Abstract
Aim: To determine the incidence of ocular toxicity of preservatives with glaucoma medications.
Methods: A prospective epidemiological survey was carried out in 1999 by 249 ophthalmologists on 4107 patients. Ocular symptoms, conjunctiva, cornea, and eyelids were assessed. A χ2 test was used for differences between preserved eye drops (P) and preservative free eye drops (PF).
Results: 84% patients used P, 13% received PF, and 3% a combination of P and PF eye drops. All symptoms were more prevalent with P than with PF drops (p<0.001): discomfort upon instillation (43% versus 17%), and symptoms between instillations such as burning-stinging (40% versus 22%), foreign body sensation (31% versus 14%), dry eye sensation (23% versus 14%), tearing (21% versus 14%), and eyelid itching (18% versus 10%). An increased incidence (>2 times) of ocular signs was seen with P eye drops. The prevalence of signs and symptoms was dose dependent, increasing with the number of P drops. A reduction in the symptoms and signs was observed when patients changed from P to PF eye drops (p<0.001).
Conclusions: Symptoms and signs are less prevalent when PF drops are used. Moreover, most of the adverse reactions induced by P glaucoma medication are reversible after removing preservatives.
Keywords: preservatives, glaucoma, ocular surface damage
Medical treatment is considered an effective way of controlling glaucoma in its initial stage.1 For treatment to be effective, side effects need to be minimised to promote compliance and allow continuation of therapy. Furthermore, topical medication should not inhibit the future success of surgical treatment of glaucoma.
The benefits of reducing microbial contamination through use of preservatives are offset by the known ocular side effects of preservatives.2 The toxic action of preservatives on the ocular surface has been widely demonstrated in vitro as well as in vivo, in both humans and animals.3–5 Studies have shown that preservative free timolol and carteolol eye drops are less toxic for the ocular surface,6–8 suggesting that the side effects of β blocker eye drops are mainly the result of the presence of preservatives.
Preservatives decrease the stability of the precorneal tear film.5,9–12 They have a detergent effect on the lipid layer,13 resulting in increased evaporation. Preservatives also destabilise the tear film indirectly by decreasing the density of goblet cells in the conjunctival epithelium.11,14,15 Any destabilisation compromises the ability of the tear film to provide protection and trophic factors to the cornea. Understandably, worsening of pre-existing dry eye is a common complication associated with the use of eye drop solutions containing preservatives.16–18
Subtle signs of ocular toxicity, such as superficial punctate keratitis, indicate chronic cell injury that can well have long term consequences. In the cornea, application of preservatives induces reduction in cell proliferation and viability. Hence, corneal healing is impaired19 and the epithelial barrier compromised.6
Histopathological and impression cytology studies of the conjunctiva have demonstrated inflammation, squamous metaplasia, and subconjunctival fibrosis in the conjunctiva and Tenon's capsule associated with the use of topical preservatives.7,20–26 These side effects are dose dependent and increase with frequency of instillation.27
Benzalkonium chloride inhibits proliferation of trabecular cells at a concentration of 0.00002% in in vitro models.28 In humans, this cytotoxicity constitutes a potential risk but has neither been reported nor investigated systematically. Nevertheless, inflammatory reactions may be seen in the trabeculum, particularly in glaucoma patients undergoing topical multidrug treatment or long term topical treatment. These disorders, similar to those seen in the conjunctiva, may be due at least in part to the presence of preservatives. They could therefore directly affect trabecular filtration, and thus the course of the glaucoma itself.27
It has been shown that failure of surgical treatment is mainly linked to the duration and the extent of previous medical treatment.29–32 The failure of surgical treatment seems to be associated with inflammation of the ocular surface structures,25 a feature more frequent in patients treated over the long term with glaucoma eye drops.25,26 Hence, it is suspected that the toxicity of the preservatives contained in glaucoma eye drops has a role in the failure of surgical treatment.33
Preservatives induce, according to their nature, an allergic reaction but more frequently a cytotoxic reaction (90%).34 The degree and type of allergy incurred depends upon patient factors and the type of preservative. Benzalkonium salts, which are considered moderately sensitising,35,36 usually result in contact allergies and delayed hypersensitivity reactions. Further, long term use of these agents may result in a form of conjunctival scarring known as drug induced pemphigoid,37,38 in which chronic allergic reaction leads to a marked and self sustaining inflammatory process.
While the ocular toxicity of preservatives has been demonstrated both in animals and in humans in numerous studies, few epidemiological data are available with regard to the nature and the frequency of these complications in glaucoma patients.
This study examined a large population being treated for chronic open angle glaucoma in ophthalmological practice. Its aim was to investigate the nature of the side effects of topical β blockers of varying dose, both preserved and preservative free in terms of symptoms and ocular signs of irritation. The study design was developed following a pilot study of 919 patients.39
MATERIALS AND METHODS
This study was a practice based prospective epidemiological study examining a population currently treated by topical glaucoma medication. Ophthalmologists in private practice enrolled patients under their management, presenting with ocular hypertension and/or chronic open angle glaucoma, and receiving medical treatment. Enrolment was based on routine attendance for glaucoma management. The investigators received case report forms and a leaflet with instructions. The observations required were clinical evaluations commonly employed in routine ophthalmic examination.
During this cross sectional observational survey, the patients attended for one visit. The ophthalmologist in case of necessity (in particular, treatment change) could plan a second visit. At the first visit, demographic data and information on treatment history were collected: age, sex, and duration of the treatment. At each visit, a questionnaire was completed about the number of preserved and preservative free eye drops used, and ocular symptoms: discomfort or pain upon instillation, and symptoms between instillations (foreign body sensation, stinging or burning sensation, dry eye sensation, tearing, eyelid itching). Clinical examination of the palpebral and bulbar conjunctiva, cornea and eyelids was undertaken. Superficial punctate keratitis was evaluated as “absent,” “mild” (a few points), “severe” (more than 25% of the corneal surface).
No change in the therapeutic practices by the ophthalmologist was requested but the patient's treatment could be modified depending on their clinical status. Any change to the prescription was at the discretion of the ophthalmologist. If marked signs or symptoms of ocular toxicity were observed, preservative free eye drops could be considered. In France, at the time of the study, a number of medications were available in preservative free form (Table 1). These were either available as unit dose solutions or multidose bottles with a membrane system preventing contamination of preservative free formulations for up to 1 month after opening. More formulations were available in preserved formulations, primarily providing smaller increments in the available dosages. Carbonic anhydrase inhibitors, sympathomimetics, prostaglandins, and combination drops were only available in preserved formulations.
Table 1.
Glaucoma medications available in France at the time of the study in preserved and preservative free form
| Preserved eye drops (%) | Preservative free eye drops (%) | |
| β Blockers | ||
| Timolol | 0.1, 0.25, 0.50 | 0.1, 0.25, 0.50 |
| Betaxolol | 0.1, 0.25, 0.3, 0.5, 0.6 | 0.25, 0.5 |
| Carteolol | 0.5, 1, 2 | – |
| α2 Agonists | ||
| Apraclonidine | 0.5 | 1 |
| Brimonidine | 0.2 | – |
| Sympathomimetics | ||
| Dipivefrin | 0.1 | – |
| Miotics | ||
| Pilocarpine | 0.5, 1, 2 | 1, 2 |
| Carbonic anhydrase inhibitors | ||
| Dorzolamide | 2 | – |
| Prostaglandin analogues | ||
| Latanoprost | 50 μg/ml | – |
Combination formulations (preserved form only): dorzolamide/timolol, pilocarpine/timolol, pilocarpine/carteolol.
Data were described by mean, standard deviation and range for quantitative variables, and by proportion for qualitative variables. At the first visit, a χ2 test was used to test for differences in prevalence of signs and symptoms between preserved (P) and preservative free (PF) eye drops. At the second visit, depending upon the change in treatment, patients were separated into subgroups and the results obtained at visits 1 and 2 were compared in order to assess the effect of these changes on ocular tolerance.
RESULTS
A total of 4107 patients were enrolled in 1999 by 249 ophthalmologists. Of this number, only 1181 patients needed to make a second visit.
At the first visit, patients were mainly women (58%) with a mean age of 66 years (SD 12, range 10–96). About 80% of patients were between the age of 50 and 80 years. The patients had been treated with eye drops for a median of 3.9 years (0–46). The majority of prescribed medications were preserved: 3469 (84%) patients used preserved (P) glaucoma eye drops, 552 (13%) received one preservative free (PF) medication, the remaining patients (3%) were treated with a combination of P and PF treatments. The majority of practitioners used both P and PF medications (79.1%) from time to time.
Of the 4107 patients surveyed at the first visit, 57% reported at least one symptom some time after instillation. Discomfort on instillation (40%) was the most commonly reported symptom followed by symptoms between instillations—burning and stinging (37%), foreign body sensation (28%), dry eye sensation (22%), tearing (20%), and eyelid itching (17%).
There were also objective signs of ocular irritation such as conjunctival hyperaemia (38%), conjunctival follicles (20%), and superficial punctate keratitis (18%).
Comparison at the first visit between P and PF eye drops showed that all symptoms occurred more frequently in patients treated with P eye drops than in those receiving PF eye drops (Table 2, p<0.001). Discomfort upon instillation was up to 2.5 times more prevalent in those using P eye drops: 43% versus 17% (p<0.001, χ2 test). A difference in incidence of symptoms favouring PF drops (p<0.001, χ2 test) was found for every symptom (Table 2).
Table 2.
Frequency of symptoms reported by patients treated with preserved and preservative free eye drops at the first visit
| Preserved eye drops (n=3469) | Preservative free eye drops (n=552) | |
| Discomfort upon instillation | 43% | 17%* |
| Foreign body sensation | 31% | 14%* |
| Stinging or burning sensation | 40% | 22%* |
| Dry eye sensation | 23% | 14%* |
| Tearing | 21% | 14%* |
| Eyelid itching | 18% | 10%* |
| Presence of symptoms of irritation between instillations | 61% | 36%* |
*Preservative free versus preserved comparison: p<0.001 (χ2 test).
Signs of ocular surface damage were also reported more frequently in patients treated with P eye drops than in patients using PF eye drops (Table 3, p<0.001). The presence of at least one conjunctival sign was reported in close to half of the patients treated with preserved eye drops but only a quarter of those treated with PF eye drops. For the cornea, the prevalence either of mild or severe superficial punctate keratitis was significantly higher in patients treated with preserved eye drops. The frequency of blepharitis and eczema was also significantly higher in patients treated with P eye drops. In particular, the frequency of eczema of the eyelids, while remaining low, was markedly increased (3.6-fold) in patients treated with P eye drops.
Table 3.
Frequency of ocular signs in patients treated with preserved and preservative free eye drops at the first visit
| Preserved eye drops (n=3469) | Preservative free eye drops (n=552) | |
| Presence of conjunctival signs | 49% | 26%* |
| Conjunctival redness | 41% | 20%* |
| Conjunctival follicles | 22% | 11%* |
| Fluorescein staining in the nasal bulbar conjunctiva | 13% | 5%* |
| Presence of an SPK | 19% | 9%* |
| Superficial punctate keratitis | ||
| Mild | 17% | 8.9%* |
| Severe | 2% | 0.6%* |
| Presence of at least one palpebral sign | 22% | 9%* |
| Anterior blepharitis | 16% | 7%* |
| Posterior blepharitis (meibomiitis) | 7% | 3%* |
| Eczema | 6% | 1%* |
*Preservative free versus preserved comparison: p<0.001 (χ2 test).
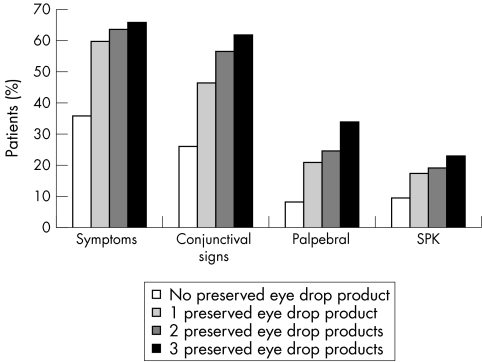
Frequencies of the symptoms and objective signs (conjunctival, corneal, or palpebral signs) increased as a function of the number of P eye drops used by the patient (Fig 1).
Figure 1.
Prevalence of signs and symptoms—number of preserved eye drops used at the first visit.
Of the patients enrolled, 1181 patients needed to make a second visit for a medical reason. They had the same demographic and pathological characteristics as the entire initial population. The mean interval between the two visits was 4 months.
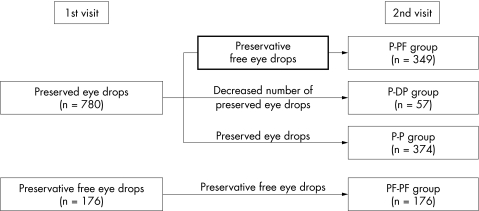
The precise number of eye drops used was recorded in 956 patients. These patients could be classified and analysed in four main subgroups according to the change of their treatment after the first visit (Fig 2).
Figure 2.
Treatment modification after the first visit (n=956).
For 349 patients, treatment was changed from one or more P eye drops to exclusively PF eye drops at the end of visit 1 as in this group signs and symptoms of ocular irritation were high at visit 1 (group P-PF). For these patients, the frequencies of all signs and symptoms were markedly decreased at visit 2 (Table 4). The prevalence of all symptoms decreased 2.7-fold to 5.2-fold. The most frequently observed sign, conjunctival hyperaemia, decreased from 61% to 16%. All these changes were statistically significant (p<0.001).
Table 4.
Frequency of symptoms and signs at visits 1 and 2 in P-PF group
| Visit 1 (preserved) | Visit 2 (preservative free) | ||||
| No* | (%) | No* | (%) | p Value | |
| Patient symptoms | |||||
| Discomfort upon instillation | 196/340 | 57.6% | 40/343 | 11.7% | <0.001 |
| Patients presenting with at least one symptom between instillations | 283/342 | 82.7% | 123/344 | 35.8% | <0.001 |
| Ocular signs found at the clinical examination (patients presenting with at least one) | |||||
| Palpebral sign | 122/342 | 35.7% | 50/346 | 14.5% | <0.001 |
| Conjunctival sign | 233/338 | 68.9% | 74/338 | 21.9% | <0.001 |
| Superficial punctate keratitis | 85/334 | 25.4% | 18/337 | 5.3% | <0.001 |
*Number of patients for which the variable had been recorded.
For 57 patients treated with preserved eye drops before the first visit, the number of preserved eye drop products was reduced but at least one preserved eye drop product continued to be used (group P-DP). In this group, the frequency of the signs and symptoms of ocular irritation was high at visit 1. At visit 2, the proportion of patients reporting symptoms decreased as did objective signs between the two visits (Table 5, p<0.001).
Table 5.
Frequency of symptoms and signs at visit 1 and visit 2 in P-DP group
| Visit 1 (preserved) | Visit 2 (preservative free) | ||||
| No* | (%) | No* | (%) | p Value | |
| Patient symptoms | |||||
| Discomfort upon instillation | 28/57 | 50.9% | 8/56 | 14.3% | <0.001 |
| Patients presenting with at least one symptom between instillations | 48/57 | 84.2% | 31/57 | 54.4% | <0.001 |
| Ocular signs found at the clinical examination (patients presenting with at least one) | |||||
| Palpebral signs | 25/57 | 43.9% | 7/57 | 12.3% | <0.001 |
| Conjunctival signs | 44/56 | 78.6% | 25/57 | 43.9% | <0.001 |
| Superficial punctate keratitis | 13/57 | 22.8% | 5/56 | 8.9% | <0.001 |
*Number of patients for which the variable had been recorded.
In the two groups of patients (PF-PF and P-P), whose treatment was not modified, there was no change in the frequency of symptoms and objective signs of eye irritation at the second visit (p>0.05 for all criteria).
DISCUSSION
Results of this survey show a high prevalence of ocular surface impairment in glaucomatous patients in daily ophthalmological practice. To the best of our knowledge, no other survey on both symptoms experienced and ocular changes with topical medication in glaucomatous patients is available for comparison with these results.
Van Beek et al40 conducted a prospective observational study examining the side effects of β blockers used in glaucoma therapy in general ophthalmological practice. They recorded a small number of ocular side effects (34 defined cases, 1.51 cases per 1000 patient years). Interestingly, the nature of these reactions was similar to those reported in our study—periorbital dermatitis or blepharitis, conjunctivitis, conjunctival hyperaemia, and punctate keratitis. It is important to note that van Beek et al sought only cases where the ophthalmologist altered the medication because of side effects. It is likely that ocular irritation and subtle signs of ocular damage were not detected by this method. Our findings would cast doubt on the observation that topical β blocker therapy is associated with few clinically important side effects.40 This survey shows that ocular surface impairment is not a marginal phenomenon in glaucoma patients but rather occurs in a large number of patients and therefore constitutes a real healthcare concern.
One of the merits of this study is that it covered a large population examined by close to 250 ophthalmologists in France. In general, the majority of medications for glaucoma therapy were preserved. However, most practitioners used both preserved and preservative free medications, limiting the bias of individual ophthalmologists. There were, however, some limitations inherent to this observational study. It is possible that patients will not show interest in participating in the clinical trial if they have no concerns about their medications, thereby biasing the sample to problematic patients. However, this effect would be minimal as participating in the study would not constitute significant inconvenience or impact their treatment. Also, unlike prospective clinical trials, the drug types used and the dosages are not controlled. Treatment regimens could be classified into groups of sufficient sample size to allow meaningful statistical comparison.
Unavoidably, the patients and the investigators were not masked as to the type of drop they were using (preserved or preservative free). Those who were aware of changing to a preservative free medication may have felt encouraged to report improved symptoms. However, the positive response to a change to preservative free medication was supported by the observations at the first visit where medication had not been altered. In both instances patients who used preservative free medications had fewer symptoms and this was corroborated by less frequent signs of ocular surface irritation.
The demographic characteristics of the glaucomatous patients are similar to those found in other surveys in France.41 Moreover, the fact that almost half the patients included reported symptoms between instillations is in agreement with the study conducted by De Jong et al, in which 10 out of 21 patients treated with preserved eye drops presented some signs of ocular irritation.6 In addition, the presence of symptoms and of objective signs of irritation was correlated.
This epidemiological study has demonstrated that ocular irritation is a very common condition in glaucomatous patients treated with eye drops. Even if no data are available on the ocular state of control patients of the same age without treatment, the striking difference in the frequency of symptoms between patients treated with preserved eye drops and preservative free eye drops is greatly in favour of the hypothesis that this is not a common natural feature in patients of that age.
The frequency of both symptoms and objective signs of ocular surface irritation was higher in patients treated with preserved eye drops compared to preservative free eye drops. A comparable conclusion was drawn by Höh.42 In a study by Zimmerman (cited by Höh42), the frequency of eye irritation after a few weeks of treatment was higher with preserved (29%) than with preservative free (10%) eye drops. The results of this survey show that use of preserved eye drops greatly increases the frequency of ocular irritation in glaucoma patients. Moreover, the frequency of signs and symptoms is correlated with the number of preserved eye drops used. This is in agreement with reports in the literature, which showed increased ocular damage with higher doses.23 It is acknowledged that while information about patient compliance with a prescribed medication regimen was not collected, non-compliance was likely to be distributed evenly throughout the patients assessed.
In this survey, a change from a preserved to preservative free glaucoma eye drop, or even a reduction in the number of preserved eye drops used is associated with a significant decrease in the frequency of signs and symptoms of ocular irritation. This also shows that preservative adverse reactions are reversible and that removing preservatives is of benefit to glaucoma patients. Similar findings are reported in the literature.6,40 De Jong et al's study included 21 glaucomatous patients treated with eye drops containing benzalkonium chloride.6 Withdrawal of the preservative led to partial normalisation of the permeability of the corneal epithelium and to decrease or disappearance of the symptoms in eight of the 10 patients complaining of a sensation of burning or dry eye. Moreover, Gordon observed the disappearance of corneal signs after discontinuation of a treatment by eye drops in 22 of 65 patients (34%) presenting with superficial punctate keratitis of unknown origin.43 An improvement in symptoms after withdrawal of the preservative was also observed in the management of dry eye syndrome,44–46 a pathology frequently found in glaucomatous patients.47
The toxic effect of preservatives is very rapid in vitro: 0.007% benzalkonium chloride induces the lysis of 50% of cultured epithelial cells in less than 2 minutes.48 The damage to the ocular surface observed during the treatment with preserved antiglaucoma eye drops probably reflects an imbalance between mucosal regeneration and daily low grade cytotoxicity of the preservative. This intolerance and repeated toxic impairment of the ocular surface may further result in chronic inflammation and conjunctival infiltration by inflammatory cells.27 The conjunctival epithelium is a very reactive tissue on which apoptotic (that is, drug induced) and immune (that is, cytokine mediated) phenomena are closely correlated.49–51 Hence, the disappearance of the signs of superficial irritation within a few weeks following preservative withdrawal is logical and could explain the results of this epidemiological survey.
Therapeutic habits in glaucoma treatment have changed and ophthalmologists prescribe preservative free β blockers more frequently. In this survey preservative free glaucoma medication were in use in 15% of patients and an additional 18% were changed to PF drops at a follow up visit.
In the present study, nearly half the patients experienced symptoms of ocular irritation with their glaucoma medication corroborated by observation of objective signs of ocular surface irritation. A large proportion of these symptoms could be ascribed to preservatives as demonstrated by previous studies, and strongly supported by this survey. The phenomenon was frequent and not limited to a small category of allergic patients. It is probably due to a direct toxic effect on eye structures, as widely demonstrated in animals and in vitro experiments. Moreover, the toxicity of preserved eye drops is strongly suspected to impair the efficacy of subsequent surgery for the glaucoma, which constitutes a real healthcare concern.
The exclusive use of preservative free eye drops or even a reduction of the number of preserved eye drops used clearly reduces the signs of ocular surface irritation in glaucoma patients. Overall, preservative free eye drop products have a significant medical advantage.
Acknowledgments
We would like to thank Mrs Agnés Lanoue and Mr Sylvain Bouton for their help during this investigation.
REFERENCES
- 1.Bohn RL, Gurwitz JH, Yeomans SM, et al. Which patients are treated for glaucoma? An observational analysis. J Glaucoma 2000;9:38–44. [DOI] [PubMed] [Google Scholar]
- 2.Wilcon LA. To preserve or not to preserve, is that the question? Br J Ophthalmol 1996;80:583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Saint Jean M, Debbasch C, Brignole F, et al. Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr Eye Res 2000;20:85–94. [DOI] [PubMed] [Google Scholar]
- 4.Burstein NL. Corneal cytotoxicity of topically applied drugs, vehicles and preservatives. Surv Ophthalmol 1980;25:15–30. [DOI] [PubMed] [Google Scholar]
- 5.Burstein NL. The effects of topical drugs and preservatives on the tears and corneal epithelium in dry eye. Trans Ophthalmol Soc UK 1985;104:402–9. [PubMed] [Google Scholar]
- 6.De Jong C, Solwijk T, Kuppens E, et al. Topical timolol with and without benzalkonium chloride; epithelial permeability and autofluorescence of the cornea in glaucoma. Graefes Arch Clin Exp Ophthalmol 1994;232:221–4. [DOI] [PubMed] [Google Scholar]
- 7.Mietz H, Niesen U, Krieglstein GK. The effect of preservatives and antiglaucomatous medication on the histopathology of the conjunctiva. Graefes Arch Clin Exp Ophthalmol 1994;232:561–5. [DOI] [PubMed] [Google Scholar]
- 8.Pisella P J, Fillacier K, Elena P, et al. Comparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbits. Ophthalmic Res 2000;32:3–8. [DOI] [PubMed] [Google Scholar]
- 9.Baudouin C, de Lunardo C. Short term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol 1998;82:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norn MS, Opauszki A. Effects of ophthalmic vehicles on the stability of the precorneal tear film. Acta Ophthalmol 1977;55:23–32. [DOI] [PubMed] [Google Scholar]
- 11.Yalvaç IS, Gedikog̃lu G, Yaragöz Y, et al. Effects of antiglaucoma drugs on ocular surface. Acta Ophthalmol Scand 1995;73:246–8. [DOI] [PubMed] [Google Scholar]
- 12.Kuppens EVMJ, de Jong CA, Stolwijk TR, et al. Effect of timolol with and without preservative on the basal tear turnover in glaucoma. Br J Ophthalmol 1995;79:339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson WS, Duncan AJ, Jay JL. Effect of benzalkonium chloride on the stability of the precorneal tear film in rabbit and man. Br J Ophthalmol 1975;59:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herreras JM, Pastor JC, Calonge M, et al. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology 1992;99:1082–8. [DOI] [PubMed] [Google Scholar]
- 15.Rolando M, Brezzo V, Giordano G, et al. In: van Bijsterveld OP, Lemp MA, Spinelli D, eds. Symposium on the lacrimal system. Singapore, March 1990. Amsterdam: Kugler & Ghedini, 1991:87–91.
- 16.Holly FJ. Tear film formation and rupture: an update. In: Holly FJ, ed. The precorneal tear film in health diseases and contact lens wear. Dry Eye Institute Inc, Lubbock, 1986:634–45.
- 17.Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and Chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci 1980;19:308–13. [PubMed] [Google Scholar]
- 18.Marquardt R, Schubert T. Beeinflussung der Tränenfilmaufreisszeit (BUT) durch Betablocker-Augentropfen ohne Konservierungstoffe. Klin Monatsbl Augenheilkd 1991;199:75–8. [DOI] [PubMed] [Google Scholar]
- 19.Grant RL, Acosta D. Prolonged adverse effects of benzalkonium chloride and sodium dodecyl sulfate in a primary culture system of rabbit corneal epithelial cells. Fundam Appl Toxicol 1996;33:71–82. [DOI] [PubMed] [Google Scholar]
- 20.Baudouin C, Garcher C, Haouat N, et al. Expression of inflammatory membrane markers by conjunctival cells in chronically treated patients with glaucoma. Ophthalmology 1994;101:454–60. [DOI] [PubMed] [Google Scholar]
- 21.Aritük N, Öge I, Baris S, et al . The effects of antiglaucomatous agents on conjunctiva used for various durations. Int Ophthalmol 1997;20: 57–62. [DOI] [PubMed] [Google Scholar]
- 22.Brandt JD, Wittpen JR, Katz LJ, et al . Conjunctival impression cytology in patients with glaucoma using long term topical medication. Am J Ophthalmol 1991;112:297–301. [DOI] [PubMed] [Google Scholar]
- 23.Becquet F, Goldschild M, Moldovan MS, et al . Histopathological effects of topical ophthalmic preservatives on rat corneoconjunctival surface. Curr Eye Res 1998;17:419–25. [DOI] [PubMed] [Google Scholar]
- 24.Williams DE, Nguyen KD, Shapourifar-Tehrani S, et al . Effects of timolol, betaxolol, and levobunolol on human Tenon's fibroblasts in tissue culture. Invest Ophthalmol Vis Sci 1992;33:2233–41. [PubMed] [Google Scholar]
- 25.Broadway DC, Grierson I, O'Brien C, et al. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol 1994;112:1437–45. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood MB, Grierson I, Millar L, et al. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon's capsule in glaucomatous patients. Ophthalmology 1989;96:327–35. [DOI] [PubMed] [Google Scholar]
- 27.Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs. Human and animal studies. Ophthalmology 1999;106:556–63. [DOI] [PubMed] [Google Scholar]
- 28.Kawa JE, Higginbotham EJ, Chang IL, et al. Effects of antiglaucoma medications on bovine trabecular meshwork cells in vitro. Exp Eye Res 1993;57:557–65. [DOI] [PubMed] [Google Scholar]
- 29.Lavin MJ, Wormald RP, Migdal CS, et al. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol 1990;108:1543–8. [DOI] [PubMed] [Google Scholar]
- 30.Longstaff S, Wormald RPL, Mazover A, et al. Glaucoma triple procedures: efficay of intraocular pressure control and visual outcome. Ophthalmic Surg 1990;21:786–93. [PubMed] [Google Scholar]
- 31.Miller MH, Rice NS. Trabeculectomy combined with irradiation for congenital glaucoma. Br J Ophthalmol 1991;75:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva Br J Ophthalmol 1993;77:590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baudouin C. Side effects of antiglaucomatous drugs on the ocular surface. Curr Opin Ophthalmol 1996;7:80–6. [DOI] [PubMed] [Google Scholar]
- 34.Wilson FM. Adverse external ocular effects of topical ophthalmic medications. Surv Ophthalmol 1979;24:57–88. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs T, Meinert A, Aberer W, et al. Benzalkonium chloride—a relevant contact allergen or irritant? Results of a multicentre study of the German Contact Allergy Group. Houtzart 1993;44:699–702. [PubMed] [Google Scholar]
- 36.Goh CL. Contact sensitivity to topical antimicrobials. II. Sensitising potentials of some topical antimicribials. Contact Dermatitis 1989;21:166–71. [DOI] [PubMed] [Google Scholar]
- 37.Fiore PM, Jacobs IH, Goldberg DB. Drug-induced pemphigoid. A spectrum of diseases. Arch Ophthalmol 1987;105:1660–3. [DOI] [PubMed] [Google Scholar]
- 38.Bernauer W, Broadway DC, Wright P. Chronic progressive conjunctival cicatrisation. Eye 1993;7:371–8. [DOI] [PubMed] [Google Scholar]
- 39.Levrat F, Pisella PJ, Baudouin C. Tolérance clinique des collyres antiglaucomateux conservés et non conservés J Fr Ophtalmol 1999;22:186–91. [PubMed] [Google Scholar]
- 40.Van Beek LM, de Keizer RJ, Polak BC, et al. Incidence of ocular side effects of topical β blockers in the Netherlands. Br J Ophthalmol 2000;84:856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bour T, Blanchard F, Segal A. Données épidémiologiques sur le GPAO et son traitement dans la Marne. J Fr Ophtalmol 1993;16:367–79. [PubMed] [Google Scholar]
- 42.Höh H. Nicht konserviert Timolol-Augentropfen—Lokalanästhesische Wirkung und subjektive Verträglichkeit. Ophthalmologica 1990;200:89–97. [DOI] [PubMed] [Google Scholar]
- 43.Gordon J, Johnson PJ. Fibronectin Study Group—discontinuation of topical ophthalmic medications promotes healing of non-healing corneal epithelial defects. Invest Ophthalmol Vis Sci 1991;32:1071. [Google Scholar]
- 44.Brewitt H, Joost P. Klinische Studie zur Wirksamkeit eines nicht konservierten Tränenersatzmittels. Klin Monatsbl Augenheilkd 1991;199:161–4. [DOI] [PubMed] [Google Scholar]
- 45.Caffery BE, Josephson JE. Is there a better “comfort drop”? J Am Optom 1990;61:178–82. [PubMed] [Google Scholar]
- 46.Laflamme MY, Swieca R. A comparative study of two preservative-free tear substitutes in the management of severe dry eye. Can J Ophthalmol 1988;23:174–6. [PubMed] [Google Scholar]
- 47.Liotet S, Van Bijsterveld OP, Blétry O, et al. In: Rapport Soc Fr Ophtalmol Paris 1987:365–73.
- 48.Takahashi N. Quantitative cytotoxicity of preservatives evaluated in cell culture with Chang's human conjunctival cells. Effects of temperature on cytotoxicity. Jpn J Ophthalmol 1982;26:234–8. [PubMed] [Google Scholar]
- 49.Brignole F, De Saint Jean M, Goldschild M, et al. Expression of Fas-Fas ligand antigens and apoptotic marker APO2.7 by the human conjunctival epithelium. Positive correlation with class II HLA DR expression in inflammatory ocular surface disorders. Exp Eye Res 1998;67:687–97. [DOI] [PubMed] [Google Scholar]
- 50.Bourcier T, De Saint Jean M, Brignole F, et al. Expression of CD40 and CD40 ligand in human conjunctival epithelium. Invest Ophthalmol Vis Sci 2000;41:120–6. [PubMed] [Google Scholar]
- 51.De Saint-Jean M, Brignole F, Bringuier AF, et al. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci 1999;40:619–30. [PubMed] [Google Scholar]