Abstract
Aim: To study the histological effects of cyclodiode laser treatment in humans, and to compare these findings with the clinical course, treatment response, complications, and indications for enucleation.
Method: Detailed histological examination of nine enucleation specimens was undertaken in conjunction with a retrospective review of patient case notes.
Results: Retreatments had been undertaken in three cases. Although all globes showed damage to pars plicata, intact ciliary processes within the treatment zone were present in all cases. Pars plana injury was also noted in two thirds of cases. Inflammation was mild. Ciliary epithelial proliferation was seen in most cases with increasing time following treatment, in a disorganised pattern, without replication of the ciliary epithelial bilayer. No regeneration of the ciliary processes with fibrovascular cores was found. The three patients with good IOP control at enucleation had all had multiple diode treatments. Neither phthisis nor sympathetic ophthalmia was seen.
Conclusions: Diode laser cyclophotocoagulation produces very characteristic injury to pars plicata, which frequently extends into pars plana, but with only mild persisting inflammation. Ciliary processes are, however, frequently spared within the treatment zone and may account for early or late treatment failure.
Keywords: cyclodestructive procedures, cyclodiode, cyclophotocoagulation, enucleation, glaucoma
Cyclodestructive therapies in the past have included cyclocryotherapy, which is recognised to have significant morbidity. This includes phthisis in up to 12% of treated eyes,1 retinal detachment,2 deterioration in visual acuity in 60%,1 sympathetic ophthalmia,3–5 inflammation that is difficult to control,6 subretinal fibrosis,7 lens subluxation,8 anterior segment necrosis,9–11 neurotrophic corneal ulcer, and cataract. Cyclocryopexy has a spot size of 7–8 mm because of the spreading effect, compared with 1 mm for laser.12
Neodymium:YAG (Nd:YAG) laser cyclophotocoagulation has not been without complications, including sympathetic ophthalmia13,14 and retinal detachment.15 More recently, transscleral diode laser cyclophotocoagulation (“cyclodiode”), which appears to be better tolerated with less inflammation, has been used in refractory advanced glaucoma.16–19 The Diode Laser Ciliary Ablation Study Group reported a cumulative probability of success (as defined by greater than 20% reduction of IOP from baseline or IOP <22 mm Hg) of 72% at 1 year and 52% at 2 years in patients with severe recalcitrant glaucoma.16 However, some morbidity has been recorded including neurotrophic corneal defects20 and scleral perforation.21
Comparative clinical studies have been performed in humans22,23 and rabbits.24 Histopathological studies of enucleation specimens following laser cyclophotocoagulation (diode and Nd:YAG laser) and cyclocryotherapy have been performed in humans25–29 and animals.12,30–35 We have previously reported histopathology of two cases of clinical failure following diode laser cyclophotocoagulation.36 This study examines histological outcomes in nine cases in humans, and correlates this with the clinical course in each case.
METHODS
A retrospective study of case notes was undertaken, in conjunction with detailed histological examination of eyes in which cyclodiode treatment had been performed and which had subsequently required enucleation. Details of clinical course, treatment complications, and indication for enucleation were noted. These data were compared with histological findings and an attempt was made to deduce clinicopathological correlates.
A standard procedure for examination of globes was followed. The superior calotte was removed from the globe, and the opened globe was examined under a dissecting microscope. The globe was then processed and was serially sectioned though the globe including pupil optic nerve sections. In all cases, at least 12 serial sections were examined and in some globes up to 20. Once exact treatment zones were determined from chart review, sections were correlated with the clock hours of treated ciliary body and attempt made to assess damage in treated versus untreated zones. Histological damage to pars plicata was graded as mild (≤⅓ of processes destroyed), moderate (>⅓–⅔ of processes destroyed) or severe (>⅔ to total destruction) in the area of treatment. Damage to pars plana was graded according to the extent of epithelial injury with mild (≤⅓ of epithelium destroyed), moderate (>⅓–⅔ of epithelium destroyed) or severe (>⅔ to total destruction).
RESULTS
The globes from nine enucleations of patients who had received cyclodiode laser were studied. Details are listed in Table 1. All patients were white with four females and five males. Age ranged from 31–80 years (median 65 years). All patients received diode laser therapy (Oculight SLx laser and G-probe, Iris Medical Instruments, Mountain View, CA, USA) with three patients receiving two or more treatments (Table 1). Diode therapy ranged from 180°–360° with power ranging from 1500 mW to 2200 mW and application duration ranging from 1500 to 2100 ms (Table 1). The time to enucleation ranged from 2 weeks to 4 years 1 month after diode (median 7 months). Causes of glaucoma were neovascular glaucoma in four, chronic open angle glaucoma in one, chronic angle closure following penetrating keratoplasty in one, epithelial downgrowth in two, and absolute pseudoexfoliation glaucoma in one.
Table 1.
Clinical features and histopathology of globes with diode laser cyclophotocoagulation
| Case | Age/ sex | Cause of glaucoma | Type of treatment | Time of treatment prior to enucleation | Clinical response* | Pars plicata injury | Pars plana injury | Other complications | Phthisis | Reason for enucleation |
| 1 | F/80 | Neovascular (CRVO) | Diode 360° 40 × 1500 ms × 1500 mW |
8 months | IOP 78→22 Late rise →48 |
Moderate | Not affected | – | No | Perforated microbial keratitis; high IOP |
| 2 | F/64 | Absolute PXF | Diode 360° 40 × 1500 ms × 1500 mW |
5 months | IOP 44→19 IOP 34 @ 4 weeks IOP 50 @ 6 weeks IOP 40 @ 4 weeks |
Moderate to severe | Severe | – | No | Blind, painful, high IOP |
| 3 | F/64 | Epithelial downgrowth post PK ×2; sutured PCIOL | Diode 180° 4–10 o'clock: 23 burns × 1500 ms × 1500 mW | 12 months | IOP 56→10 Rise to 22–30 @ 4 months |
Severe | Mild | – | No | Recurrent high IOP, rapidly enlarging cystic bleb |
| 4 | M/65 | Chronic angle closure glaucoma after penetrating keratoplasty. | Diode ×3 #1 270° 12–9 o'clock, 30 × 15002 † #2 180° 10–4 o'clock, 20 × 15002; #3 180° 3–9 o'clock, 2000 mW × 1500 ms |
2 years 1 month 1 year 7 months 11 months |
#1 no IOP change; #2 IOP 50→18, but PK failed, IOP→ 28 #3 IOP→17 |
Severe | Severe | Scleral damage | No | Painful, with unresolved inferior herpes simplex virus keratitis (IOP controlled) |
| 5 | M/71 | R COAG | Diode 270° 3–12 o'clock 2200 mW × 18 shots |
7 months | IOP 48→50 @ 3 and 4 months | Moderate to severe | Moderate | Necrosis of iris base, apposed to angle | No | High IOP Total hyphaema,-blood stained cornea |
| 6 | M/44 | Neovascular | Diode 270° 12–9 o'clock, 2000 ms × 1700–2000 mW |
4 months | IOP 42→20; IOP increased again to 48 @ 4 months |
Moderate | Not affected | Iris base necrosis Scleral damage | No | Blind, painful, total retinal detachment |
| 7 | M/74 | Neovascular post plaque for choroidal MM; failed L trab. | Diode 180° 10–4 o'clock; 20 × 15002 |
4 years 1 month | IOP response poor; 40+ at enucleation |
Mild | Not affected | – | No | Recurrent inflammation?active tumour, blind painful eye |
| 8 | F/31 | Neovascular, tractional retinal detachment (surgery including silicone oil), chronic uveitis | Diode × 4 #1 inf 180° 22 × 15002 #2 lat 180° 20 × 15002 #3 3–12 o/clock 1510mW × 2100 ms #4 360° 1750–2000 ms × 1500–2000 mW |
#1 6 months #2 4 months #3 6 weeks #4 2 weeks |
#1 26→21 #2 45→38→16 #3 38→11→40 #4 40→21→14 |
Moderate to severe | Mild | Iris necrosis; Scleral damage | No | Persisting pain despite IOP control |
| 9 | M/75 | R traumatic aphakia; epithelial downgrowth post 2° ACIOL |
Diode ×2 #1 Inf 180° 2000 mW × 1500 ms; #2 Nasal 180° 20002 |
#1 7 months>br>#2 5 months | IOP 42→16, but 35 @ 6 weeks; #2 Rx →IOP 14, 16 |
Moderate to severe | Mild | – | No | Persisting pain due to corneal decompensation despite IOP control |
*Clinical response is measured as IOP (mm Hg); †15002 = 1500 mW × 1500 ms. CRVO = central retinal vein occlusion, PXF = pseudoexfoliation, PK = penetrating keratoplasty, PCIOL = posterior chamber intraocular lens, COAG = chronic open angle glaucoma, MM = malignant melanoma, Trab = trabeculectomy, ACIOL = anterior chamber intraocular lens.
Pathology
Pars plicata
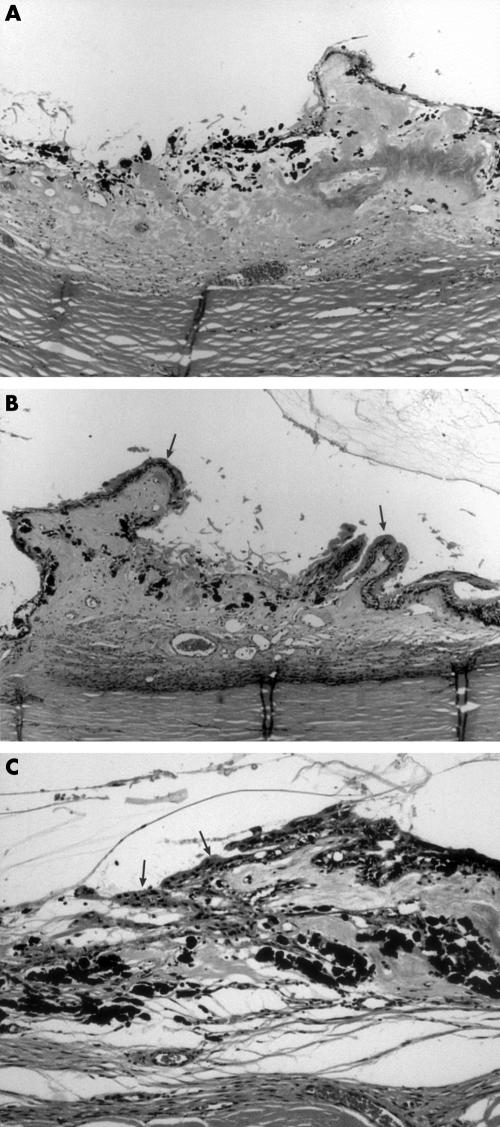
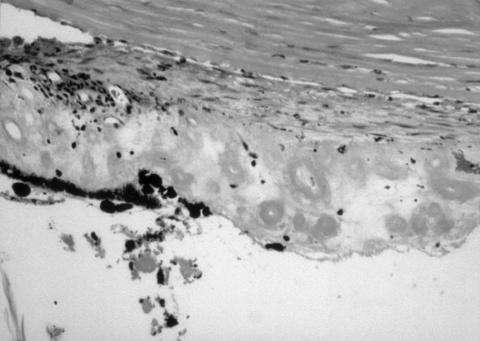
Pars plicata damage was seen in all nine patients. This injury was characteristic for diode laser allowing recognition of such treatment, even before the clinical history was known. The damage comprised destruction of pigmented and non-pigmented ciliary epithelium and capillaries in the ciliary processes with pigment clumping, coagulative necrosis, and extensive destruction of ciliary muscle with moderate reduction in vascularity (Fig 1A and B). Injury was severe in two, moderate to severe in four, moderate in two, and mild in one. All of the moderately to severely affected cases had unequal damage around the circumference of treated areas with some foci showing total loss of ciliary processes but other foci with some sparing of some ciliary processes anteriorly, posteriorly, or centrally (Fig 1B). In three cases, a small amount of residual outer longitudinal muscle was noted. Some proliferation of non-pigmented epithelium over the surface of the necrotic masses was seen (Fig 1C), but this was only mild in cases with short intervals to enucleation (<7 months). The proliferation was always disorganised with masses of flattened epithelium with no reconstitution of ciliary processes with fibrovascular core and normal bilayered epithelial architecture. Vessels were sparse in the damaged pars plicata even at over 4 years, the longest time since treatment.
Figure 1.
(A) Low power photomicrograph of pars plicata showing severe, total destruction of ciliary processes with pigment clumping, loss of vessels, and little epithelial regeneration in case 5, 7 months after diode treatment. Haematoxylin and eosin. Original magnification ×40. (B) Low power photomicrograph of pars plicata showing damaged central zone with sparing of anterior and posterior processes (arrows) in case 5, 7 months after diode treatment. Haematoxylin and eosin. Original magnification ×40. (C) Medium power photomicrograph of haphazardly regenerated non-pigmented epithelium (arrows) over damaged pars plicata with underlying pigment clumping in case 3, 12 months after diode treatment. Haematoxylin and eosin. Original magnification ×100.
Pars plana injury
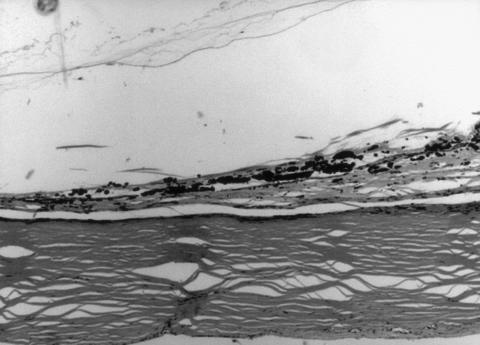
Pars plana injury was seen in six patients with complete sparing in three. Patient 2 has been previously reported by us.36 Damage consisted of loss of normal epithelium, pigment clumping, and destruction of muscle and most vessels. Two were graded as severe, one moderate, and three mild. In one of the severe cases (patient 2), the pars plana was extensively damaged (Fig 2) but adjacent pars plicata was totally spared over 180°. Some epithelial proliferation in disorganised masses was seen in cases with longer intervals to enucleation. The outer longitudinal muscle was spared in most cases.
Figure 2.
Low or medium power photomicrograph of severely damaged pars plana with no residual or regenerated epithelium and pigment clumping in case 2, 5 months after diode. Haematoxylin and eosin. Original magnification ×40.
Iris root
Iris root was damaged in three of the eight cases where this could be assessed (patient 1 lost iris tissue as a result of corneal perforation before enucleation). All patients with iris injury had neovascular glaucoma on histopathology, although one had a history of chronic open angle glaucoma (patient 5). The iris root for about 2 mm was totally necrotic with loss of iris pigment, muscle, and vessels (Fig 3) and this was contiguous with adjacent severe injury to pars plicata.
Figure 3.
Medium power photomicrograph of damaged iris base with no residual pigment epithelium, necrosis of stroma and vessels, adjacent to severe injury to pars plicata, in case 5, 7 months after diode. Haematoxylin and eosin. Original magnification ×100.
Sclera
Sclera in the path of the laser showed damage in three cases (patients 4, 6, and 8). Two of these patients had had multiple (three to four) diode treatments. The damaged sclera showed scarring with increased fibroblast density and increased vascularity in the area (Fig 4).
Figure 4.
Low power photomicrograph of scarred sclera (between arrowheads) with increased density of fibroblasts and new vessels overlying severely damaged pars plicata with total destruction of ciliary processes and pigment clumping in case 6, 4 months after diode treatment. Haematoxylin and eosin. Original magnification ×40.
Perivascular lymphocytes were seen in sclera and episclera, with pigment seen in macrophages around vessels in two patients. No difference in scleral pigmentation was evident between cases with and without scleral injury.
Inflammation
Inflammation in the ciliary body was only seen in a minority of cases (33%) (patients 3, 7, 8). In each case, this may have been due to confounding factors. The first had undergone penetrating keratoplasty, cataract extraction, and posterior chamber intraocular lens placement with moderate iridocyclitis related to the haptic and subsequent epithelial ingrowth. The second had had 131I plaque therapy for choroidal malignant melanoma (but with persisting intermittent episodes of clinically evident inflammation) and a later cataract extraction. Patient 8 had clinical chronic uveitis. Mild perivascular lymphocytic infiltration was noted in sclera and episclera in the three patients with scleral injury. The remaining patients had no inflammation in the uvea or sclera.
Phthisis
None of the globes was phthisical.
Correlation of number of treatments and power with IOP at time of enucleation
The three patients with normal IOP at enucleation had all had multiple treatments with at least one of these at higher power (2000 mW). The other six patients with high IOP at enucleation had a single treatment, with power of 1500 mW in four and higher power (1700–2200 mW) in the other two.
DISCUSSION
This study undertakes retrospective review of nine globes enucleated following cyclodiode procedures. Most of these eyes were removed because of problems directly related to raised IOP. This creates a potential for selection bias in this series, in that the eyes examined are often treatment failures, and their histopathological findings may be different from those from the successful eyes, which are not available to examine. However, at least three eyes with diode therapy (Nos 4, 8, and 9) in this study had successful control of IOP at enucleation, and the pathological findings in these cases are not qualitatively different from the other six diode cases (Table 1).
Cyclodiode produces very characteristic injury with focal damage and minimal inflammation. Pars plicata is usually severely affected with non-specific destruction of ciliary pigmented and non-pigmented epithelium and capillaries in the ciliary processes. This damage to the ciliary processes is presumed to result in reduced aqueous production and lowering of IOP. However, loss of ciliary processes is often incomplete, with sparing of some processes within the field of treatment. This may explain both a persistently elevated IOP in some patients, and that control may be achieved with retreatment, by filling in treatment gaps. The sparing of processes is presumably a function of spot size and recommended spacing of burns with the standard footplate.
Some epithelial regeneration in pars plicata was noted after diode therapy, and was more marked with increasing time since treatment. However, this epithelium, which was always disorganised and lacked a well developed pigment epithelial layer, may have been non-functional from a secretory viewpoint, as the normal architecture of ciliary processes with typical bilayered epithelium and fibrovascular core was never restored. Despite initial control of IOP, a late rise in IOP was seen in a number of cases (1, 2, 6, 8, 9) some months after treatment. In all of these cases, there were foci of spared ciliary processes within the treatment areas, when multiple sections of the globe were examined, and even circumferential treatments spared some ciliary processes (cases 1, 2, 8). It is interesting to note that the three cases with well controlled IOP at enucleation (cases 4, 8, and 9) all had more than one, and in one case four, diode laser treatments, including higher power for at least one of the retreatments. However, even these cases showed some sparing of ciliary processes.
Pars plana is affected in two thirds of diode cases (6/9), and severely in 20%. Damage to pars plana was unintentional given the footplate design of the G-probe, which matches the curve of the globe, abutting the limbus to centre the fibre optic 1.2 mm behind the limbus. However, the site of the ciliary body is not uniform for all eyes, and the position in relation to the limbus may vary with axial length.37 We have previously reported a case of pars plana injury due to diode laser36 and have proposed that an increase in uveoscleral outflow may explain a good clinical IOP lowering response despite treatment delivered to pars plana alone.36,38
The characteristic early clinical response to diode laser cyclophotocoagulation involves a profound decrease in IOP within the first few days.39 The origin of this phenomenon is unexplained, but it has been postulated that early inflammation may be responsible for the decrease, although the clinical level of inflammation is routinely low. Only one case of early post-treatment enucleation was examined in our study—case 8, at 2 weeks—and the level of inflammation was slight despite good IOP control, although admittedly after repeated treatments.
Almost all of the reported experimental studies of cyclophotocoagulation in animals have involved Nd:YAG laser rather than diode laser.12,30–35 The study of Brancato and colleagues of diode laser in rabbits examined the treated eyes at only one time point, 24 hours after treatment,32 whereas we studied eyes enucleated at longer intervals of 2 weeks to 4 years after cyclodestruction. The major pathology in rabbit eyes at 24 hours was coagulative necrosis of epithelium and stroma of pars plicata as well as vascular congestion and thrombosis. This correlates well with the later stages of loss of ciliary processes with pigment clumping and depletion of vessels seen in our human cases.
Injury to other ocular tissues with diode laser cyclophotocoagulation is limited. Iris base contiguous with damaged pars plicata was damaged in three patients with neovascular glaucoma and may have been related to poor diode footplate positioning, the effect perhaps enhanced by energy absorption in adjacent hyphaema (case 5). It is unlikely that this iris necrosis relates to an acute episodic rise in IOP, since the pupillary margin, which is at the end of the circulation, is usually damaged in that instance, rather than iris root. Scleral damage was noted in only three cases (33%). These three had all received high energy or repeat treatments, although the case with four treatments (case 8) showed only mild scleral changes.
Histological examination in this series of patients with refractory glaucoma and enucleation has shown that diode laser is associated with much less long term inflammation of intraocular structures than Nd:YAG laser or cryotherapy6,26–29 (and unpublished observations). As a more focal cyclodestructive technique, contact diode laser treatment results in preservation of some ciliary processes within the treatment zone. This may contribute to an explanation of both a lack of IOP response in some cases, or a late recrudescence of elevated IOP in others. Of other potential causes of IOP recovery, renewed function of secretory ciliary epithelium in areas of damage appears unlikely on histological appearances, and recovery of vascular elements is sparse and inconsistent. Histology is not helpful in determining whether variations in uveoscleral outflow or early suprachoroidal cleft formation occur and contribute to IOP changes. Evidence of sympathetic ophthalmia or phthisis was not seen in this series.
Footnotes
The authors have no proprietary interest related to this paper.
REFERENCES
- 1.Brindley G, Shields MB. Value and limitation of cyclocryotherapy. Graefes Arch Clin Exp Ophthalmol 1986;224:545–8. [DOI] [PubMed] [Google Scholar]
- 2.Wagle NS, Freedman SF, Buckley EG, et al. Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. Ophthalmology 1998;105:1921–6. [DOI] [PubMed] [Google Scholar]
- 3.Sabates R. Choroiditis compatible with the histopathologic diagnosis of sympathetic ophthalmia following cyclocryotherapy of neovascular glaucoma. Ophthalmic Surg 1988;19:176–82. [PubMed] [Google Scholar]
- 4.Harrison TJ. Sympathetic ophthalmia after cyclocryotherapy of neovascular glaucoma without ocular penetration. Ophthalmic Surg 1993;24:44–6. [PubMed] [Google Scholar]
- 5.Biswas J, Fogla R. Sympathetic ophthalmia following cyclocryotherapy with histopathologic correlation. Ophthalmic Surg Lasers 1996;27:1035–8. [PubMed] [Google Scholar]
- 6.Hurvitz LM, Spaeth GL, Zakhour I, et al. A comparison of flurbiprofen, dexamethasone and placebo on cyclocryotherapy-induced inflammation. Ophthalmic Surg 1984;15:394–9. [PubMed] [Google Scholar]
- 7.Kao SF, Morgan CM, Bergstrom TJ. Subretinal fibrosis following cyclocryotherapy. Case report. Arch Ophthalmol 1987;105:1175–6. [DOI] [PubMed] [Google Scholar]
- 8.Pearson PA, Baldwin LB, Smith TJ, Lens subluxation as a complication of cyclocryotherapy. Ophthalmic Surg 1989;20:445–56. [PubMed] [Google Scholar]
- 9.Sharp DC, Bell RA, Cruess AF. Anterior segment necrosis following cryotherapy. Can J Ophthalmol 1982;17:268–70. [PubMed] [Google Scholar]
- 10.Krupin T, Johnson MF, Becker B. Anterior segment ischaemia after cyclocryotherapy. Am J Ophthalmol 1977;84:426–8. [DOI] [PubMed] [Google Scholar]
- 11.Krupin T, Mitchell KB, Becker B. Cyclocryotherapy in neovascular glaucoma. Am J Ophthalmol 1978;86:24–6. [DOI] [PubMed] [Google Scholar]
- 12.Schubert HD, Federman JL. A comparison of CW ND:YAG contact trans-scleral cyclophotocoagulation with cryopexy. Invest Ophthalmol Vis Sci 1989;30:536–47. [PubMed] [Google Scholar]
- 13.Edwards DP, Brown SV, Higginbotham E, et al. Sympathetic ophthalmia following neodymium:YAG cyclotherapy. Ophthalmic Surg 1989;20:544–6. [PubMed] [Google Scholar]
- 14.Bechrakis NE, Muller-Stolzenburg NW, Helbig H, et al. Sympathetic ophthalmia following laser cyclocoagulation. Arch Ophthalmol 1994;112:80–4. [DOI] [PubMed] [Google Scholar]
- 15.Geyer O, Neudorfer M, Lazar M. Retinal detachment as a complication of neodymium:yttrium aluminium garnet laser cyclophotocoagulation. Ann Ophthalmol 1993;25:170 –2. [PubMed] [Google Scholar]
- 16.Kosoko O, Gaasterland DE, Pollack IP, et al. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 1996;103:1294–302. [DOI] [PubMed] [Google Scholar]
- 17.Bloom PA, Tsai JC, Sharma K, et al. “Cyclodiode”. Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 1997;104:1508–19. [DOI] [PubMed] [Google Scholar]
- 18.Walland MJ. Diode laser cyclophotocoagulation: longer term follow-up of a standardized treatment protocol. Clin Exp Ophthalmol 2000;28:263–7. [DOI] [PubMed] [Google Scholar]
- 19.Spencer AF, Vernon SA. “Cyclodiode”: results of a standard protocol. Br J Ophthalmol 1999;83:311–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SM. Neurotrophic corneal defects after diode laser cycloablation. Am J Ophthalmol 1998;126:725–7. [DOI] [PubMed] [Google Scholar]
- 21.Sabri K, Vernon SA. Scleral perforation following trans-scleral cyclodiode. Br J Ophthalmol 1999;83:502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Araie M, Yunita A, et al. Transscleral Nd:YAG laser cyclophotocoagulation versus cyclocryotherapy. Graefes Arch Clin Exp Ophthalmol 1991;229:33–6 [DOI] [PubMed] [Google Scholar]
- 23.Ulbig MW, McHugh DA, McNaught AI, et al. Clinical comparison of semiconductor diode versus neodymium:YAG noncontact cyclo photocoagulation. Br J Ophthalmol 1995;79:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higginbotham EJ, Harrison M, Zou XL. Cyclophotocoagulation with the transscleral contact Nd:YAG laser versus cyclocryotherapy in rabbits. Ophthalmic Surg 1991;22:27–30. [PubMed] [Google Scholar]
- 25.Ferry AP. Histopathologic observations on human eyes following cyclocryotherapy for glaucoma. Trans Am Acad Ophthalmol Otolaryngol 1977;83:90–113. [PubMed] [Google Scholar]
- 26.Ferry AP, King MH, Richards DW. Histopathologic observations on human eyes following neodymium:YAG laser cyclophotocoagulation for glaucoma. Trans Am Ophthalmol Soc 1995;93:315–31. [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh P, Wilson DJ, Samples JR, et al. A clinicopathologic correlative study of noncontact trans-scleral ND:YAG cyclophotocoagulation. Am J Ophthalmol 1993;115:597–602 [DOI] [PubMed] [Google Scholar]
- 28.Brancato R, Trabucchi G, Verdi M, et al. Diode and Nd:YAG laser contact transscleral cyclophotocoagulation in a human eye: a comparative histopathologic study of lesions produced using a new fibre optic probe. Ophthalmic Surg 1994;25:607–11. [PubMed] [Google Scholar]
- 29.Shields SM, Stevens JL, Kass MA, et al. Histopathological findings after Nd:YAG transcleral cyclophotocoagulation. Am J Ophthalmol 1988;106:100–1. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg LF, Karalekas DP, Krupin T, et al. Cyclocryotherapy and noncontact Nd:YAG laser cyclophotocoagulation in cats. Invest Ophthalmol Vis Sci 1996;37:2029–36. [PubMed] [Google Scholar]
- 31.Kontic D, Buschmann W. [Experimental and clinical examinations of the effect of cryosurgery of intraocular pressure.] Albrecht Von Graefes Arch Klin Exp Ophthalmol 1981;216:167–76. [DOI] [PubMed] [Google Scholar]
- 32.Brancato R, Leoni G, Trabucchi G, et al. Histopathology of continuous wave neodymium:yttrium aluminum garnet and diode laser contact transscleral lesions in rabbit ciliary body. A comparative study. Invest Ophthalmol Vis Sci 1991;32:1586–92. [PubMed] [Google Scholar]
- 33.Echelman DA, Nasisse MP, Shields MB, et al. Influence of exposure time on inflammatory response to neodymium:YAG cyclophotocoagulation in rabbits. Arch Ophthalmol 1994;112:977–81. [DOI] [PubMed] [Google Scholar]
- 34.Schubert HD, Federman Jl. The role of inflammation in CW ND:YAG contact trans-scleral photocoagulation and cryopexy. Invest Ophthalmol Vis Sci 1989;30:543–9 [PubMed] [Google Scholar]
- 35.Van der Zypen E, England C, Fankhauser F, et al. The effect of transscleral laser cyclophotocoagulation on rabbit ciliary body vascularization. Graefes Arch Clin Exp Ophthalmol 1989;227:172–9. [DOI] [PubMed] [Google Scholar]
- 36.Walland MJ, McKelvie PA. Diode laser cyclophotocoagulation: histopathology in two cases of clinical failure. Ophthalmic Surg Lasers 1998;29:852–6. [PubMed] [Google Scholar]
- 37.Frieling E, Dembinsky B. Morphometry of the ciliary body using ultrasound biomicroscopy. Ophthalmologe 1995;92:745–9. [PubMed] [Google Scholar]
- 38.Liu G-J, Mizukawa A, Okisaka S. Mechanism of intraocular pressure decrease after transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Res 1994;26:65–79. [DOI] [PubMed] [Google Scholar]
- 39.Gaasterland D, Pollack IP. Initial experience with a new method of laser transscleral cyclophotocoagulation for ciliary ablation in severe glaucoma. Trans Am Ophthalmol Soc 1992;90:225–46. [PMC free article] [PubMed] [Google Scholar]