that are grown in 20% oxygen. Figure 3A shows a high number of BrdU positive and a low number of SABG labelled cells in both cell lines at early PDL when grown in 20% oxygen. With increasing PDL, the proportion of SABG positive cells increased and the number of BrdU labelled cells was dramatically reduced. Both cell lines grown in 40% oxygen that had ceased proliferation, showed a high proportion of SABG positive cells and a low number of BrdU positive cells. The increase of SABG positive cells and decrease of BrdU positive cells was linearly associated in both cell lines in 20% oxygen and 40% oxygen (Fig 3B), which corresponds to the report of Dimri et al.6 Moreover, the slope of fitting lines for 20% oxygen and 40% oxygen were nearly identical.
Figure 2.
The combined SABG and BrdU labelling assay in WI38 and RPE340 cells. (A) RPE340 cells in 20% oxygen at PDL 34. (B) WI38 cells in 20% oxygen at PDL 34. (C) RPE340 cells in 20% oxygen at PDL 58. (D) WI38 cells in 20% oxygen at PDL 41. (E) RPE340 cells in 40% oxygen at PDL 48. (F) WI38 cells in 40% oxygen at PDL 37. Small arrows: cells positive for BrdU only, large arrows: cells positive for SABG only, arrowheads: cells positive for both stainings, long arrows: cells containing pigment granules. Scale bar, 100 μm.
Figure 3.
(A) Analysis of SABG and BrdU labelled cells. Solid columns are the percentage of SABG positive cells and open columns are the percentage of BrdU positive cells against the total number of cells counted for each condition. RPE340 cells with starting PDLs of 35, 45, and 52 were measured in 40% oxygen at PDL 47, 51, and 54, respectively. WI38 cells measured in 40% oxygen at PDL 37 and 39 started the incubation at 34 and 38, respectively. (B) The association between the numbers of SABG positive cells and BrdU positive cells. Solid line represents the line fit for the plots of RPE340 cells grown in 20% oxygen. Broken line represents the line fit for the plots of WI38 cells grown in 20% oxygen.
DISCUSSION
The data show a reduction in proliferative capacity with increasing replicative age when RPE340 cells were grown in 20% oxygen, and an accelerated inhibition of proliferation by 40% oxygen that resulted in a shortened replicative lifespan. An altered morphological phenotype and SABG activity at late PDL accompanied these changes when grown in 20% oxygen. Likewise, identical morphological changes, SABG activity, and loss of BrdU incorporation indicative of senescence, developed in RPE340 cells at an earlier PDL compared with when grown in 20% oxygen.
The major characteristics of senescence include irreversible replicative exhaustion, typical enlarged and flattened cell morphology, critical shortening of chromosomal telomeres, and an altered gene expression phenotype.16,17 Recently, Dumont et al demonstrated that WI38 cells treated daily with a low dose of tert-butylhydroperoxide (t-BHP) lost replicative potential and developed senescent biomarkers including SABG activity.4 These results suggest that oxidative stress induces premature senescence. With mild hyperoxia treatment an accelerated shortening of mean telomere length, a major defining factor of replicative senescence, has been demonstrated in both RPE340 and WI38 cell lines.3,14 Saretzki et al found a similar gene expression pattern between senescent and mild hyperoxic treated fibroblasts.18 Our data showing SABG expression with 40% oxygen further support the hypothesis that mild hyperoxia induces premature senescence. The proportion of increased SABG positive cells and decreased BrdU positive cells in 40% oxygen was identical to cells grown in 20% oxygen, which indicates that the increase of SABG active cells in 40% oxygen reflects the decrease of replicative capacity of cells by oxidative stress. In conclusion, RPE340 and WI38 cells grown in mild hyperoxia exhibit SABG expression at earlier PDL than in ambient oxygen, which suggests the induction of premature senescence by oxidative stress. Since the RPE in vivo is exposed to high oxygen fluxes, the development of senescence by oxidative stress could play a part in ageing of the RPE and possibly age related macular degeneration.
Acknowledgments
Grant support: This work was supported by National Institutes of Health grants NIH/EY00344 (JTH), NIH/EY06473 (LMH), UCD Health System Awards (JTH), Manpower Award (JTH), an unrestricted RPB grant from Research to Prevent Blindness to the Department of Ophthalmology, and Nippon Eye Bank Association.
Commercial relationships: None.
Abbreviations
BrdU, 5-bromo-2`-deoxyuridine
PDL, population doubling level
RPE, retinal pigment epithelium
SABG, senescent associated β galactosidase
References
- 1.Gille JJ, Joenje H, Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res 1992;275:405–14. [DOI] [PubMed] [Google Scholar]
- 2.Honda S, Hjelmeland LM, Handa JT, The use of hyperoxia to induce chronic mild oxidative stress in RPE cells in vitro. Mol Vis 2001;7:63–70. [PubMed] [Google Scholar]
- 3.Von Zglinicki T, Saretzki G, Docke W, et al. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 1995;220:186–93. [DOI] [PubMed] [Google Scholar]
- 4.Dumont P, Burton M, Chen QM, et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med 2000;28:361–73. [DOI] [PubMed] [Google Scholar]
- 5.Honda S, Hjelmeland LM, Handa JT, Oxidative stress—induced single-strand breaks in chromosomal telomeres of human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci 2001;42:2139–44. [PubMed] [Google Scholar]
- 6.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 1995;92:9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reznikoff CA, Yeager TR, Belair CD, et al. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res 1996;56:2886–90. [PubMed] [Google Scholar]
- 8.Serrano M, Lin AW, McCurrach ME, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997;88:593–602. [DOI] [PubMed] [Google Scholar]
- 9.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998;279:349–52. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga H, Handa JT, Aotaki-Keen A, et al. Beta-galactosidase histochemistry and telomere loss in senescent retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1999;40:197–202. [PubMed] [Google Scholar]
- 11.Sigal SH, Rajvanshi P, Gorla GR, et al. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol 1999;276:G1260–72. [DOI] [PubMed] [Google Scholar]
- 12.Mishima K, Handa JT, Aotaki-Keen A, et al. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci 1999;40:1590–3. [PubMed] [Google Scholar]
- 13.Shelton DN, Chang E, Whittier PS, et al. Microarray analysis of replicative senescence. Curr Biol 1999;9:939–45. [DOI] [PubMed] [Google Scholar]
- 14.Honda S, Weigel A, Hjelmeland LM, et al. Induction of telomere shortening and replicative senescence by cryopreservation. Biochem Biophys Res Commun 2001;282:493–8. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga H, Handa JT, Gelfman CM, et al. The mRNA phenotype of a human RPE cell line at replicative senescence. Mol Vis 1999;5:39. (http://www.molvis.org/molvis/v5/p39/) [PubMed] [Google Scholar]
- 16.Rubin H, Cell aging in vivo and in vitro. Mech Ageing Dev 1997;98:1–35. [DOI] [PubMed] [Google Scholar]
- 17.Campisi J, The biology of replicative senescence. Eur J Cancer 1997;33:703–9. [DOI] [PubMed] [Google Scholar]
- 18.Saretzki G, Feng J, von Zglinicki T, et al. Similar gene expression pattern in senescent and hyperoxic-treated fibroblasts. J Gerontol A Biol Sci Med Sci 1998;53:B438–442. [DOI] [PubMed] [Google Scholar]