Abstract
Aim: To study the suitability of corneas from very old donors for graft after banking and their clinical and endothelial outcomes in recipients.
Methods: 419 corneas stored in organ culture were divided into group 1, donors under 85 years (330 corneas) and group 2, “very old” donors aged 85 years and over (89 corneas). Endothelial cell density (ECD) before and after organ culture, discard rate before and after storage, and clinical and endothelial outcomes of the 196 penetrating keratoplasties (PKP) (158 in group 1 and 38 in group 2) were compared in a prospective longitudinal study.
Results: Initial ECD was lower in group 2 than in group 1 and elimination for low ECD was more frequent in group 2 (respectively 38% v 20.2%, p=0.001). At the end of storage, because very old corneas lost fewer ECs than younger ones (respectively 4.2% v 9.5%, p=0.022), ECD was comparable between the two groups. The corneas of very old donors had a poorer macroscopic appearance at procurement and during surgery. Despite this, in grafted patients, overall graft survival in groups 1 and 2 (respectively 87.4% v 80.6%, p=0.197), visual acuity, and ECD did not differ at completion of the study (mean follow up 25 months).
Conclusion: This study suggests that endothelial cell count during banking ensures that functional and cellular results of PKPs are not dramatically influenced by very old donor age. Considering Europe's ageing population, the very elderly should not be deemed off limits for corneal procurement.
Keywords: penetrating keratoplasty, organ culture, old age, graft survival, corneal endothelium
L ife expectancy in industrialised countries keeps on rising. The French National Institute of Statistics and Economic Research estimates that, in 20 years' time, the number of people in France aged 85 years or more will have practically doubled, and that by 2020 life expectancy at birth will be 86.4 years for women and 77.9 years for men. The French Graft Agency1 reports that, although in recent years the number of procured corneas has increased and the number of waiting patients has fallen sharply, corneal procurement in France remains far from adequate, as about 20% of demand must still be imported from foreign banks.2
To enlarge the potential donor pool, it was logical, for corneas as for other organs and tissues,3,4 to procure from increasingly old donors. Several studies have examined the influence of donor age on graft survival in terms of transparency5–14; others have considered graft outcome in terms of endothelial cell density (ECD).15–22 These studies made contradictory findings, probably because of elements of bias: retrospectiveness, involvement of several surgeons, incomplete patient follow up. In particular, none of these studies presented a population of donors as old as ours. Lastly, these studies presented confounding factors—the two main ones being the matching of corneas with recipient age or indications. Before endothelial examinations were made possible by the general use of corneal storage techniques instead of “fresh” grafting, surgeons understandably grafted corneas from young donors. These examinations are currently performed at the eye banks, making it possible to discard corneas with an excessively low ECD irrespective of donor age. Despite this, there is still a bias against corneas from very old donors, particularly in the United States.23
To date, no prospective study has examined both the outcome for corneas from very old donors after organ culture storage and post-graft clinical and endothelial outcome in recipients. We thus made a comparative study of two cornea groups, one being from very old donors aged 85–100 years. The corneas were monitored longitudinally at each stage of transplantation, from procurement and storage to penetrating keratoplasty (PKP) in recipients, without a policy of age matching donors and recipients.
MATERIALS AND METHODS
Study design
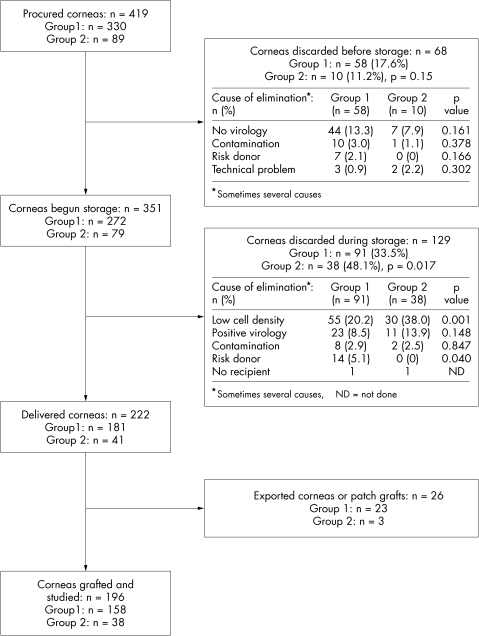
The 419 corneas (211 donors) procured consecutively at Saint-Etienne teaching hospital were analysed prospectively over a 38 month period (1 November 1996 to 31 December 1999). The outcome of all corneas from procurement to grafting is given in Figure 1.
Figure 1.
Outcome of 419 corneas from procurement to graft and causes of exclusion before and after organ culture. Group 2 corneas were discarded more often than those in group 1, mainly due to inadequate ECD (n =number of corneas).
Given the dependence of each donor's two corneas, the study of cornea quality during procurement and storage was performed on one of two eyes chosen at random, except for three “single eye” donors and six untreated eyes of donors operated on for cataracts. Further, 24 corneas from eyes having undergone cataract surgery were the subject of a special study. The statistical analysis thus covered 202 of the 419 procured corneas.
For the study of the recipients, 24 corneas distributed to other hospitals, preventing follow up, and two patch grafts were excluded. Fifteen of 196 patients grafted in our hospital received two or three corneas. As these were not independent observations, we considered for statistical analysis only the first graft. The statistical analysis thus covered 179 recipients.
Corneas at procurement and during storage
The corneas were divided into two groups according to donor age: group 1 (330 corneas, 79%) comprised 166 donors aged under 85 years (mean 67.3 (SD 15.7), median 73, range 16–84); group 2 (89 corneas, 21%) comprised 45 donors aged 85 years and over (89.4 (3.4), median 89, range 85–100). The overall mean age of donors was 72.1 (16.7) years.
Corneoscleral buttons were procured by an ophthalmology student within 24 hours after donor death. For donors having undergone cataract surgery, the following data were noted: type of surgery, site and size of incision, presence or absence of an anterior chamber lens (ACL), or posterior chamber lens (PCL). At the time of procurement, each cornea underwent a macroscopic assessment using a portable slit lamp. The presence of the following five characteristics was checked: epithelial oedema or abrasion, stromal oedema, localised stromal opacity(ies), gerontoxon, descemetic membrane folds. In addition, overall cornea quality was assessed with a three level score taking account of all these parameters. Corneas with a healthy epithelium, a clear stroma, and without gerontoxon or Descemet folds, were deemed “very good”; those with an oedematous and/or abrased epithelium, and whose stroma exhibited a moderate oedema and/or a gerontoxon and/or few descemetic folds, were “good”; those with a major stromal oedema and/or numerous Descemet folds were “acceptable.”
Corneas were immediately organ cultured in Inosol medium (Opsia, Toulouse, France) at +31°C. Besides the microbiological safety tests performed at the start and end of organ culture, the central endothelium was evaluated by induced dilatation of intercellular spaces and trypan blue (0.4%), a first time at 2–4 days after receipt, and a second time 1–3 days before delivery. The examinations were performed using a light microscope, always by the same technician. They comprised measurement of endothelial cell density (ECD) and of percentage cell death, and an appraisal of the endothelium with grading of the following parameters: anisocytosis (low/moderate/severe), pleomorphism (low/moderate/severe), mortality in the folds (none/moderate/severe), presence of rosette patterns (yes/no). The technician performed an overall assessment of corneal quality, using a three level score that took account of ECD and mosaic morphology. The corneas were graded “very good” when the ECD exceeded 2400 cells/mm2, “good” when the ECD was between 2400 and 2200 cells/mm2 with low or moderate anisocytosis and/or pleomorphism, and “acceptable” when the ECD was between 2400 and 2200 cells/mm2, but there was major anisocytosis and/or pleomorphism or when the ECD was between 2200 and 2000 cells/mm2. The percentage of endothelial cell (EC) loss during storage was calculated for each cornea. At the end of storage, the corneas were placed in Exosol deswelling medium (Opsia) for 2 days. Corneas were discarded if at the end of storage they displayed one of the following three criteria: ECD less than 2000 cells/mm2, EC mortality greater than 2%, EC loss greater than 20%.
Recipient characteristics
All corneas were allocated without age matching. Recipient data were studied: age, sex, graft indication (divided into keratoconus; aphakic or pseudophakic bullous keratopathy, Fuchs' dystrophy; lattice dystrophy; herpes; regraft; other), visual acuity (VA), presence of an ocular hypertony or known glaucoma, history of rejection, history of non-immunological graft failure. We thought it important for this study to have recipient subgroups: on one hand, those with low ECD (bullous keratopathy, Fuchs', regrafts) and those with high ECD (keratoconus, lattice dystrophy, herpes); and on the other hand, recipients with a high risk of rejection (corneal vascularisation in two or more quadrants, history of rejection), the remaining recipients being low risk.
PKP characteristics and graft follow up
All PKPs were performed by two surgeons (PG and JM) unaware of donor age. Surgical procedures comprised simple PKPs (67%) or combined surgery (PKP + manual extracapsular technique + PCL in 29% of cases, PKP + ACL removal or exchange in 4% of cases), always of 8.25 mm diameter (95%) or 7.25 mm (5%). During the operation, the two surgeons graded the quality of the graft using a three level score identical to that used by the procuring physician.
The grafted patients were reviewed prospectively by one of the department physicians 1, 3, 6, 9, and 12 months postoperatively, then every 6 months. The following data were studied: graft transparency (yes/no), best corrected VA during follow up and at term, keratometric astigmatism, endothelial rejection (yes/no), intraocular pressure. In each case, ECD and morphometry were evaluated using a non-contact specular microscope assisted by an image analysis system (Topcon SP 2000).
Statistical analysis
The percentages of the two donor age groups were compared using a χ2 test and Fisher's exact test in the case of too small populations. Comparison of means between the two groups were made using Student's t test. The graphs showing graft survival (defined as maintained transparency) were generated using the Kaplan-Meier method, and the two groups were compared with the log rank test. Recipients lost to follow up were analysed using the last available visit data. Statistical analysis was done with the Statistical Package for the Social Sciences with p<0.05 deemed significant.
RESULTS
Initial cornea quality
The procurer's ratings were higher for group 1 corneas than for group 2 corneas, which were respectively deemed “very good” in 34% and 10% of cases (p=0.002), with fewer descemetic folds (49.7 v 73.8%, p=0.005) and fewer gerontoxons (47.1 v 78.6%, p=0.001). Death procurement times were comparable (Table 1).
Table 1.
Corneal characteristics and qualitative assessment done by the procurer. Analysis covered 202 corneas deemed to be independent events
| Group 1 (n=160) | Group 2 (n=42) | p Value | |
| Donor age (years) | |||
| Mean (SD) | 67.3 (15.7) | 89.4 (3.4) | 0.0001 |
| Range | 16–84 | 85–100 | |
| Median | 73 | 89 | |
| Time death procurement (hours, mean (SD)) | 12.5 (7.7) | 12.9 (8.1) | 0.806 |
| Non-heart beating/heart beating donors (%) | 92/8 | 100/0 | 0.74 |
| Macroscopic appearance (%) | |||
| Epithelial impairment* | 27.7 | 28.6 | 0.915 |
| Presence of stromal oedema* | 10.3 | 7.1 | 0.769 |
| Presence of stromal opacity(ies)* | 1.9 | 2.4 | 0.856 |
| Presence of descemetic folds* | 49.7 | 73.8 | 0.005 |
| Presence of gerontoxon* | 47.1 | 78.6 | 0.001 |
| Cornea quality score (%) (very good/good/acceptable)* | 34/38/28 | 10/49/41 | 0.008 |
*Five missing data.
At the start of organ culture, ECD was higher in group 1 than in group 2 (respectively 2217 (SD 425) (range 803–2984) v 2022 (362) cell/mm2 (range 1116–2637), p=0.011). The two groups did not differ in mortality or in qualitative parameters of the endothelium (Table 2).
Table 2.
Endothelial assessment at start of storage. Some corneas were eliminated immediately after storage. Analysis thus covered the 170 corneas deemed to be independent events
| Group 1 (n=132) | Group 2 (n=38) | p Value | |
| Initial ECD cells/mm2 | |||
| Mean (SD) | 2217 (425) | 2022 (362) | 0.011 |
| Range | 803–2984 | 1116–2637 | |
| Mortality rate (mean (SD))* | 0.45 (0.90) | 0.56 (0.71) | 0.533 |
| Qualitative assessment of endothelium (%) | |||
| Anisocytosis (low/moderate/severe)* | 50/35/15 | 43/24/33 | 0.058 |
| Pleomorphism (low/moderate/severe)* | 56/28/16 | 65/22/13 | 0.657 |
| Mortality in folds (low/moderate/high)* | 8/88/4 | 6/88/6 | 0.828 |
| Presence of rosette patterns (no/yes)* | 68/32 | 67/33 | 0.989 |
*Four missing data due to difficult visualisation of the endothelium.
Fifteen donors (24 corneas) had undergone cataract surgery: two with bilateral ACL, three with unilateral ACL, one with bilateral aphakia, six with bilateral PCL, and three with unilateral PCL. Group 2 contained more corneas from eyes having undergone cataract surgery (12.4 v 4%, p=0.003). All eyes had been operated on with a large incision using a manual technique. Twenty one corneas (87.5%) had insufficient ECD at the start of storage: 1041 (287) for the seven with ACL, and 1426 (251) cells/mm2 for 14 with PCL. Three of the 24 corneas (12.5%) had sufficient ECD and had been grafted. Note that all three were from the very old donor group (one from a 90 year old donor with PCL, and two from a 100 year old aphakic donor) and were clear at the term of our study.
Cornea elimination (Fig 1)
Of all the procured corneas, 68 were discarded shortly after receipt at the bank, with a comparable discard rate between the two age groups (17.6% v 11.2%, p=0.15). The main cause of elimination was non-performance of a virological test because of a haemolysed or insufficiently large blood sample.
A total of 129 corneas were then discarded during organ culture, slightly more of them from old donors (48.1% v 33.5%, p=0.017). The main cause was low ECD, which was more frequent in group 2 than in group 1 (respectively 38 v 20.2%, p=0.001). Note that none in group 2 was discarded because of donor medical contraindication.
Delivered corneas
Endothelial characteristics of delivered corneas at the beginning and end of organ culture are presented in Table 3. Whereas storage times in the two groups were comparable (about 13 days), group 1 corneas lost more cells during storage than those in group 2: 9.5% (8.2%) v 4.2% (11.4%), respectively (p=0.022). There was thus no difference in final ECD at delivery between group 1 and group 2 corneas (respectively 2102 (288) v 2026 (309) cells/mm2, p=0.306). The characteristics of the endothelium (morphology, mortality) and the overall score to assess the final quality of the delivered corneas did not differ between the two groups.
Table 3.
Endothelial assessment of corneas at the start and end of storage. Some corneas were eliminated during storage. Analysis thus covered the 112 corneas deemed to be independent events
| Group 1 (n=93) | Group 2 (n=19) | p Value | |
| Storage time (days, mean (SD)) | 13.0 (4.0) | 13.1 (3.8) | 0.916 |
| ECD (cells/mm2, mean (SD)) | |||
| Start of storage | 2336 (357) | 2135 (393) | 0.030 |
| End of storage | 2102 (288) | 2026 (309) | 0.306 |
| Cell loss (%, mean (SD))* | 9.5 (8.2) | 4.2 (11.4) | 0.022 |
| Mortality rate (mean (SD)) | |||
| Start of storage* | 0.19 (0.42) | 0.12 (0.46) | 0.539 |
| End of storage | 0.87 (0.52) | 0.61 (0.45) | 0.058 |
| Final qualitative analysis (%) | |||
| Anisocytosis (low/moderate/severe)* | 60/37/3 | 47/42/11 | 0.318 |
| Pleomorphism (low/moderate/severe)* | 60/34/6 | 69/26/5 | 0.761 |
| Mortality in folds (absent/moderate/high)* | 7/92/1 | 0/100/0 | 0.473 |
| Presence of rosette patterns (no/yes)* | 76/24 | 84/16 | 0.555 |
| Final quality score (%) (very good/good/acceptable) | 33/47/20 | 16/68/16 | 0.214 |
*Two missing data due to difficult visualisation of the endothelium.
Cornea allocation (Table 4)
Table 4.
Cornea allocation to recipients (n=179 recipients)
| Group 1 (n=143) | Group 2 (n=36) | p Value | |
| Recipient age (years, mean (SD) range) | 60.5 (20) (18–91) | 65.6 (18) (20–88) | 0.139 |
| Recipient sex % (n) | 0.348 | ||
| Male | 44 (63) | 53 (19) | |
| Female | 56 (80) | 47 (17) | |
| Recipient indication % (n) | |||
| Keratoconus | 27 (39) | 11 (4) | 0.042 |
| Lattice | 7.5 (11) | 5.5 (2) | 0.659 |
| Herpes | 4 (6) | 0 (0) | 0.602 |
| Fuchs' | 7 (10) | 22 (8) | 0.012 |
| Aphakic or pseudophakic keratopathy | 47.5 (68) | 55.5 (20) | 0.391 |
| Regraft | 5 (7) | 3 (1) | 0.583 |
| Other | 1.5 (2) | 3 (1) | ND |
| Other recipient characteristics % (n) | |||
| Poor endothelium | 61 (87) | 83 (30) | 0.011 |
| High risk of rejection | 12 (17) | 6 (2) | 0.372 |
| Previous glaucoma | 10 (14) | 19.5 (7) | 0.144 |
| History of failure | 15.5 (22) | 25 (9) | 0.173 |
| LogMAR preoperative BCVA (mean (Snellen) (DS)) | 1.72 (20/1000) | 1.65 (20/1000) | 0.484 |
| 0.47 | 0.62 | ||
BCVA = best corrected visual acuity, ND = not done as population too small.
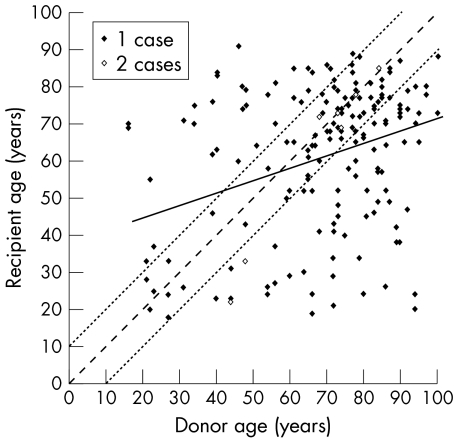
Although a weak correlation (r=0.334, p<0.01) was found between donor and recipient ages (Fig 2), recipients' mean age was not statistically different between the two cornea groups: group 1: 60.5 (20) (range 18–91) v group 2: 65.6 (18) years (range 20–88), p=0.139. Neither did the two groups differ in terms of sex, VA, rejection risk, previous glaucoma, or history of non-immunological graft failure or of rejection. But group 1 corneas were allocated slightly more often to keratoconus hosts (27% v 11%, p=0.042) and group 2 corneas to Fuchs' dystrophy hosts (22% v 7%, p=0.012). Overall, slightly more group 2 corneas were thus allocated to the subgroup of “poor” endothelium recipients (83% v 61%, p=0.011).
Figure 2.
Distribution of recipient age in relation to donor age (n = 179). Note there is only a weak correlation (r = 0.334, p<0.01) between the two, as we did not deliberately age match. Solid line: straight line of linear regression; fine dotted lines: age matching of more or less than 10 years. Less than half the corneas (42.5%) were matched in this way.
Preoperative quality score of corneas
In the surgeons' preoperative assessment of graft quality, corneas from group 1 and group 2 were deemed “very good,” “good,” and “acceptable” in 30%, 63%, and 7% v 7%, 74%, and 19% of cases, respectively. There were thus more very good corneas in group 1 than in group 2 (p=0.022).
Clinical and endothelial outcome in recipients (Table 5)
Table 5.
Clinical and cellular outcome in recipients at term (n=179 recipients)
| Group 1 (n=143) | Group 2 (n=36) | p Value | |
| Global graft survival (%) | 87.4 | 80.6 | 0.197 |
| LogMAR BCVA (mean (Snellen) (SD)) | |||
| Term of study | 0.64 (20/100)(0.65) | 0.87 (20/160)(0.75) | 0.069 |
| During follow up | 0.51 (20/63)(0.53) | 0.59 (20/80)(0.52) | 0.422 |
| Term astigmatism (dioptres, mean (SD)) | 3.7 (2.0) | 3.01 (2.0) | 0.085 |
| Rejection % (n) | 16 (23) | 33 (12) | 0.020 |
| Hypertony % (n) | 13 (19) | 14 (5) | 0.924 |
| Term ECD (cells/mm2, mean (SD), min − max)* | 1183 (463)(370 − 2677) | 1078 (549)(239 − 1989) | 0.352 |
| Surface variation coefficent (%, mean (SD))* | 28.2 (7.0) | 28.7 (7.4) | 0.706 |
| Hexagonality coefficient (%, mean (SD))* | 56.2 (17.4) | 53.2 (18.5) | 0.410 |
| Follow up (month, mean (SD), range) | 25.8 (12.6)(0–50) | 22.9 (12.7)(2–47) | 0.223 |
*Measurements performed on 154 grafts clear at term.
BCVA = Best corrected visual acuity.
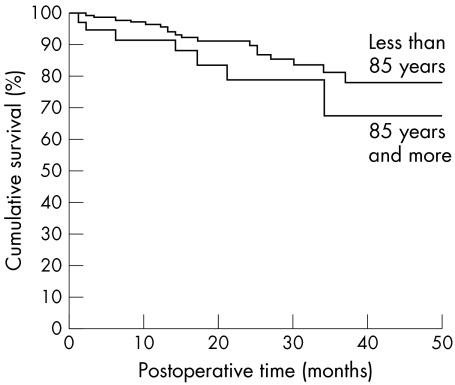
Eighteen recipients were lost (9.2%; 10 not followed, eight dead). There was no significant difference in overall graft survival (Fig 3) between group 1 and 2, respectively 87.4% v 80.6% (p=0.197), VA, astigmatism, percentage of hypertony with comparable follow up. Only the percentage of rejection, generally reversible, was higher in group 2 (33% v 16%, p=0.024).
Figure 3.
Cornea survival according to donor age group. At the end of our study, there was no significant difference in the overall graft survival between the two groups (log rank: p=0.197).
At the end of our study, ECD was not statistically different in groups 1 and 2, with 1183 (463) v 1078 (549) cells/mm2 (p=0.352) respectively; nor were the morphological characteristics of the endothelial mosaic.
DISCUSSION
In this study, we showed that the corneas procured from very old donors, after selection by EC count, had a clinical and endothelial outcome comparable with that of the younger donors' corneas.
The growing need for corneas14,24–27 coupled with safety requirements, particularly those related to the large number of serological examinations and more stringent donor selection in respect of prion diseases,28,29 is accentuating the imbalance between supply and demand. Given population ageing in all industrialised countries, the most effective method to increase corneal procurement is to raise the upper age limit for donors. Although no age limit is set by the European Eye Bank Association (EEBA)30 or the Eye Bank Association of America, some banks, especially outside Europe, do not procure above a certain age.31 This attitude, which can be justified in the absence of endothelial examinations, may also stem partly from economic concerns, as the examinations are costly and a higher proportion of corneas from old donors is discarded.23,32 In our bank, this extra cost is negligible as we systematically do an endothelial examination at the start of organ culture. This allows us, if necessary, to avoid pointless storage.
The EEBA's 2001 register30 reveals a mean donor age range of 62 (7.4) years (range 36–77) (Table 6), which corresponds to the mean age of donors in group 1. In this respect group 2 is an exception, with a mean age of near 90 years. Our mean donor age is particularly high for several reasons. Firstly, the French population is ageing. Likewise, we do not procure from minors because of difficulties in obtaining family consent. Lastly, our series includes very few corneas from multiorgan procurements. Here again, given that seeking consent for cornea procurement can arouse opposition in some families to the donation of other organs, in particular the heart and kidneys, we advised our procurement team to make the cornea request to only the best disposed families.33
Table 6.
Data on donor age and storage method of the 74 European eye banks listed in the EEBA's 2001 register.30 43 of the 74 banks procure corneas over the age of 85 years, with the extreme of 104 years of age being in the bank of Barcelona (Spain). Mean donor age range of 36–77 years. Note that only three banks have mean ages that exceed our own in this study: 74, 75, and 77 years for, respectively, the banks of Barcelona, Ravenna (Italy), and Berlin University (Germany)
| Country | Town | Storage methods | Average age (years) | Min age | Max age | Received corneas |
| Austria | Graz | OC | 67 | 27 | 89 | 101 |
| Innsbruck | OC/4°C | 58 | 15 | 87 | 102 | |
| Salzburg | OC | 63 | 31 | 91 | 264 | |
| Vienna | OC/4°C | 56 | 19 | 88 | 666 | |
| Belgium | Edegem | OC | 69 | 14 | 90 | 178 |
| Gent | OC/4°C | 54 | 16 | 95 | 95 | |
| Leuven | OC | 58 | 18 | 79 | 213 | |
| Liége | 4°C | 70 | 18 | 89 | 443 | |
| Bulgaria | Sofia | 4°C | 51 | 18 | 76 | 424 |
| Croatia | Zagreb | OC/4°C | 45 | 10 | 78 | 105 |
| Czech Republic | Prague | 4°C | 50 | 5 | 65 | 965 |
| Denmark | Arhus | OC | 71 | 17 | 98 | 804 |
| France | Besançon | OC | 61 | 8 | 94 | 473 |
| Bordeaux | OC | 49 | 17 | 76 | 107 | |
| Brest | OC/4°C | 64 | 5 | 89 | 236 | |
| Clermont-Ferrand | OC | 68 | 18 | 94 | 115 | |
| Dijon | OC | 64 | 19 | 96 | 62 | |
| Grenoble | OC | 62 | 15 | 93 | 198 | |
| Lille | OC | 55 | nc | nc | 118 | |
| Limoges | OC | 64 | 19 | 96 | 129 | |
| Paris Banque Française | OC | nc | nc | nc | 691 | |
| Paris St Antoine | OC | 72 | 26 | 97 | 544 | |
| Poitiers | OC | 54 | 17 | 84 | 65 | |
| Rouen | OC | 58 | 21 | 87 | 124 | |
| St Etienne | OC | 67 | 21 | 97 | 218 | |
| Tours | OC | 71 | 37 | 91 | 162 | |
| Germany | Aachen | OC | 61 | 7 | 87 | 462 |
| Berlin Charity | OC/4°C | 54 | 6 | 86 | 155 | |
| Berlin University | 4°C | 77 | 68 | 88 | 16 | |
| Dusseldorf | OC | 62 | 0 | 99 | 773 | |
| Erlangen | 4°C | 62 | 8 | 91 | 336 | |
| Essen | OC | 57 | 22 | 87 | 80 | |
| Greifswald | OC | 57 | 18 | 79 | 46 | |
| Halle-Saale | OC | 58 | 17 | 89 | 150 | |
| Hamburg | OC | 65 | 24 | 91 | 723 | |
| Hanover | OC | 59 | 26 | 85 | 136 | |
| Homburg | OC/4°C | 61 | 19 | 88 | 109 | |
| Kiel | OC | 65 | 7 | 94 | 413 | |
| Munich | OC/4°C | 58 | 10 | 75 | 1078 | |
| Munster | OC | 47 | 20 | 72 | 136 | |
| Wiesbaden | OC/4°C | nc | nc | nc | nc | |
| Greece | Thessaloniki | 4°C | 46 | 15 | 70 | 58 |
| Hungary | Budapest | OC | 59 | 17 | 87 | 747 |
| Debrecen | 4°C | 67 | 43 | 89 | 152 | |
| Ireland | Dublin | OC | 41 | 5 | 81 | 102 |
| Israel | Tel Hashomer | 4°C | 62 | 17 | 81 | 164 |
| Italy | Bologna | 4°C | nc | nc | nc | nc |
| Cosenza | 4°C | nc | nc | nc | 26 | |
| Lucca | 4°C | nc | nc | nc | 1967 | |
| Monza | 4°C | 62 | 12 | 92 | 760 | |
| Pavia | 4°C | 60 | 9 | 90 | 330 | |
| Ravenna | 4°C | 75 | nc | nc | 161 | |
| Venice | OC/4°C | 66 | 1 | 99 | 4206 | |
| Netherlands | Amsterdam | OC | 63 | 5 | 80 | 2381 |
| Poland | Lublin | 4°C | 49 | 17 | 72 | 387 |
| Warsaw | OC/4°C | 56 | 17 | 87 | 282 | |
| Russia | Moscow | 4°C | 42 | 18 | 62 | 717 |
| Russia | Moscow | 4°C | 42 | 18 | 62 | 717 |
| Slovakia | Bratislava | 4°C | 51 | 20 | 77 | 567 |
| Spain | Barcelona | OC/4°C | 74 | 7 | 104 | 1656 |
| Oviedo | 4°C | 45 | 14 | 61 | 81 | |
| Santander | 4°C | 42 | 17 | 69 | 20 | |
| Sweden | Molndal | OC | 69 | 25 | 89 | 166 |
| Lund | OC | 58 | 32 | 84 | 63 | |
| Orebro | OC | 71 | 18 | 90 | 214 | |
| Stockholm | OC | 68 | 19 | 94 | 112 | |
| Switzerland | Berne | OC | 52 | 3 | 80 | 172 |
| Lausanne | OC | 59 | 8 | 94 | 120 | |
| Zurich | OC/4°C | 70 | 22 | 95 | 370 | |
| Turkey | Istanbul | 4°C | 36 | 5 | 77 | 221 |
| UK | Bristol | OC | 61 | 0 | 101 | 2149 |
| East Grinstead | 4°C | 66 | 17 | 92 | 481 | |
| London Keratec Bank | OC | 69 | 11 | 87 | 209 | |
| London Moorfields | 4°C | 62 | 4 | 92 | 535 | |
| Manchester | OC | 63 | 1 | 93 | 1191 |
OC = organ culture, 4°C=+4°C storage, nc = not communicated.
For our patients we legitimately prefer corneas of “very good” macroscopic appearance. At procurement and during surgery, the macroscopic appearance of corneas from the older donors is poorer. The poor condition of the epithelium, which is often shredded or oedematous at the time of procurement, and the more frequent endothelial folds found in often dehydrated elderly donors, may adversely affect the procurers' judgment. In particular, the frequency of gerontoxons certainly contributed to the poor grades given to the old corneas. However, gerontoxon affects neither the transparency of the central cornea nor the endothelium.32,34 In total, despite the poorer macroscopic appearance of the oldest corneas, visual outcome and survival in recipients were not significantly affected.
In the coming years, because of population ageing,35,36 patients who have had cataract surgery will make up a significant proportion of potential donors. In our series, none of the corneas from pseudophakic donors with ACL had a sufficient initial ECD, unlike two from an aphakic donor and one with PCL. It should also be noted that none of the donors with PCL had undergone surgery by phacoemulsification. In the coming years, it seems likely that the generalised use of phacoemulsification techniques37 will mean that this postoperative pool will provide acceptable very old donors.
The overall cornea discard rate in our series was high (47% = 129 + 68/419). This rate remains comparable, however, with that of Armitage,38 who recorded 55% cornea elimination for a donor age band of 80 years and more. This is of course explained by our high mean donor age. The cornea discard rate due to low ECD was substantially higher in group 2 of very old donors than in group 1 (respectively 48.1% v 33.5%, p<0.001). Chu et al recorded comparable rates of endothelium related elimination out of 8207 eyes: 63% in donors aged 65 years and over, and only 36% in the those under 65 years.39 Note that a significant proportion of corneas from the younger donor group was in fact discarded because of a state of intellectual deterioration, which raised at least a theoretical doubt about Creuzfeldt-Jakob disease, which was never the case for the donors aged over 85 years.
The corneas from very old donors displayed a lower ECD at the start of banking than that of the younger donors, in agreement with the physiology of EC loss estimated at a mean 0.6% per year.40 But this difference disappeared at the end of storage, because of a considerably lower cell loss for the very old corneas. These results agree with those of Borderie et al41 and Armitage and Easty,38 who noted EC loss during storage was all the higher because the start of storage ECD was high or the donors were young. The overall assessment of cornea quality made by the technician at the end of organ culture highlighted no difference between the two groups either. This might suggest that old ECs are more resistant than young ones to organ culture. Redbrake et al42 showed that the metabolism of corneas from old donors allowed better use of glucose as an energy source than that of young donors. Armitage and Easty,38 in their study of 9250 organ cultured corneas, also concluded that it was preferable, given cell parity, to graft a cornea from an old donor because an old donor's ECD is more stable during storage.
The recipients in the two groups had similar preoperative VA, which shows there was no preferential allocation of old corneas to recipients liable to have a poorer functional outcome. However, we slightly more often grafted—although this is not the rule—the relatively young corneas in recipients with high preoperative cell density, including those with keratoconus, and the old corneas in recipients with low cell density, including those with Fuchs' dystrophy. For this reason, although there was no age matching policy in our institution, a weak correlation (r=0.334) appeared with age. Despite this bias, which should have substantially penalised the future of grafts using very old corneas, we found no evidence of a difference either in graft survival or in VA, with follow up being comparable for the two groups. Other studies have reported such a result, but none included so many very old donors.5,7,10,14,39,43,44 Only two studies have reported the adverse influence of advanced donor age. Wilhelmus et al45 found an increased risk of primary failure in grafts of donors aged 70 years and over. Ing et al21 suggested the risk of graft failure due to idiopathic endothelial failure increased with donor age, but only after 10 years' follow up. But this last study is difficult to compare with ours because its mean donor age was 39 years, with an extreme of 72 years.
We were surprised to find a significantly higher rate of endothelial rejection in group 2, as recipients in both groups ran the same risk of rejection. Rejection is accepted to be most frequent in patients receiving corneas from paediatric donors, which are considered to be more immunogenic.46,47 But the immunological mechanisms involved in those series, which comprised corneas from child donors with high immunogenicity, probably differ from those in our study, which mainly covered old donor corneas. The mechanisms resulting in the highest frequency of rejected corneas from very old donors, which Sanfilippo et al48 also noted for donors aged over 50 years, remain unexplained.
We found no evidence, with a mean follow up of about 2 years, that donors' very old age influenced the ECD in recipients. Our study agrees with several others which established that corneas from old donors, selected by EC count, were capable of prolonged survival in the recipient.15–17,49 Conversely, some authors showed that EC behaviour of corneas from young donors was better post graft than that of old donors. Musch et al18 and Bourne et al,19 both noted lower postoperative cell loss at 1 year for grafts from donors aged under 25 years. But both studies comprised particularly young donors, who represent only a very small proportion of potential donors in France and in Europe as a whole.50 A double blind randomised prospective study comparing the longer term outcome of grafts in two age groups (under 50 years v over 50 years) including a larger number of recipients with normal ECD is in progress. It should confirm that cornea procurement from very elderly donors is legitimate because the endothelial examination permits selection of corneas whose ultimate optical quality and cell survival are highly acceptable.
This still too little known fact deserves to be disseminated in order to allay the reticence of all those involved in procurement and grafting: the care teams present in the final moments of life; the hospital coordination teams that organise procurement; procurers; surgeons; and even donor families or potential donors themselves, who often believe that their great age is an obstacle to cornea donation.
Video Reports (www.bjophthalmol.com).
Capsule staining and mature cataracts: a comparison of indocyanine green and trypan blue dyes. D F Chang
Pearls for implanting the Staar toric IOL. D F Chang
An intraocular steroid delivery system for cataract surgery. D F Chang
Evaluation of leucocyte dynamics in mouse retinal circulation with scanning laser ophthalmoscopy. Heping Xu, A Manivannan, Garry Daniels, Janet Liversidge, Peter F Sharp, John V Forrester, Isabel J Crane
Dipetalonema reconditum in the human eye. T Huynh, J Thean, R Maini
REFERENCES
- 1.Medical and scientific committee report of the French Graft Agency. Procurement and graft in France. 2000:318–36.
- 2.Houssin D. Organ shortage: a public health crisis. What is the French state doing about it? Transplant Proc 1997;29:3197–8. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez Romero C, Moreno Gonzalez E, et al. Use of octogenarian livers safely expands the donor pool. Transplantation 1999;68:572–5. [DOI] [PubMed] [Google Scholar]
- 4.Kumar MS, Panigrahi D, Dezii CM, et al. Long-term function and survival of elderly donor kidneys transplanted into young adults. Transplantation 1998;65:282–5. [DOI] [PubMed] [Google Scholar]
- 5.Forster RK, Fine M. Relation of donor age to success in penetrating keratoplasty. Arch Ophthalmol 1971;85:42–7. [DOI] [PubMed] [Google Scholar]
- 6.Abbott RL, Forster RK. Determinants of graft clarity in penetrating kerotoplasty. Arch Ophthalmol 1979;97:1071–5. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins MS, Lempert SL, Brown SI. Significance of donor age in penetrating keratoplasty. Ann Ophthalmol 1979;11:974–6. [PubMed] [Google Scholar]
- 8.Volker-Dieben HJ, D'Amaro J, Kok-van Alphen CC. Hierarchy of prognostic factors for corneal allograft survival. Aust NZ J Ophthalmol 1987;15:11–18. [DOI] [PubMed] [Google Scholar]
- 9.Andersen J, Ehlers N. The influence of donor age and post mortem time on corneal graft survival and thickness when employing banked donor material (a five-year follow-up). Acta Ophthalmol (Copenh) 1988;66:313–17. [DOI] [PubMed] [Google Scholar]
- 10.Boisjoly HM, Tourigny R, Bazin R, et al. Risk factors of corneal graft failure. Ophthalmology 1993;100:1728–35. [DOI] [PubMed] [Google Scholar]
- 11.Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology 1994;101:1536–47. [DOI] [PubMed] [Google Scholar]
- 12.Vail A, Gore SM, Bradley BA, et al. Corneal graft survival and visual outcome. A multicenter Study. Corneal Transplant Follow-up Study Collaborators. Ophthalmology 1994;101:120–7. [DOI] [PubMed] [Google Scholar]
- 13.Chang SD, Pecego JG, Zadnik K, et al. Factors influencing graft clarity. Cornea 1996;15:577–81. [PubMed] [Google Scholar]
- 14.Williams KA, Muehlberg SM, Lewis RF, et al. Influence of advanced recipient and donor age on the outcome of corneal transplantation. Australian Corneal Graft Registry. Br J Ophthalmol 1997;81:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruusuvaara P. Effects of corneal preservation, donor age, cadaver time and postoperative period on the graft endothelium. A specular microscopic study. Acta Ophthalmol (Copenh) 1979;57:868–81. [DOI] [PubMed] [Google Scholar]
- 16.Culbertson WW, Abbott RL, Forster RK. Endothelial cell loss in penetrating keratoplasty. Ophthalmology 1982;89:600–4. [DOI] [PubMed] [Google Scholar]
- 17.Abbott RL, Fine M, Guillet E. Long-term changes in corneal endothelium following penetrating keratoplasty. A specular microscopic study. Ophthalmology 1983;90:676–85. [DOI] [PubMed] [Google Scholar]
- 18.Musch DC, Meyer RF, Sugar A. Predictive factors for endothelial cell loss after penetrating keratoplasty. Arch Ophthalmol 1993;111:80–3. [DOI] [PubMed] [Google Scholar]
- 19.Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am J Ophthalmol 1994;118:185–96. [DOI] [PubMed] [Google Scholar]
- 20.Musch DC. Donor age and corneal endothelial cell density. Invest Ophthalmol Vis Sci 1995;36:259. [PubMed] [Google Scholar]
- 21.Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology 1998;105:1855–65. [DOI] [PubMed] [Google Scholar]
- 22.Borderie VM, Scheer S, Touzeau O, et al. Donor organ cultured corneal tissue selection before penetrating keratoplasty. Br J Ophthalmol 1998;82:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck RW, Gal RL, Mannis MJ, et al. Is donor age an important determinant of graft survival? Cornea 1999;18:503–10. [DOI] [PubMed] [Google Scholar]
- 24.Maeno A, Naor J, Lee HM, et al. Three decades of corneal transplantation: indications and patient characteristics. Cornea 2000;19:7–11. [DOI] [PubMed] [Google Scholar]
- 25.Dobbins KR, Price FW Jr, Whitson WE. Trends in the indications for penetrating keratoplasty in the midwestern United States. Cornea 2000;19:813–6. [DOI] [PubMed] [Google Scholar]
- 26.Patel NP, Kim T, Rapuano CJ, et al. Indications for and outcomes of repeat penetrating keratoplasty, 1989–1995. Ophthalmology 2000;107:719–24. [DOI] [PubMed] [Google Scholar]
- 27.Wiffen SJ, Hodge DO, Bourne WM. The effect of contact lens wear on the central and peripheral corneal endothelium. Cornea 2000;19:47–51. [DOI] [PubMed] [Google Scholar]
- 28.Zeidler M, Brown P. More patients should be excluded from being tissue donors. BMJ 1998;316:1170–1. [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy RH, Hogan RN, Brown P, et al. Eye banking and screening for Creutzfeldt-Jakob disease. Arch Ophthalmol 2001;119:721–6. [DOI] [PubMed] [Google Scholar]
- 30.European Eye Bank Association Directory. 7th ed. 1999.
- 31.Farge EJ, Silverman LM, Khan MM, et al. The impact of state legislation on eye banking. Arch Ophthalmol 1994;112:180–5. [DOI] [PubMed] [Google Scholar]
- 32.Moyes AL, Holland EJ, Palmon FE, et al. Tissue utilization at the Minnesota Lions' Eye Bank. Cornea 1995;14:571–7. [PubMed] [Google Scholar]
- 33.Gain P, Thuret G, Pugniet JL, et al. Obtaining cornea donation consent by telephone. Transplantation 2002;(in press). [DOI] [PubMed]
- 34.Kotulak JC, Brungardt T. Age-related changes in the cornea. J Am Optom Assoc 1980;51:761–5. [PubMed] [Google Scholar]
- 35.Hirvela H, Luukinen H, Laatikainen L. Prevalence and risk factors of lens opacities in the elderly in Finland. A population-based study. Ophthalmology 1995;102:108–17. [DOI] [PubMed] [Google Scholar]
- 36.Lundstrom M, Stenevi U, Thorburn W. Cataract surgery in the very elderly. J Cataract Refract Surg 2000;26:408–14. [DOI] [PubMed] [Google Scholar]
- 37.Ravalico G, Tognetto D, Palomba MA, et al. Corneal endothelial function after extracapsular cataract extraction and phacoemulsification. J Cataract Refract Surg 1997;23:1000–5. [DOI] [PubMed] [Google Scholar]
- 38.Armitage WJ, Easty DL. Factors influencing the suitability of organ-cultured corneas for transplantation. Invest Ophthalmol Vis Sci 1997;38:16–24. [PubMed] [Google Scholar]
- 39.Chu W, Dahl P, O'Neill MJ. Benefits of specular microscopy in evaluating eye donors aged 66 and older. Cornea 1995;14:568–70, 634. [PubMed] [Google Scholar]
- 40.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci 1997;38:779–82. [PubMed] [Google Scholar]
- 41.Borderie V, Laroche L, Vedie F, et al. [Penetrating keratoplasty after graft preservation in organ culture at +37 degrees centigrade. 1-year results.] J Fr Ophtalmol 1995;18:570–7. [PubMed] [Google Scholar]
- 42.Redbrake C, Becker J, Salla S, et al. The influence of the cause of death and age on human corneal metabolism. Invest Ophthalmol Vis Sci 1994;35:3553–6. [PubMed] [Google Scholar]
- 43.Chipman ML, Basu PK, Willett PJ, et al. The effects of donor age and cause of death on corneal graft survival. Acta Ophthalmol (Copenh) 1990;68:537–42. [DOI] [PubMed] [Google Scholar]
- 44.Sharif KW, Casey TA. Penetrating keratoplasty for keratoconus: complications and long-term success. Br J Ophthalmol 1991;75:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelmus KR, Stulting RD, Sugar J, et al. Primary corneal graft failure. A national reporting system. Medical Advisory Board of the Eye Bank Association of America. Arch Ophthalmol 1995;113:1497–502. [DOI] [PubMed] [Google Scholar]
- 46.Palay DA, Kangas TA, Stulting RD, et al. The effects of donor age on the outcome of penetrating keratoplasty in adults. Ophthalmology 1997;104:1576–9. [DOI] [PubMed] [Google Scholar]
- 47.Vail A, Gore SM, Bradley BA, et al. Influence of donor and histocompatibility factors on corneal graft outcome. Transplantation 1994;58:1210–16. [PubMed] [Google Scholar]
- 48.Sanfilippo F, MacQueen JM, Vaughn WK, et al. Reduced graft rejection with good HLA-A and B matching in high-risk corneal transplantation. N Engl J Med 1986;315:29–35. [DOI] [PubMed] [Google Scholar]
- 49.Zacks CM, Abbott RL, Fine M. Long-term changes in corneal endothelium after keratoplasty. A follow-up study. Cornea 1990;9:92–7. [PubMed] [Google Scholar]
- 50.European Eye Bank Association Directory. 9th ed. 2001.