Retinal toxicity attributable to intravitreal use of aminoglycosides for endophthalmitis has been reported. Campochiaro and Conway reported 101 cases of retinal damage due to intravitreal aminoglycosides.1 Amikacin, an aminoglycoside, is in widespread use in the United Kingdom for the treatment of Gram negative organisms in endophthalmitis. We report a case of macular toxicity following the use of intravitreal amikacin for postoperative endophthalmitis, outline mechanisms of retinal toxicity, and offer alternatives to amikacin. We believe that enough evidence now exists to support a change in the current Royal College of Ophthalmologists' endophthalmitis treatment guidelines that are based on the Endophthalmitis Vitrectomy Study.2
Case Report
A 69 year old white woman presented 1 day after uneventful right phacoemulsification and intraocular lens implantation with a vision of counting fingers (CF), a relative afferent pupil defect, hypopyon with anterior chamber fibrin, and normal intraocular pressure. We were unable to visualise the fundus although a red reflex was noted. Ultrasound examination showed patchy increased vitreous reflectivity with a flat retina and choroid. Pars plana vitreous tap of 0.2 ml was performed followed by injection of 1 mg/0.1 ml of vancomycin and 0.4 mg/0.1 ml of amikacin. Sterile dilution was conducted with typewritten instructions. The globe never became tense. The ocular inflammation resolved on a daily regimen of oral prednisolone 60 mg, oral ciprofloxacin 750 mg four times daily, topical ofloxacin 0.3% 2 hourly, topical dexamethasone 0.1% 2 hourly, and topical atropine 1% once daily. Vision however remained CF because of angiographically proved macular ischaemia and vascular occlusion (Figs 1 and 2). There was no microbiological growth from the vitreous sample.
Figure 1.
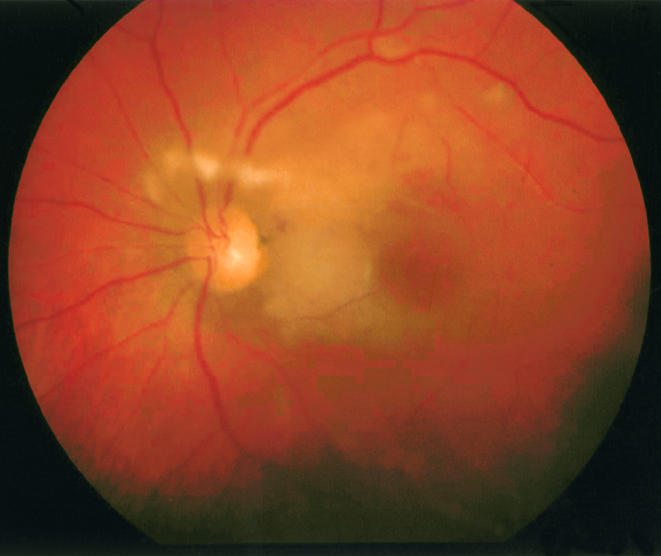
Fundus photograph showing diffuse posterior pole retinal cloudy swelling, cotton wool spots, and scattered intraretinal haemorrhages.
Figure 2.
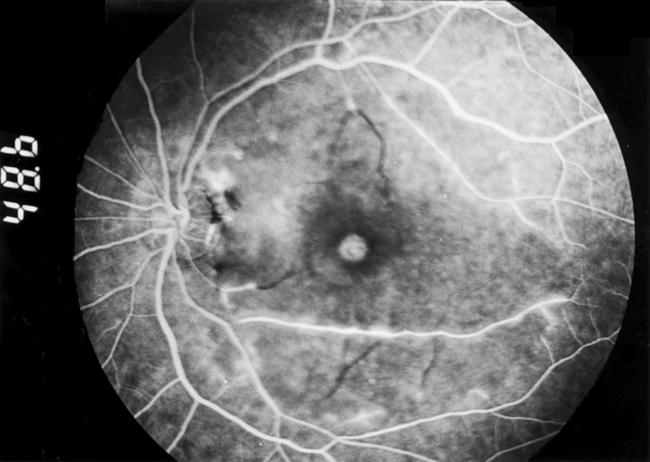
Fluorescein angiogram at 48 seconds showing diffuse macular arteriolar occlusion with staining of the arteriolar walls along the inferior and superior arcades.
Comment
The rationale for amikacin use relates to the relatively high incidence of Gram negative isolates in endophthalmitis (6.4–26.2%)3–5 and the broad spectrum of action with low resistance offered by this agent. Amikacin has largely superseded gentamicin because of its perceived safety profile. Our case and others reported in the literature6–8 serve to notify clinicians of the serious retinal toxicity occurring with amikacin use, which is possibly a class related phenomenon.
Hypotheses for toxicity include dilution errors, raised post-injection intraocular pressure, concomitant use of high concentrations of subconjunctival amikacin, and variations in vitreous concentration. Local variation in concentration may play a part if the agent is directly injected into the posterior vitreous space instead of the gel itself. Another possibility for macular predilection is that this is the dependent part in the supine patient during pars plana injection.
Large studies offer alternatives to aminoglycosides on the basis of culture sensitivities.3–5, 9 Suggestions include ceftazidime, cefotaxime, and ciprofloxacin. Sensitivities of Gram negative organisms to ceftazidime range from 61% to 100% according to published studies.3–5, 9 It appears that in most studies 2 mg (0.1 ml of 20 mg/ml solution) of ceftazidime has in vitro effectiveness equipotent to or greater than 0.4 mg amikacin.3–5, 9 Primate studies have shown that intravitreal ceftazidime is non-toxic at doses of 2.25 mg.10 Ceftazidime is already in clinical use in the United Kingdom.6 There have been no reported cases of retinal toxicity with intravitreal ceftazidime use in humans.11
With mounting evidence against amikacin we support a change in current UK treatment guidelines. Choices will remain controversial until the incidence of toxicity for both amikacin and ceftazidime is determined by a prospective randomised controlled study; however, on the evidence currently available we suggest that ceftazidime should replace amikacin as the first line agent of choice against Gram negative organisms in postoperative endophthalmitis.
References
- 1.Campochiaro PA, Conway BP. Aminoglycoside toxicity—a survey of retinal specialists: implications for ocular use. Arch Ophthalmol 1991;109:946–50. [DOI] [PubMed] [Google Scholar]
- 2.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomised trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 1995;113:1479–96. [PubMed] [Google Scholar]
- 3.Kunimoto DY, Das T, Sharma S, et al. Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Endophthalmitis Research Group. Am J Ophthalmol 1999;128:240–2. [DOI] [PubMed] [Google Scholar]
- 4.Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 1996;122:1–17. [DOI] [PubMed] [Google Scholar]
- 5.Donahue SP, Kowalski RP, Eller AW, et al. Empiric treatment of endophthalmitis. Are aminoglycosides necessary? Arch Ophthalmol 1994;112:45–7. [DOI] [PubMed] [Google Scholar]
- 6.Jackson TL, Williamson TH. Amikacin retinal toxicity. Br J Ophthalmol 1999;83:1199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seawright AA, Bourke RD, Cooling RJ. Macula toxicity after intravitreal amikacin. Aust N Z J Ophthalmol 1996;24:143–6. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Dada T. Preretinal haemorrhages: an unusual manifestation of intravitreal amikacin toxicity. Aust NZ J Ophthalmol 1999;27:435–6. [DOI] [PubMed] [Google Scholar]
- 9.Irvine WD, Flynn HW Jr, Miller D, et al. Endophthalmitis caused by gram-negative organisms. Arch Ophthalmol 1992;112:1450–4. [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Green WR. Toxicity of intravitreous ceftazidime in primate retina. Arch Ophthalmol 1992;110:1625–9. [DOI] [PubMed] [Google Scholar]
- 11.Lim JI, Campochiaro PA. Successful treatment of gram-negative endophthalmitis with intravitreous ceftazidime. Arch Ophthalmol 1992;110:1686. [DOI] [PubMed] [Google Scholar]


