Abstract
Aims: To investigate the correlation between the level of angiotensin II and vascular endothelial growth factor (VEGF) in the vitreous fluid and the severity of proliferative diabetic retinopathy (PDR).
Methods: During vitreoretinal surgery at the Tokyo Women's Medical University, vitreous fluid samples were obtained from 51 eyes of diabetic patients with PDR, six eyes of diabetic patients without retinopathy, and 16 eyes of non-diabetic patients with ocular disease (controls). The VEGF levels in vitreous fluid and plasma were determined by enzyme linked immunosorbent assay, while angiotensin II levels were measured by radioimmunoassay.
Results: The vitreous fluid levels of VEGF and angiotensin II were significantly higher in patients with PDR than in non-diabetic patients or diabetic patients without retinopathy (all p<0.0001). The vitreous fluid level of angiotensin II was significantly correlated with that of VEGF (p<0.0001), and the vitreous concentrations of both VEGF and angiotensin II were significantly higher in patients with active PDR than in those with quiescent PDR (p<0.0001 and p=0.0005, respectively).
Conclusion: The authors found that both angiotensin II and VEGF levels were significantly higher in the vitreous fluid of patients with PDR than in that of non-diabetic patients or diabetic patients without retinopathy, and that the levels of both angiotensin II and VEGF were elevated in the active stage of PDR. These findings suggest that angiotensin II contributes to the development and progression of PDR in combination with VEGF.
Keywords: proliferative diabetic retinopathy, angiotensin II, vascular endothelial growth factor, angiogenesis
A ngiogenesis is the major feature in the pathogenesis of proliferative diabetic retinopathy (PDR).1 In this condi tion, retinal neovascularisation has a catastrophic effect on vision by causing vitreous haemorrhage, retinal detachment with formation of a fibrovascular membrane, and eventual blindness.2,3 The factors that stimulate the growth of retinal blood vessels have not been fully defined, but circumstantial evidence indicates that this not only involves angiogenic cytokines such as vascular endothelial growth factor (VEGF) but also vasoactive hormones such as angiotensin II.4,5
Angiotensin II has a proliferative effect and has been reported to regulate the growth of vascular smooth muscle cells6 and to stimulate the induction of various growth factors.7,8 Recent studies have suggested that abnormalities of the renin-angiotensin system (RAS) may also play a part in the progression of diabetic retinopathy.9–14 Inhibition of angiotensin converting enzyme (ACE) has been reported to be associated with a reduction of PDR,15,16 suggesting that suppression of the RAS may be of value for preventing and treating retinal neovascularisation. The hypothesis that an ocular RAS is involved in the development of PDR is supported by evidence that all components of the RAS are present in the retina10,13,14 and that angiotensin II, the effector molecule of this system, has angiogenic activity.5,17
These findings prompted us to examine whether angiotensin II plays a part in the development of PDR in combination with VEGF, which is considered to be the most potent factor in promoting angiogenesis. Therefore, we investigated the relation between the levels of angiotensin II and VEGF in the vitreous fluid of diabetic patients as well as the correlation between these factors and the severity of PDR. The present study revealed that angiotensin II and VEGF levels in the vitreous fluid were correlated with the severity of PDR and that the vitreous levels of these two molecules were also correlated with each other. Furthermore, both angiotensin II and VEGF were elevated in the active stage of PDR. Angiotensin II may induce neovascularisation via a paracrine effect on VEGF in diabetic patients with PDR.
MATERIALS AND METHODS
Patients
Undiluted vitreous fluid samples were harvested at the start of vitrectomy after informed consent was obtained from each subject following an explanation of the purpose and potential adverse effects of the procedure. This study was performed in accordance with the 1975 Declaration of Helsinki, as revised in 1983. Vitreous fluid samples were obtained from 51 patients with PDR, six diabetic patients without diabetic retinopathy, and 16 non-diabetic patients with ocular disease. Vitrectomy was performed on the 51 patients with PDR for the following reason: 27 had vitreous and/or preretinal haemorrhage, 17 had retinal detachment, and seven had macular heterotropia with proliferative tissues. The cases with macular heterotropia hoped to undergo the surgery because of the disturbed vision. The six diabetic patients without diabetic retinopathy included four with macular hole and two with epiretinal membrane, while the 16 non-diabetic patients included 12 with macular hole and four with epiretinal membrane (none of these 16 patients had proliferative vitreoretinopathy). Exclusion criteria for this study were: (1) treatment with an ACE inhibitor or an angiotensin II receptor antagonist (ARA), (2) previous ocular surgery, and (3) a history of ocular inflammation. All patients with PDR underwent retinal photocoagulation (mean 922 shots; range 342–1682 shots) before vitreous surgery. Vitrectomy was performed at the Tokyo Women's Medical University.
Fundus findings
Preoperative and operative fundus findings were recorded for each subject. The severity of diabetic retinopathy was assessed by standardised fundus colour photography and fluorescein angiography (FAG), which were performed with a Topcon TRC-50IA fundus camera, an image-net system (Tokyo Optical Co Ltd, Japan), and a preset lens with a slit lamp. Diabetic retinopathy was graded according to the modified Early Treatment Diabetic Retinopathy Study (ETDRS) retinopathy severity scale.18,19
PDR was classified as active (30 eyes) if there were new preretinal capillaries and as quiescent (21 eyes) if the vasoproliferation only consisted of large vessels within the membrane at the time of surgery.4,20
Sample collection
Samples of vitreous fluid were collected into sterile tubes and were rapidly frozen at −80°C. Those samples were obtained at the time of vitreoretinal surgery, with the protocol for sample collection being approved by the institutional review board and with all patients giving informed consent.
Plasma samples were also collected from the 51 patients with PDR. Blood was immediately placed on ice and subjected to centrifugation at 3000 × g for 5 minutes at 4°C, after which the plasma was rapidly frozen at −80°C until assay. The institutional review board also approved the protocol for blood collection.
Measurement of VEGF, angiotensin II, and ACE levels
Both VEGF and angiotensin II were measured in vitreous samples from the same eye as well as in the plasma. The VEGF concentration was measured by an enzyme linked immunosorbent assay (ELISA) for human VEGF (R&D Systems, Minneapolis, MN, USA).21 This VEGF kit was able to detect two of the four VEGF isoforms (VEGF121 and VEGF165), probably because these two shorter VEGF isoforms are secreted and the two longer isoforms are cell associated. The assay was performed according to the manufacturer's instructions. A standard solution (100 μl) or sample (10 or 100 μl) was added to the wells of a 96 well plate coated with an immobilised monoclonal antibody. After incubation, the plate was washed and the enzyme labelled antibody was added. After further incubation, the plate was washed again and the substrate was added. The reaction was stopped after colour had developed by adding the stop solution, and the optical density was determined at 450 and 620 nm using an absorption spectrophotometer (Titertek Multiscan MCC/340; ICN, Tokyo, Japan). A standard curve was plotted from the measurements made with the standard solution (from 15.6 to 1000 pg/ml), and the concentration of VEGF in each sample was determined from this curve. The VEGF levels in vitreous fluid and plasma were within the detection range of the assay, since the minimum detectable concentration was 15.6 pg/ml (coefficient of variation (CV) intra-assay 3.5%, CV interassay 5.8%).
Vitreous and plasma angiotensin II levels were determined by radioimmunoassay (RIA)22 and serum ACE levels were determined by the method of Kasahara.23 For the measurement of angiotensin II, 0.5 ml of vitreous fluid or plasma was mixed with 2.5 ml of ethanol. After centrifugation at 2000 g for 15 minutes at 4°C, the supernatant was dried under nitrogen gas at 37°C. The dried samples were reconstituted with 0.5 ml of assay buffer, and assay was performed with an angiotensin II RIA kit (Nichols, CA, USA). Measurement of ACE activity was performed with an ACE colour kit (Fujirebio, Tokyo, Japan). Serum (0.05 ml) was mixed with 0.5 ml of p-hydroxybenzoyl-glycyl-l-histidyl-l-leucine. After incubation, the reaction was stopped when colour had developed by adding the stop solution and the optical density was determined at 505 nm using an absorption spectrophotometer (AU600; Olympus, Tokyo, Japan). The angiotensin II and ACE levels in vitreous fluid and plasma were within the detection range of these assays, since the minimum detectable concentration was 4.0 pg/ml (CV intra-assay 3.8%, CV interassay 6.0%) and 2.5 pg/ml (CV intra-assay 4.0%, CV interassay 6.2%), respectively.
Statistical analysis
All analyses were performed with sas System 6.12 software (SAS Institute Inc, Cary, NC, USA).24 Data are presented as the frequency or mean (SD). Data with a skewed distribution were transformed to a logarithmic scale, and the geometric mean was calculated together with 1 SD below and 1 SD above the mean on that scale. Analysis of variance (ANOVA) was used to test for statistically significant differences among the groups and the Turkey-Kramer multiple comparison test was also applied when appropriate. Correlations were tested using Spearman's rank correlation coefficients. A two tailed p value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Vitreous levels of VEGF and angiotensin II
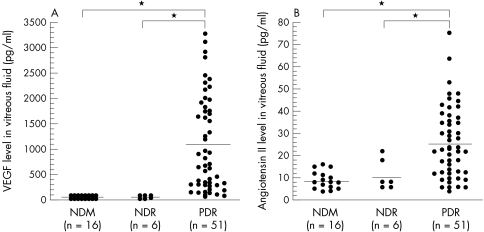
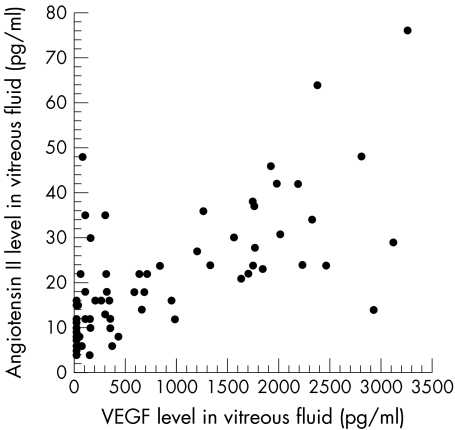
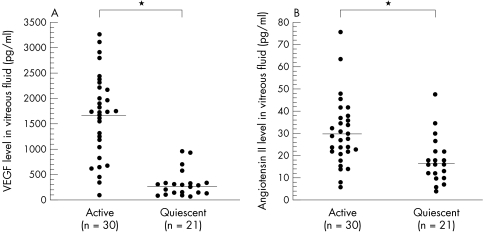
The diabetic patients included 31 men and 26 women mean age 60.7 (SD 10.5) years, with a diabetes duration of 16.2 (6.3) years and an HbA1c level of 7.6% (2.3%). The 16 patients with non-diabetic ocular disease included eight men and eight women aged 63.4 (6.5) years. There was no significant difference in age between the diabetic and non-diabetic patients (p=0.2364). Vitreous fluid concentrations of VEGF were significantly elevated in the samples from patients with PDR (1135.2 pg/ml (75.6 to 3280.0)) when compared with the samples from non-diabetic patients (19.3 pg/ml (15.6 to 40.6)) (p<0.0001) or diabetic patients without retinopathy (49.9 pg/ml (23.4 to 105.0)) (p<0.0001) (Fig 1A). Vitreous fluid concentrations of angiotensin II were also significantly elevated in the patients with PDR (25.0 pg/ml (4.0 to 76.0)) when compared with non-diabetic patients (8.9 pg/ml (4.0 to 16.0)) (p<0.0001) or diabetic patients without retinopathy (11.3 pg/ml (6.0 to 22.0)) (p<0.0001) (Fig 1B). There was a significant relation between the vitreous fluid concentration of VEGF and that of angiotensin II (ρ=0.702, p<0.0001) (Fig 2). Furthermore, the vitreous fluid concentrations of VEGF and angiotensin II in the patients with active PDR (VEGF: 1697.3 pg/ml (107.0 to 3280.0)); angiotensin II: 30.4 pg/ml (6.0 to 76.00) were significantly higher (p<0.0001 and p=0.0005, respectively) than in the patients with quiescent PDR (VEGF: 332.2 pg/ml (75.6 to 968.0); angiotensin II: 17.4 pg/ml (4.0 to 48.0)) (Fig 3A and B).
Figure 1.
(A) VEGF concentrations in the vitreous fluid of non-diabetic patients (NDM), diabetic patients without retinopathy (NDR), and PDR patients (PDR) (*p<0.0001). (B) Angiotensin II concentrations in the vitreous fluid of non-diabetic patients (NDM), diabetic patients without retinopathy (NDR), and PDR patients (PDR) (*p<0.0001).
Figure 2.
Relation between the vitreous fluid levels of VEGF and angiotensin II in PDR patients and diabetic patients without retinopathy (ρ=0.702, p<0.0001).
Figure 3.
(A) VEGF levels in the vitreous fluid of patients with active PDR and quiescent PDR (*p<0.0001). (B) Angiotensin II levels in the vitreous fluid of patients with active PDR and quiescent PDR (*p=0.0005).
Vitreous and plasma levels of VEGF, angiotensin II, and ACE
The vitreous fluid concentration of VEGF was significantly higher than the plasma VEGF level (50.9 pg/ml (15.6 to 396.0)) in the patients with PDR (p<0.0001) (Table 1). The vitreous fluid concentration of angiotensin II was also significantly higher than the plasma angiotensin II level (17.5 pg/ml (4.0 to 46.0)) in the PDR patients (p =0.0106) (Table 1). Plasma angiotensin II levels showed a significant correlation with the vitreous fluid levels of both VEGF and angiotensin II (ρ=0.596, p<0.0001 and ρ=0.755, p<0.0001, respectively). The plasma ACE level (14.2 pg/ml (2.5 to 26.0)) was significantly correlated with the vitreous fluid level of angiotensin II (ρ=0.372, p=0.0013), but was not significantly correlated with that of VEGF (ρ=0.223, p=0.0594). There was no significant relation between plasma and vitreous fluid VEGF levels (ρ=0.128, p=0.1490). There was also no significant relation between HbA1c (7.6 % (4.8 to 12.3)) and the vitreous levels of VEGF or angiotensin II (ρ=0.220, p=0.1712 and ρ=0.253, p=0.0626, respectively).
Table 1.
Comparison of VEGF and angiotensin II concentrations in vitreous fluid and plasma in diabetic patients with PDR
| Vitreous fluid | Plasma | p Value | No | |
| VEGF | 1135.2 (837.7) | 50.9 (38.9) | <0.0001 | 51 |
| Angiotensin II | 25.0 (14.3) | 17.5 (9.6) | 0.0106 | 51 |
DISCUSSION
The present study showed that both angiotensin II and VEGF levels were increased in the vitreous fluid of patients with PDR and were correlated with the severity of diabetic retinopathy. In addition, angiotensin II and VEGF showed a statistically significant correlation with each other and the vitreous fluid level of angiotensin II was elevated in the active stage of PDR. We showed that not only the vitreous level of VEGF but also that of angiotensin II was significantly elevated in PDR patients when compared with non-diabetic patients or diabetic patients without retinopathy. Angiotensin II has been shown to promote the growth of capillary vessels in the chorioallantoic membrane25 and to stimulate new vessel formation in the rabbit cornea.5 A protective effect of an ACE inhibitor and of an ARA AT1 receptor on hyperoxia induced and normoxia induced neovascularisation has been demonstrated in newborn mice.17 However, continuous transvitreal infusion of angiotensin II alone produced retinal artery constriction, but not new vessel formation from the retina to the vitreous in the cat eye.26 Angiotensin II not only has a growth promoting effect, but also stimulates the induction of many cytokines and growth factors.7,8 Therefore, angiotensin II may affect neovascularisation in combination with other cytokines or growth factors.
In the present study, the vitreous level of angiotensin II was statistically correlated with that of VEGF. It has been suggested that induction of VEGF mRNA most probably occurs through transcriptional regulation. Receptors for angiotensin II are present on endothelial cells, and angiotensin II acts to stimulate endothelial cell growth and upregulate VEGF mRNA expression.27 Moreover, angiotensin II may potentiate VEGF induced angiogenic activity in the retina through increased expression of the VEGF receptor Flk-1/KDR.28 The effect of angiotensin II on VEGF expression was completely inhibited by an ARA.29 There is a possibility that angiotensin II might influence VEGF elicited signal transduction or post-transcriptional regulation of KDR.28 The functioning of the ocular RAS is not yet clear. However, our results and previous studies have suggested that an autocrine-paracrine relation may exist between angiotensin II and VEGF in ocular tissues. The capacity of VEGF to act as a potent angiogenic agent suggests that an angiotensin II induced increase of VEGF production could have a key role in the occurrence of neovascularisation in PDR. Further investigations will be needed to clarify the ocular interactions between angiotensin II and VEGF as well as the role of angiotensin II during neovascularisation in PDR.
It seems logical for the vitreous fluid levels of angiotensin II and VEGF to vary with the severity of PDR. In fact, we found that the vitreous fluid levels of both angiotensin II and VEGF were significantly higher in active PDR than in quiescent PDR. The levels of both angiotensin II and VEGF in the vitreous fluid seem to increase during active neovascularisation and to decrease in the absence of neovascularisation, because we classified the severity of PDR according to the activity of neovascularisation in this study. It was previously reported that the vitreous fluid level of VEGF was higher in active PDR than in quiescent PDR and that VEGF played a major part in mediating intraocular neovascularisation in diabetic retinopathy.4,20 However, to our knowledge, the present study provides the first evidence that the vitreous fluid levels of angiotensin II are elevated in the active stage of PDR.
It is still unclear whether production of angiotensin II can occur in ocular tissues. From our results, it cannot be said whether ocular angiotensin II is located intracellularly or extracellularly and it is also impossible to determine whether angiotensin II is synthesised locally in the eye or sequestered from the plasma. Sequestration is not very likely since that would imply a specific uptake process. The local concentration of angiotensin II in the retinal microvasculature is reported to be higher than the serum and vitreous fluid levels.11,13 In the present study, the vitreous fluid level of angiotensin II was significantly higher than the plasma level, but the statistical difference was small. Furthermore, the plasma levels of angiotensin II and ACE were significantly correlated with the vitreous fluid level of angiotensin II. The level of angiotensin II in the ocular fluid from normal porcine eyes is low to undetectable, in contrast with the relatively high levels in surrounding ocular tissues such as the RPE and choroids.13 Breakdown of the blood-retinal barrier (BRB) may facilitate diffusion of angiotensin II from the blood into the vitreous fluid. Since the vitreous can be considered the repository for products originating from the retina, a high level of angiotensin II might well be explained by its production and secretion from the retina. Accordingly, angiotensin II may be produced locally in ocular tissues, but little of this angiotensin II may leak into the ocular fluid under normal conditions and only when the BRB is disrupted will angiotensin II reach the vitreous fluid in high concentrations.13 The patients with active PDR in the present study had a hyperfluorescein pattern on FAG just before surgery. These results and previous reports suggest that disruption of the BRB may lead to elevation of the vitreous fluid concentration of angiotensin II. ACE inhibitors have been reported to maintain the BRB in diabetic patients and to have a favourable effect on diabetic retinopathy.15,16,30 It may be possible that angiotensin II in the vitreous fluids derived both from production in ocular tissues (such as the retinal pigment epithelium-choroid complex) and via disruption of the BRB.
The underlying biochemical cause of PDR would seem to be chronic hyperglycaemia, suggesting that HbA1c might show a positive relation with the angiotensin II concentration in vitreous fluid. However, there was no significant correlation between them in the present study. The VEGF concentration also showed no significant relation with the HbA1c value. Because glycaemic control was improved in some patients before vitreous surgery, the HbA1c level at the time of the operation did not necessarily indicate their long term glycaemic control, so we could not assess the relation of hyperglycaemia to angiotensin II or VEGF based on these results.
In summary, the present study showed that the concentrations of both angiotensin II and VEGF in the vitreous fluid of patients with PDR were significantly higher than those in non-diabetic patients or diabetic patients without retinopathy. Vitreous fluid levels of angiotensin II were significantly correlated with those of VEGF. Moreover, the levels of both VEGF and angiotensin II were higher in active PDR than in quiescent PDR. These findings support our contention that angiotensin II plays a part in the neovascularisation process of PDR via stimulation of VEGF. The therapeutic implication is that inhibition of the RAS may be beneficial for the treatment of PDR. Indeed, the beneficial effects of ACE inhibition for patients with PDR have recently been shown by the EUCLID study15 and the Steno study.16 Our results support the possibility that the treatment with an ACE inhibitor or ARA may effectively prevent the development of PDR. We hope to investigate the possibility that ACE inhibitor or ARA inhibit angiotensin II in eyes and also inhibit the progression of retinopathy to PDR in the next stage.
Video Reports (www.bjophthalmol.com) .
Capsule staining and mature cataracts: a comparison of indocyanine green and trypan blue dyes. D F Chang
Pearls for implanting the Staar toric IOL. D F Chang
An intraocular steroid delivery system for cataract surgery. D F Chang
Evaluation of leucocyte dynamics in mouse retinal circulation with scanning laser ophthalmoscopy. Heping Xu, A Manivannan, Garry Daniels, Janet Liversidge, Peter F Sharp, John V Forrester, Isabel J Crane
Dipetalonema reconditum in the human eye. T Huynh, J Thean, R Maini
Acknowledgments
This study was supported by Health Science Research Grants (No 10060101, to HF, HY, and SH) from the Ministry of Health and Welfare, Research on Eye and Ear Sciences, Immunology, Allergy and Organ Transplantation in Japan.
We thank Drs Shigehiko Kitano, Erika Shimizu, Kensuke Haruyama, and Shinko Nakamura for their assistance in collecting the vitreous and plasma samples and in performing the ophthalmological examinations. We also thank Drs Yasuhiko Iwamoto and Naoko Iwasaki for their assistance in performing the internal medical examinations. We finally would like to thank Katsunori Shimada for his assistance in conducting the statistical analyses.
Abbreviations
ACE, angiotensin converting enzyme
ARA, angiotensin II receptor antagonist
BRB, blood-retinal barrier
ELISA, enzyme linked immunosorbent assay
FAG, fluorescein angiography
KDR, kinase insert domain containing receptor
PDR, proliferative diabetic retinopathy
RIA, radioimmunoassay
RAS, renin-angiotensin system
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med 1995;73:333–46. [DOI] [PubMed] [Google Scholar]
- 2.Moss SE, Klein R, Klein BEK. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998;105:998–1003. [DOI] [PubMed] [Google Scholar]
- 3.Fong DS, Ferris FL, Davis MD, et al. Causes of severe visual loss in the Early Treatment Diabetic Retinopathy Study: ETDRS report no.24. Am J Ophthalmol 1999;127:137–41. [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331:1480–7. [DOI] [PubMed] [Google Scholar]
- 5.Fernadez LA, Twickler J, Mead A. Neovascularization produced by angiotensin?. J Lab Clin Med 1985;105:141–5. [PubMed] [Google Scholar]
- 6.Daemen MJ, Lombardi DM, Bosman FT, et al. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 1991;68:450–6. [DOI] [PubMed] [Google Scholar]
- 7.Itoh H, Mukoyama M, Pratt RE, et al. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin? J Clin Invest 1993;91:2268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delafontaine P, Lou H. Angiotensin II regulates insulin-like growth factor I gene expression in vascular smooth muscle cells. J Biol Chem 1993;268:16866–70. [PubMed] [Google Scholar]
- 9.Danser AHJ, van den Dorpel MA, Deinum J, et al. Renin, prorenin, and immunoreactive rennin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab 1989;68:160–7. [DOI] [PubMed] [Google Scholar]
- 10.Deinum J, Derkx FHM, Danser AHJ, et al. Identification and quantification of renin and prorenin in the bovine eye. Endocrinology 1990;126:1673–82. [DOI] [PubMed] [Google Scholar]
- 11.Sramek SJ, Wallow IHL, Tewksbury DA, et al. An ocular renin-angiotensin system: immunohistochemistry of angiotensinogen. Invest Ophthalmol Vis Sci 1992;33:1627–32. [PubMed] [Google Scholar]
- 12.Feman SS, Mericle RA, Reed GW, et al. Serum angiotensin converting enzyme in diabetic patients. Am J Med Sci 1993;305:280–4. [DOI] [PubMed] [Google Scholar]
- 13.Danser AHJ, Derkx FHM, Admiraal PJJ, et al. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci 1994;35:1008–18. [PubMed] [Google Scholar]
- 14.Wagner J, Danser AHJ, Derkx FHM, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol 1996;80:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi N, Sjolie AK, Stephenson JM, et al, and the EUCLID Study Group. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. Lancet 1998;351:28–31. [DOI] [PubMed] [Google Scholar]
- 16.Gaede P, Vedel P, Parving HH, et al. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomized study. Lancet 1999;353:617–22. [DOI] [PubMed] [Google Scholar]
- 17.Lonchampt M, Pennel L, Dubault J. Hyperoxia/normoxia-driven retinal angiogenesis in mice: a role for angiotensin II. Invest Ophthalmol Vis Sci 2001;42:429–32. [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs. An extension of the modified Airlie House classification. ETDRS Report Number 10. Ophthalmology 1991;98:786–806. [PubMed] [Google Scholar]
- 19.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS Report Number 12. Ophthalmology 1991;98:823–33. [PubMed] [Google Scholar]
- 20.Katsura Y, Okano T, Noritake M, et al. Hepatocyte growth factor in vitreous fluid of patients with proliferative diabetic retinopathy and other retinal disorders. Diabetes Care 1998;21:1759–63. [DOI] [PubMed] [Google Scholar]
- 21.Hyodo I, Doi T, Endo H, et al. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer 1998;34:2041–5. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Shimamoto K, Moriguchi O, et al. A sensitive radioimmunoassay for the determination of plasma angiotensin II in human subjects. Jap Circ J 1979;43:727–33. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara Y and Ashihara Y. Colorimetry of angiotensin-converting enzyme activity in serum. Clin Chem 1981;27:1922–5. [PubMed] [Google Scholar]
- 24.SAS Institute Inc. SAS/STAT Software. Changes and Enhancements through Release.6.12, 1997.
- 25.Le Noble FA, Hekking JW, Van Straaten HW, et al. Angiotensin II stimulates angiogenesis in the chorio-allantoic membrane of the chick embryo. Eur J Pharmacol 1991;195:305–6. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood EJ, Fantes F, Davis EB, et al. The response of retinal vasculature to angiotensin. Invest Ophthalmol Vis Sci 1987;28:676–82. [PubMed] [Google Scholar]
- 27.Chua CC, Hamdy RC, Chua BHL. Upregulation of vascular growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta 1998;1401:187–94. [DOI] [PubMed] [Google Scholar]
- 28.Otani A, Takagi H, Suzuma K, et al. Angiotensin potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res 1998;82:619–28. [DOI] [PubMed] [Google Scholar]
- 29.Moravski CJ, Kelly DJ, Cooper ME, et al. Retinal neovascularization is prevented by blocked of the rennin-angiotensin system. Hypertension 2000;36:1099–104. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert RE, Kelly DJ, Cox AJ, et al. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia 2000;43:1360–7. [DOI] [PubMed] [Google Scholar]