Abstract
Aim: To evaluate the relation between the quantitative measurement of vitreous fluorescein with fluorescein angiography and retinopathy in diabetic patients with and without clinically significant macular oedema (CSMO).
Methods: In a prospective cross sectional study, passive permeability and active, outward transport of fluorescein across the blood-retinal barrier were quantitated with vitreous fluorometry in 61 eyes from 48 patients with CSMO and 22 fellow eyes without CSMO, after exclusion of eyes with previous macular laser treatment and vitreous liquification. All patients were recruited from the university hospital's outpatient clinic. Retinopathy and fluorescein angiograms were evaluated on 60 degree photographs.
Results: The passive permeability in CSMO was significantly correlated with the severity of leakage on fluorescein angiograms (r=0.73), the level of retinopathy (r=0.61), and visual acuity (r=0.45). Significant differences between eyes with CSMO and eyes without CSMO were found for passive permeability (p<0.001), fluorescein leakage (p<0.001), visual acuity (p=0.02), and retinopathy (p=0.002).
Conclusion: Passive permeability of fluorescein quantitated with vitreous fluorometry was correlated both with semiquantitative fluorescein angiography and retinopathy, and a significant increase in passive permeability was found when comparing eyes with CSMO to eyes without CSMO. No such pattern was found for the active transport indicating that passive and not the outward, active transport is the factor of most importance in the development of CSMO.
Keywords: diabetes, retinopathy, blood-retinal barrier, fluorescein, oedema, vitreous fluorimetry, angiography
Clinically significant macular oedema (CSMO) develops with time in 10–15% of diabetic patients.1,2 The tight blood-retinal barrier is damaged as the result of loss of anchor proteins in tight junctions and trans-endothelial vesicular transport in the capillary endothelial cells and/or the retinal pigment epithelium3,4 leading to an increase in the passive leakage of water and electrolytes and retinal thickening. Quantifying the passive leakage could be valuable in clinical investigations in addition to semiquantitative photographic techniques and measurements of retinal thickness. Previous studies with vitreous fluorometry have shown an increase in passive permeability both in diabetic retinopathy and macular oedema5–7; however, the contribution of retinopathy or oedema has not been analysed.
An outward, active transport of fluorescein, inhibited by competitive and metabolic inhibitors, has been demonstrated both in vitro and in vivo.8–11 Thus, CSMO could also be related to the metabolic activity of the retinal pigment epithelium as a result of changes in the active transport of electrolytes and water from the retina to the blood. If the active transport decreases, retinal oedema could theoretically appear. In contradiction to this hypothesis a previous study found the active transport to be significantly increased in those with CSMO compared to healthy subjects and nearly unchanged compared to a small number of eyes without CSMO.12
The purpose of the present study was to evaluate passive permeability and active transport quantitated with vitreous fluorometry in relation to retinopathy in eyes with and without CSMO and to compare fluorometry with semiquantitative fluorescein angiography.
SUBJECTS AND METHODS
Fifty three patients with CSMO in at least one eye were screened consecutively for a prospective study; 19 patients had type 1 and 34 had type 2 diabetes. The patients were recruited from the outpatient clinic of the department of ophthalmology at Herlev Hospital, University of Copenhagen, and the majority of the patients were regularly followed at the Steno Diabetes Center before referral to the department.
Exclusion criteria were proliferative retinopathy, cataract or pseudophakia, macular laser, and vitreous haemorrhage. In addition, vitreous detachment and/or posterior vitreous liquification led to exclusion as calculation of passive and active transport is not possible in such eyes with the present methodology.13 Evaluated from the vitreous scans at 30 minutes, 16 eyes (five patients with liquification in both eyes) were excluded a priori because of vitreous liquification or posterior vitreous detachment.
Eighty three eyes from 48 patients fulfilled the inclusion/exclusion criteria, CSMO was found in 61 eyes and less or no retinal thickening in 22 eyes.
Clinical data
The mean age of patients was 57 years (range 28–71) and duration of the disease 13 years (range 1–43). HbA1c was 8.8 (range 1–12), and systolic and diastolic blood pressures were 144 mm Hg (range 105–195) and 82 mm Hg (range 68–97), respectively, calculated as the mean of measurements every 3 months 1 year before the study.
Clinically significant macular oedema
CSMO was graded according to the ETDRS criteria as retinal thickening within 500 μm of the fovea, as hard exudates at/or within the same 500 μm if associated with retinal thickening, and as a >1 optic disc area of retinal thickening if any part of the oedematous area is within 1 disc diameter from the fovea. The grading was performed with biomicroscopy by an experienced retinal specialist and by a grader on stereoscopic fundus photographs. In five cases of disagreement the clinical grading was given priority.
Retinopathy grading
Retinopathy was graded on 60 degree fundus photographs, using a procedure adapted to the modified Airlie House description.14 All patients had non-proliferative diabetic retinopathy (NPDR), ranging from mild to severe.
Fluorescein angiography
The filling phase of the fluorescein angiogram was recorded with a laser scanning ophthalmoscope (CLSO, Zeiss Germany) with 20 degree pictures in order to obtain maximum quality pictures of the foveal avascular zone. In all eyes examined (one eye in each patient) the diameter of the foveal avascular zone was below 1000 μm.
The later phases (2–3 minutes and 7–9 minutes) were obtained on 60 degree pictures from the Canon camera (CF-60UVi) and the severity of leakage was graded with a simplified procedure, based on the ETDRS system.15 The grading evaluates severity leakage at the geometric centre and the area of leakage in various distances from the fovea: the centre field within 500 μm, the inner field annulus between 500 μm and 1 disc diameter, and the outer field annulus between 1 and 2 disc diameters from the fovea. The far temporal field (>2 disc diameters from the fovea) was also graded. All fields were graded from standard photographs with the ETDRS classifications from 0 to 4 (0: no leakage, 1: questionable leakage, 2: definite, 3: moderate, 4: severe).
The fraction of leakage evaluated to originate from microaneurysms versus more diffuse leakage was also graded.15 The source of leakage was evaluated as either focal with >67% of the leakage originating from microaneurysms, diffuse with <33% from microaneurysms, and an intermediate group.
Vitreous fluorometry
The transport of fluorescein through the blood-retinal barrier is estimated from the preretinal fluorescein concentration curve and the concentration of free, unconjugated fluorescein in the plasma. Fluorescein is metabolised to another fluorescent molecule, fluorescein glucuronide, and in the study presented here both compounds are measured in plasma and in the vitreous with differential spectrofluorometry. In the eye, fluorescence is measured along the optical axis using an ocular fluorometer (Fluorotron, OcuMetrics, San Jose, CA, USA). After a bolus injection of 14 mg/kg disodium fluorescein, post-injection scans were performed at 30 and 60 minutes for the calculation of the passive permeability of the blood-retinal barrier, and at 7, 8, 9, and 10 hours for the active transport (four scans at each session). Blood samples were obtained at each time point plus 5 and 15 minutes.
Passive permeability
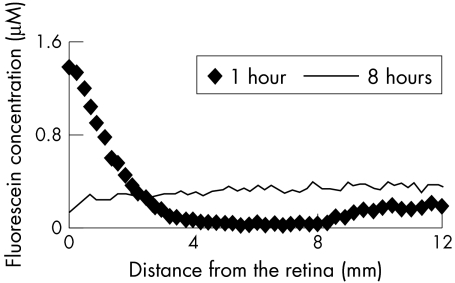
Calculation of the passive permeability and outward active transport has been described earlier. Briefly, the passive permeability of the blood-retinal barrier and the diffusion coefficient of fluorescein in the vitreous is calculated from the preretinal fluorescein concentration 1–5 mm in front of the retina (Fig 1) that is obtained at 30–60 minutes after injection. The model corrects for variations in plasma concentrations and light absorption of the lens.16–19
Figure 1.
The preretinal fluorescein concentration 1 hour and 8 hours after injection. Early after injection, the concentration is high close to the retina (0 mm) and low in the mid-vitreous. Eight hours after injection, the fluorescein concentration is more evenly distributed within the vitreous, with a small gradient with lower concentration at the retina because of active transport from the retina to the blood.
Outward, active transport
The flux of fluorescein from the plasma to the eye diminishes as a function of time and the net movement changes towards the outward direction from the vitreous to the blood.20 The preretinal gradient reverses in a way that the concentration near the retina is lower than in the centre of the vitreous (Fig 1). The outward, active transport is calculated with a simulation model from the preretinal gradient, the diffusion coefficient for fluorescein in the vitreous and the plasma values with a mathematical model.12
Comparison of fluorescein angiography and vitreous fluorometry
The correlation of leakage evaluated with fluorescein angiography to passive permeability was analysed after a correction of the angiographic leakage for the distance to the fovea, assuming that the influence of peripheral leakage on the passive permeability (measured along the optical axis of the eye) is less than the effect of central leakage. The effect of distance for the various fields in fluorescein angiography was calculated with multiple regression analysis of passive permeability versus the grade of leakage for each distance. The weight factors for various distances, derived from the multiple regression analysis, were: geometric centre: −0.05, centre field 0.07, inner subfield annulus 0.14, outer subfield annulus 0.07, far temporal field 0.09), and an arbitrary unit of leakage times distance was calculated for each eye.
Visual acuity was measured with standard, retroilluminated ETDRS charts.
Statistics
All calculations were performed with statistical software (systat 7.0). Differences between two or more groups were analysed with Student's t test or with analysis of variance. Regarding fluorescein transport all statistics and tables are based on log transformed data to normalise data distribution. The numbers in figures and tables are back transformed to the scale of the raw data. Both eyes were included if inclusion criteria were fulfilled. The correlation coefficient is calculated as Pearson's r.
The study was approved by the local medical ethics commitee. All participants gave their written informed consent after full information according to the Helsinki declaration.
RESULTS
Vitreous fluorometry and fluorescein angiography
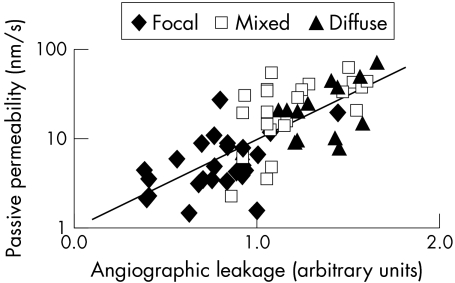
The passive permeability compared to fluorescein angiography (in arbitrary units of severity times distance from the fovea) was significantly correlated both for eyes with and without CSMO (with CSMO: r = 0.73; p <0.001, Fig 2; without CSMO: r = 0.65; p<0.001).
Figure 2.
The passive permeability versus fluorescein angiography in CSMO. The angiographic leakage is graded in arbitrary units of area of severity times distance, where the area of leakage is graded for the subfields in the ETDRS system—that is, the foveal centre, centre, inner, outer subfields, and far temporal field on 60 degree pictures. The distance factors for each subfield are calculated with multiple regression analysis as described in the methods section. The quantitative vitreous fluorometry is highly correlated with the semiquantative angiography grading (r = 0.73).
The range of passive permeability in CSMO was large (Table 1) corresponding to the variation seen in angiograms. Examples of fluorescein angiograms for two eyes, both with CSMO, in one case associated with low passive permeability and for the other case associated with high permeability are shown in Figure 3.
Table 1.
Passive permeability, active transport, and visual acuity for 61 eyes with CSMO and 22 eyes without CSMO
| Passive permeability (nm/s) | Active transport (nm/s) | Visual acuity logMAR | |
| CSMO | |||
| Mean | 11.32* | 62.20 | 0.16* |
| 95% confidence interval | 8.7 to 14.7 | 48.2 to 80.2 | 0.1 to 0.2 |
| Range | 2–72 | 2–265 | −0.26–0.9 |
| No CSMO | |||
| Mean | 3.57 | 71.95 | 0.043 |
| 95% confidence interval | 2.7 to 4.7 | 56.2 to 92.1 | −0.03 to 0.1 |
| Range | 1–13 | 23–195 | −0.2–0.6 |
*Significant differences (p<0.05).
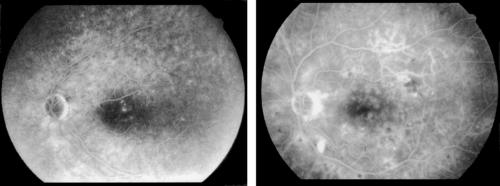
Figure 3.
Examples of a patient with a small central clinically significant macular oedema with focal leakage (left side) and a patient with more widespread and diffuse leakage (right side). Focal leakage is defined as leakage originating predominantly (> 67%) from microaneurysms, while in diffuse leakage the estimated component from microaneurysms is less than 33%. The calculated passive permeability for the example on the left was 3.5 nm/s, for the example on the right the passive permeability was 21 nm/s.
Focal leakage—that is, leakage originating primarily from microaneurysms, was associated with lower passive permeability compared to intermediate or diffuse leakage, where leakage is found adjacent to dilated capillaries often widespread in the posterior pole (Fig 3). Passive permeability for focal, intermediate, and diffuse leakage was 5.18 nm/s, 19.81 nm/s, and 21.01 nm/s, respectively, the difference between groups was significant (p <0.001; ANOVA). Active transport was not correlated with the fluorescein angiogram grading (r = 0.1).
Vitreous fluorometry and retinopathy
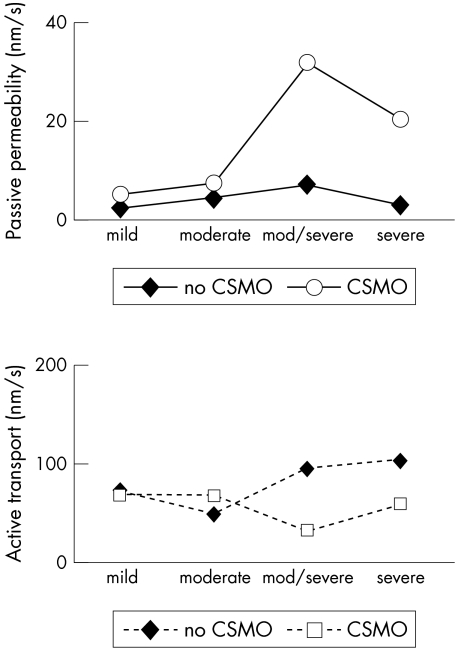
The passive permeability was significantly correlated with the level of retinopathy in CSMO (5.15 nm/s, 7.74 nm/s, 31.83 nm/s, and 20.28 nm/s for mild, moderate, moderate/severe, and severe retinopathy respectively, p <0.001; ANOVA; Fig 4) and the same was found in eyes without CSMO (2.65 nm/s, 4.59 nm/s, 6.76 nm/s, and 2.93 nm/s for mild, moderate, moderate/severe, and severe retinopathy, respectively, p = 0.045; ANOVA). The active transport was not correlated with retinopathy (p = 0.6; ANOVA).
Figure 4.
Passive permeability (top) and active transport (bottom) versus retinopathy grading on fundus photographs in eyes with and without CSMO. All eyes had non-proliferative diabetic retinopathy (NPDR) with the levels mild, moderate, moderate to severe, and severe according to the ETDRS system. In all grades, mean passive permeability is higher in oedematous eyes, the difference is significant for moderate/severe and severe retinopathy. The passive permeability was significantly correlated with the degree of retinopathy, in eyes with and without CSMO (p<0.001 and p = 0.045 respectively, ANOVA). No such pattern was found for the active transport.
Vitreous fluorometry and visual acuity
A significant correlation was found between visual acuity (ETDRS) and passive permeability for eyes with CSMO (r = 0.45 p < 0.001). No significant correlation was found with regard to active transport (r = 0.24; p = 0.07).
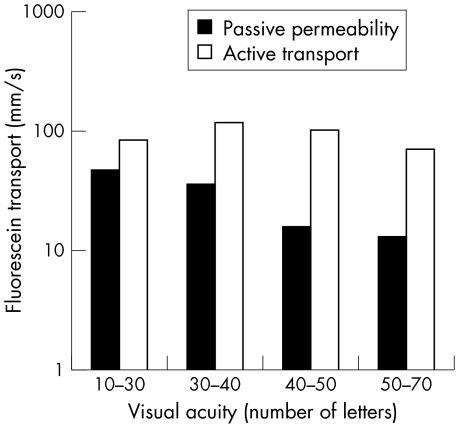
The relation between visual acuity and fluorescein transport is illustrated in Figure 5 with a simplified visual acuity scale (below 30 letters (20/63), from 30 to 40 letters, 40 to 50 letters, and 50 letters or more (that is, from 20/25 and better)). For the active transport, no clear pattern is seen, as the largest active transport corresponds to a modest loss of visual acuity.
Figure 5.
Passive permeability and active transport versus visual acuity given as number of letters from the ETDRS chart (55 letters equals 20/20, five letters equals one line). A significant correlation was found between passive permeability and visual acuity (Pearson's r = 0.45, p <0.001) but not for visual acuity and active transport (Pearson's r = 0.24, p = 0.07).
Clinical parameters
No significant correlations were found for fluorescein transport (neither passive nor active) and blood pressure or blood glucose (HbA1c). The passive permeability for CSMO eyes was independent of age but correlated with the duration of disease (r = −0.43, p <0.01) and larger for CSMO eyes with type 2 than for eyes with type 1 diabetes (12.3 and 7.8 nm/s respectively, p = 0.16) indicating that patients with type 2 diabetes and thus relatively short duration tend to have a high passive permeability.
The duration of disease was significantly shorter for patients with type 2 than for patients with type 1 diabetes—10.0 and 20.1 years, respectively, p <0.001.
CSMO and non-CSMO
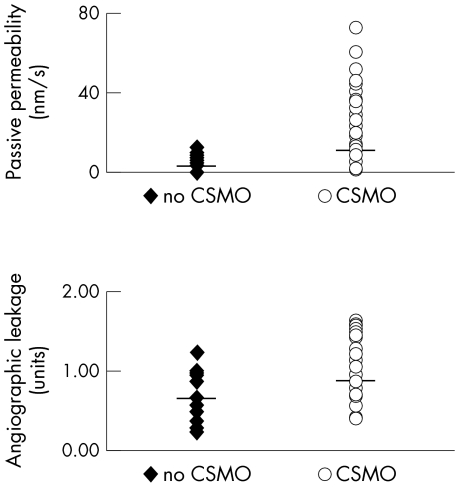
The passive permeability of fluorescein was four times larger from eyes with CSMO compared with eyes without CSMO (Ppassive 11.32 nm/s and 3.57 nm/s, respectively, p <0.001; Table 1, Fig 6).
Figure 6.
Passive permeability (top) and angiographic leakage (bottom) in eyes without CSMO and with CSMO, mean (back transformed from the logarithmic scale) is indicated with a horizontal line. The difference between eyes with CSMO and eyes without CSMO was significant for both methods (p <0.001).
The passive permeability of the metabolite fluorescein glucuronide was also larger in significant oedema (CSMO: 10.71 nm/s, no CSMO: 5.76 nm/s, p = 0.003) and the permeability of the fluorescein and fluorescein glucuronide was correlated (r = 0.7, p<0.001).
The active transport was not statistically different in eyes with and without oedema (62.12 nm/s and 71.95 nm/s respectively, p = 0.5, Table 1).
Angiographic leakage was statistically significantly different for eyes with CSMO and eyes without CSMO (p <0.001, Fig 6) with a larger overlap between the groups than for passive permeability.
Retinopathy and visual acuity. A higher degree of retinopathy was found in eyes with CSMO compared with eyes without CSMO (p = 0.002, Mann-Whitney U test).
As expected, visual acuity was significantly lower in eyes with CSMO than without (logMAR = 0.16 and 0.04 respectively, corresponding to 20/32 and 20/20, p = 0.02).
DISCUSSION
In diabetic macular oedema, the passive permeability of fluorescein, quantitated with vitreous fluorometry, was closely correlated with the leakage evaluated with angiography. The passive permeability was also significantly different in eyes with and without CSMO; thus vitreous fluorometry seems to be valuable as an alternative end point in clinical studies.
A gradual increase in permeability was seen in eyes without CSMO and mild retinopathy progressing to CSMO with severe retinopathy and both for CSMO and eyes without CSMO, the passive permeability was significantly correlated with retinopathy. A decrease in passive permeability was found at the most severe level of retinopathy compared to moderate retinopathy; however, only one eye without CSMO was found in this group. The largest increase in passive permeability was found in eyes with CSMO when retinopathy changed from moderate to moderate/severe—that is, at the appearance of intraretinal microvascular abnormalities.
The increase in permeability in relation to retinopathy corresponds to the majority of studies with vitreous fluorometry.6,7,12,21 In one previous study of diabetic patients with different levels of retinopathy, presumably without oedema, the passive permeability was not correlated with fluorescein angiography leakage and the number of background retinopathy changes (microaneurysms, hard and soft exudates, haemorrhages).22 An increase was first noted in the preproliferative and proliferative stages. A reason for the lack of correlation in mild background retinopathy could be loss of sensivity in the study, as data were analysed as the mean of right and left eye which may blur out differences in retinopathy.
In the present study, an overlap was found in both vitreous fluorimetry and with angiographic leakage between CSMO and eyes without CSMO (Fig 6), though the overlap was substantially smaller with vitreous fluorometry. This is not surprising as retinal thickening is related to the tissue oncotic pressure of various electrolytes and proteins, whereas fluorescein leakage is an estimate of blood-retinal barrier leakage for a specific molecule. Thus, some discrepancy between retinal thickening and fluorescein leakage may be expected, as also demonstrated in a study with simultaneous assessment of fluorescein leakage and objective measurement of retinal thickness.23,24 Also, in some cases fluorescein leakage is only present in a very small area and the increase in permeability is small even if the criteria of CSMO are fulfilled.
The active transport, which is substantially larger than the passive permeability, was equal in eyes with and without CSMO. However, compared to healthy subjects in a previous study using the same method, the active transport was significantly increased.12 The capacity of the active transport system in the retinal pigment epithelium is high as shown in animal studies and it is possible that the level of active transport of fluorescein in the healthy eyes is below full capacity while intraretinal changes related to retinopathy alone and/or oedema stimulate the pump activity to near full capacity.25
In severe retinopathy, the active transport in the present study decreased non-significantly in eyes with CSMO and moderate to severe level of retinopathy (Fig 4); additionally, a decrease of active transport was seen in eyes with low visual acuity (Fig 5). Thus, one might speculate whether the active transport is exhausted in late stages of macular oedema.
Blood pressure and metabolic regulation were not correlated with passive or active transport, whereas passive permeability was negatively correlated with the duration of disease.
In contrast, epidemiological studies have found that macular oedema is associated with high blood pressure and long duration of diabetes.1,2 The apparent differences from our results are probably the result of the presence of patients with type 2 diabetes, diagnosed late in relation to onset of the disease and already having retinopathy and/or CSMO at diagnosis or soon after. The expected correlation of passive permeability with blood pressure and duration cannot be expected in such patients, untreated for many years before diagnosis, similar to the study of Lawson et al,26 where blood pressure, glucose control, and duration were not associated with the severity of macular oedema evaluated as loss of visual acuity.
In summary, the breakdown of the blood-retinal barrier and the following increase in passive permeability measured with vitreous fluorometry is a dominant factor in diabetic macular oedema and, unlike active transport, the passive permeability is correlated with fluorescein angiogram leakage, retinopathy, and visual acuity. Thus, treatment of macular oedema should focus on the passive permeability. The exact mechanisms relating blood-retinal barrier breakdown to retinal thickening are not known, but recent studies have accentuated the role of vascular endothelium growth factor (VEGF) induced hyperpermeability, which seems related to changes in tight junction proteins (occludin) and upregulation of retinal adhesion molecule (ICAM-1), coincident with leucostasis.27,28 High levels of VEGF are found in proliferative retinopathy in humans and animal studies have shown an increased leakage of fluorescein from retinal vessels after injection of VEGF in the vitreous associated with an increased vesicular transport in endothelial cells.29,30 However, human studies in relation to diabetic macular oedema have not yet been completed.
Acknowledgments
The study was funded by the Danish Diabetes Association, Odense, and the Danish Eye Health Society, Copenhagen. The authors thank Hans-Henrik Petersen for photographic assistance.
REFERENCES
- 1.The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IV. Diabetic macular edema. Ophthalmology 1984;91:1464–74. [DOI] [PubMed] [Google Scholar]
- 2.Zander E, Herfurth S, Bohl B, et al. Maculopathy in patients with diabetes mellitus type 1 and type 2: associations with risk factors. Br J Ophthalmol 2000;84:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonetti DA, Barber AJ, Khin S, Lieth E, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelium growth factor decreases occludin in retinal endothelial cells. Diabetes 1998;12:1953–9. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi T, Inomata H. Ultrastructure of retinal vessels in diabetic patients. Br J Ophthalmol 1993;77:574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler C, Krogsaa B, Lund-Andersen H. Blood-retina barrier permeability and its relation to the progression of diabetic retinopathy in type I diabetes. Graefes Arch Clin Exp Ophthalmol 1991;339:442–6. [DOI] [PubMed] [Google Scholar]
- 6.Krogsaa B, Lund-Andersen H, Mehlsen J, et al. The blood-retinal barrier permeability in diabetic patients. Acta Ophthalmol Copenh 1981;59:689–94. [DOI] [PubMed] [Google Scholar]
- 7.Krogsaa B, Lund-Andersen H, Mehlsen J. The blood-retina barrier permeability versus diabetes duration and retinal morphology in insulin dependent diabetic patients. Acta Ophthalmol Copenh 1987;65:686–92. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida A, Ishiko S, Kojima M, et al. Blood-ocular barrier permeability in monkeys. Br J Ophthalmol 1992;76:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida A, Ishiko S, Kojima M. Outward permeability of the blood-retinal barrier. Graefes Arch Clin Exp Ophthalmol 1992;230:78–83. [DOI] [PubMed] [Google Scholar]
- 10.Koyano S, Arate M, Eguchi S. Movement of fluorescein and its glucuronide across retinal pigment epithelium-choroid. Invest Ophthalmol Vis Sci 1993;34:531–8. [PubMed] [Google Scholar]
- 11.Engler C, Sander B, Larsen M, et al. Probenecid inhibition of the outward trasnport of fluorescein across the blood-retina barrier. Acta Ophthalmol Copenh 1994;72:663–7. [DOI] [PubMed] [Google Scholar]
- 12.Sander B, Larsen M, Moldow B, et al. Passive and active transport of fluorescein in diabetic macular edema. Introducing a simplified mathematical model of active transport. Invest Ophthalmol Vis Sci 2001;42:433–8. [PubMed] [Google Scholar]
- 13.Moldow B, Sander B, Larsen M, et al. The effect of acetazolamide on passive and active transport of fluorescein across the blod-retina barrier in retinitis pigmentosa complicated by macular edema. Graefes Arch Clin Exp Ophthalmol 1998;236:881–9. [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98:786–807. [PubMed] [Google Scholar]
- 15.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. ETDRS report number 11. Classification of diabetic retinopathy from fluorescein angiograms. Ophthalmology 1991;98:807–22. [PubMed] [Google Scholar]
- 16.Larsen M, Dalgaard P, Lund-Andersen H. Differential spectrofluorometry in the human vitreous: blood-retina barrier permeability to fluorescein and fluorescein glucuronide. Graefes Arch Clin Exp Ophthalmol 1991;229:350–7. [DOI] [PubMed] [Google Scholar]
- 17.Larsen M, Lund-Andersen H. Lens fluorometry: light attenuation effects and estimation of total lens transmittance. Graefes Arch Clin Exp Ophthalmol 1991;229:363–70. [DOI] [PubMed] [Google Scholar]
- 18.Lund-Andersen H, Krogsaa B, La Cour M, et al. Quantitative vitreous fluorophotometry applying a mathematical model of the eye. Invest Ophthalmol Vis Sci 1985;26:698–710. [PubMed] [Google Scholar]
- 19.Dalgaard P, Larsen M. Fitting numerical solutions of differential equations to experimental data: a case study and some general remarks. Biometrics 1990;46:1097–109. [PubMed] [Google Scholar]
- 20.Engler C, Sander B, Larsen M, et al. Fluorescein transport across the human blood-retina barrier in the direction vitreous to blood. Acta Ophthalmol Copenh 1994;72:655–662. [DOI] [PubMed] [Google Scholar]
- 21.Van Schaik H, Castillo JM, Caubergh M, et al. Evaluation of diabetic retinopathy by fluorophotometry. European concerted action on ocular fluorometry. Int Ophthalmol 1998–99;22:97–104. [DOI] [PubMed] [Google Scholar]
- 22.Van Gerven J, Boot J, Lemkes H, et al. Effect of morphological abnormalities on blood-retinal barrier permeability in diabetic retinopathy. Doc Ophthalmol 1992;80:183–8. [DOI] [PubMed] [Google Scholar]
- 23.Shahidi M, Ogura Y, Blair N, et al. Retinal thickness analysis for quantitative assessment of diabetic macular edema. Arch Ophthalmol 1991;109:1115–19. [DOI] [PubMed] [Google Scholar]
- 24.Lobo CL, Bernardes RC, Cunha-Vaz JG. Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch Ophthalmol 2000;118:1364–9. [DOI] [PubMed] [Google Scholar]
- 25.Marmor MF. Mechanisms of fluid accumulation in retinal edema. Doc Ophthalmol 1999;97:239–49. [DOI] [PubMed] [Google Scholar]
- 26.Lawson PM, Hunt B, Kohner EM. Medical conditions in patients with diabetic maculopathy. Diabet Med 1985;4:245–50. [PubMed] [Google Scholar]
- 27.Antcliff R, Marshall J. The pathogenesis of edema in diabetic maculopathy. Sem Ophthalmol 1999;14:223–32. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto, Khosrof S, Bursell SE, et al. Vacular endothelial growth factor (VEGF)–induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 2000;156:1733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiello L, Bursell S, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated b protein kinase C in vivo and supressed by an orally effective beta-isoform-selective inhibitor. Diabetes 1997;46:1473–80. [DOI] [PubMed] [Google Scholar]
- 30.Hofman P, Blaauwgeers H, Tolentino M, et al. VEGF-A induced hyperpermeability of blood-retinal barrier endothelium in vivo is predominantly associated with pinocytotic vesicular transport and not with formation of fenestrations. Curr Eye Res 2000;21:637–45. [PubMed] [Google Scholar]