Abstract
Background: Tumour microvascularity is a significant determinant of prognosis for a large number of different tumours, including uveal melanoma. The development of blood vessels within these and other tumours is partly controlled by soluble pro-angiogenic cytokines, of which basic fibroblast growth factor (bFGF) and vascular endothelial growth factor-A (VEGF) are the best described.
Methods: Because VEGF has been inconsistently found within uveal melanomas and bFGF is described as an autocrine growth factor in cutaneous melanoma, the authors looked at the expression of these cytokines in uveal melanomas using immunohistochemistry and reverse transcriptase polymerase chain reaction (RT-PCR). The cross talk between uveal melanoma cells and endothelial cells was then assessed in an in vitro co-culture model.
Results: While most tumour cells expressed bFGF at the protein level by immunohistochemistry (89%), relatively few (22%) expressed VEGF, and this was of limited extent. All 20 tumours tested by RT-PCR contained mRNA for both bFGF and VEGF. Co-culture experiments using an ATP based bioassay showed that uveal melanomas could support the growth of a rat brain endothelial cell line (GPNT) and human umbilical vein endothelial cells (HUVEC), and that this could be modulated by cytokines and anti-cytokine antibodies.
Conclusion: These results suggest that angiogenesis within uveal melanoma may be the result of a complex interplay between endothelial and tumour cells, and that bFGF and VEGF could play a part.
Keywords: melanoma, eye, choroid, vascular endothelial growth factor, basic fibroblast growth factor, angiogenesis
Uveal melanoma is a rare tumour with a poor prognosis1,2 Tumours with high vascularity as assessed by microvessel count or vascular pattern have a particularly poor outcome, both in terms of mortality due to distant metastasis and loss of the eye.3–9 An inverse relation between survival and vascularity is common to many tumours10–16 and supports the hypothesis that angiogenesis is necessary for growth of both the primary tumour and its metastases.17–25
Because bFGF is known to support both the autonomous division of cutaneous melanoma cells,26–32 and is a pro-angiogenic cytokine,33,34 we asked (i) whether bFGF was present in uveal melanoma, and (ii) whether, secreted from cultured primary human melanoma, it could support or enhance the growth of endothelial cells. This is particularly relevant given that vascular endothelial growth factor-A (VEGF), the major proangiogenic growth factor described in many tumours, has not been found consistently in uveal melanoma.35–39 Neither VEGF protein nor its mRNA have been found in most studies of uveal melanoma, with only two immunohistochemical reports indicating its presence.38,39 However, a recent study using reverse transcriptase polymerase chain reaction (RT-PCR) has found VEGF mRNA in transformed uveal melanoma cell lines.40
We first investigated 51 tumours for the presence of VEGF and basic fibroblast growth factor (bFGF) protein by immunohistochemistry and compared these findings against microvessel density, tumour cell type, tumour location, and mitotic index. Non-quantitative RT-PCR was used to determine whether VEGF and/or bFGF mRNA were present in a subset of these tumours. Co-culture experiments were performed to examine the interplay between primary human uveal melanoma, these cytokines, and endothelial cells.
MATERIALS AND METHODS
Tumours
A total of 50 tumours were included in this study. The median age of the patients was 62 years (range 29–85), with 27 females and 23 males. Most tumours (39) were choroidal, with ciliary body (CB) involvement in 10 tumours and one large iris tumour. Six tumours had received previous irradiation (proton radiotherapy in three, 125I plaque in three). Two tumours underwent choroidal biopsy before enucleation, and four had extrascleral extension. Tumour size varied from 4 mm in the case of the iris tumour to 24 mm in a choroidal tumour (median 14 mm). Thirteen tumours were classified as spindle cell, 13 as epithelioid, and 24 as mixed histology. The mitotic count varied from 0–12 (median 0.5) mitoses/mm2.
Enucleated eyes were placed in DMEM cell culture medium with antibiotics (Sigma Chemical Co, Dorset, UK), received at the laboratory between 15 and 90 minutes following removal, and assessed under sterile conditions by an ocular pathologist. Following tumour localisation by transillumination, eyes were oriented on a sterile metal eye cup and cut anteroposteriorly through the tumour. The main block containing the bulk of the tumour, cornea, and optic nerve was immediately fixed in buffered 4% formaldehyde. Tumour material not required for diagnostic histopathology was removed from the remaining calotte for tissue culture or RNA extraction and the scleral part of the calotte fixed to allow histological assessment to determine intrascleral or extrascleral extension.
Immunohistochemistry
Immunocytochemistry for VEGF and bFGF was performed using 5 μm paraffin sections cut from formalin fixed blocks used for diagnostic histopathology. Because others have reported conflicting immunocytochemical data, three commercially available anti-VEGF antibodies were used as recommended by the manufacturer, and the third was used in conjunction with two methods of antigen retrieval (Table 1). Incubations were performed at room temperature unless otherwise specified. Following washing in TRIS buffered saline (TBS), non-specific antibody binding was blocked by the addition of 1.0% bovine serum albumin (BSA) in TBS, pH 7.6 for 25 minutes. Antigen retrieval techniques and primary antibody dilutions are shown in Table 1. All dilutions were made in TBS + 1.0% BSA and left on the slide at room temperature for 60 minutes in a humidified covered tray. Slides were washed three times in TBS and the second antibody added. In all cases this was a biotinylated multilink antibody (Dako Ltd, High Wycombe, UK) used at 1 in 300 dilution in TBS for 45 minutes. Following washing, the sections were incubated with a tertiary streptavidin-alkaline phosphatase reagent (Dako). In three cases, recombinant VEGF or bFGF (at concentration of 1 μg/ml) was preincubated with antibody to demonstrate specificity. The sections were again washed in TBS and incubated in Vector Red made up according to the manufacturer's instructions (Vector Laboratories, Peterborough, UK) for 15 minutes, washed and lightly counterstained with Mayer's haematoxylin for 5 seconds. Sections were viewed by direct microscopy and the positivity of the melanoma cells, other cells within the tumour, and other cells within the eye assessed qualitatively after ranking the specimens in order of greatest staining.41,42 Immunohistochemical staining was graded as 0 (no staining), 1 (weak staining of >50% cells), 2 (intermediate staining of >50% cells), 3 (strong staining of >50% of cells).
Table 1.
Antibodies used for immunohistochemistry: all were polyclonal affinity purified
| Antibody | Supplier | Catalogue number | Antibody | Dilution used | Pretreatment |
| vWF | Dako | M616 | Mouse IgG | 1:500 | Trypsin 30 minutes |
| bFGF | Calbiochem | PC194L | Goat IgG | 1:20 | Trypsin 15 minutes |
| VEGF (1) | Calbiochem | PC315 | Rabbit IgG | 1:20 | Trypsin 15 minutes |
| VEGF (2) | R&D Systems | AB-293-NA | Goat IgG | 1:200 | Trypsin 15 minutes |
| VEGF (3) | Santa Cruz | SC-152 | Rabbit IgG | 1:75 | Pressure cook in citrate buffer pH 6.0 for 3.5 minutes on high and 10 minutes on medium power, then 0.015% trypsin at 37°C for 45 minutes |
Calbiochem/Oncogene Research Products, Nottingham, UK.
R&D Systems UK Ltd, Santa Cruz, Autogen Bioclear UK Ltd, Calne, Wilts, UK.
Dako Ltd, Ely, UK.
Microvessel density
Blood vessels were counted as described by Foss et al4 in sections immunostained for von Willebrand factor VIII. Areas of high microvessel density (vascular “hot spots”) were located by scanning the entire tumour at ×100 magnification. Hot spot counting was used to maintain continuity with previous work in this field.4,6 Once a hot spot was located, the vessels were counted in three non-overlapping fields at ×400 magnification using a field area of 0.23 mm2. Results were expressed as vessels per mm2.
Reverse transcriptase β polymerase chain reaction
A further series of 20 tumour fragments obtained at dissection as described above were snap frozen in liquid nitrogen for use in reverse transcriptase polymerase chain reaction (RT-PCR) studies. These comprised 17 choroidal, two ciliary body and one choroidal/ciliary body tumours with four epithelioid, seven spindle cell, and nine mixed histology. The mitotic count varied from 0 to 20 (median 0.45) mitoses/mm2. The median age of the patients was 65 years (range 44–86) with 12 male and eight female patients. RT-PCR was performed following mechanical disruption of previously frozen primary tumour fragments in Trizol reagent (Gibco, BRL, Burlington, Ontario, Canada). One microgram of total cellular RNA was subjected to oligo dT first strand cDNA synthesis using the Maloney mouse leukaemia virus reverse transcriptase. Standard polymerase chain reaction (PCR) was then performed with primers specific for GAPDH, VEGF, and bFGF. Primer sequences are: GAPDH forward 5' - CTC TAA GGC TGT GGG CAA GGT CAT, GAPDH reverse 5' - GAG ATC CAC CAC CCT GTT GCT GTA; VEGF 5' - forward TCG GGC CTC CGA AAC CAT GA, VEGF reverse 5' - CCGT CCT CGG CTT GTC ACA TCT; bFGF forward 5' - GCT CTT AGA AGA CAT TGG AAG A , bFGF reverse 5' - GGC TTC TTC CTG CGC ATC CA. PCR conditions were set at 95°C (denaturation), and 65°C (annealing for VEGF) and 60°C (annealing for GAPDH and bFGF), and 72°C (extension for 1 minute). VEGF was amplified for 35 cycles, bFGF and GAPDH for 30 cycles. Cycle numbers were kept low to reduce the production of non-specific amplification products. Because we anticipated low copy numbers of VEGF-A we chose to amplify for an additional five cycles (n=35), none the less remaining well below 40 cycles. The specificity of these amplified products was confirmed by northern analysis (data not shown). Amplified products were then subject to agarose gel electrophoresis (1.5%) and observed using ethidium bromide staining.
Cell culture
Three primary human uveal melanoma cell cultures were derived from two epithelioid tumours and one mixed tumour following enucleation. Primary human uveal melanoma cells for culture were dissociated from blocks of tumour tissue by collagenase H digestion (1.5 mg/ml; Sigma Chemical Co Ltd, Poole, UK), washed in Dulbecco's modified Eagle's medium (DMEM, Sigma). The cells grew slowly in DMEM + 10% FBS and were passaged up to three times before sufficient cells were obtained for co-culture experiments in 24 well plates. At least 99% of the cells present were S100 positive in immunostained drop preparations.
Two types of endothelial cells were used, one an SV-40 transformed rat brain microvascular endothelial cell line (GPNT) and the other, primary human umbilical vein endothelial cells (HUVEC). GPNT was generously provided by Professor John Greenwood, Institute of Ophthalmology, University College London (UCL), and the HUVEC provided through the laboratory of Dr Ian Zachary, Department of Cardiovascular Cell Biology, UCL. Initial cell culture experiments were performed with GPNT in 96 well plates to determine the suitability of various growth media and to confirm the utility of the ATP method for measurement of effects on endothelial cell growth. The effects of various media and exogenous cytokines were assessed on these lines before co-culture. Thereafter, four dual culture experiments were performed in triplicate wells using GPNT or HUVEC.
The GPNT endothelial cell line was grown in support medium consisting of a 1:1 mixture of Ham's F10 (Sigma, Poole, UK) and alpha-MEM (Gibco, Paisley, UK) containing 10% fetal bovine serum (FBS, Gibco), 1% heat inactivated human AB serum (Sigma), 1% penicillin + streptomycin, 1% glutamine (Sigma), 5 ng/ml bFGF, and 5 mg/ml puromycin (Sigma). Plates and flasks were coated with collagen at 1:20 dilution in Hank's balanced salt solution (Sigma).
Primary endothelial cells, HUVEC, were grown in support medium consisting of EBM Clonetics modified MCBD 131 medium with 10% FBS, bovine brain extract (BBE), 1% glutamine, and 1% penicillin + streptomycin. Cells were plated onto vitronectin coated plates in this growth medium and transferred to assay medium 24 hours before co-culture.
Co-culture experiments
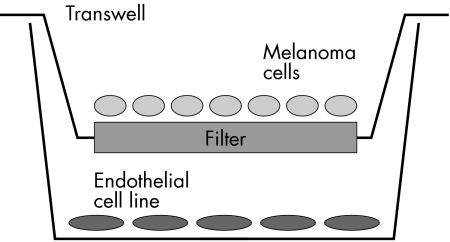
Dual cell culture experiments were performed in 24 well plates using the transwell system (Costar 3470, 0.4 μm pore size) to separate endothelial cells, grown in the lower wells as adherent monolayers, from melanoma cells, also grown as monolayers, in the overlying filter (Fig 1). Twenty four hours before initiating co-culture experiments, melanoma and endothelial cells were grown separately in assay medium. Melanoma cells were plated at a density of 20 000 cells per transwell filter (that is, upper chamber of the CoStar Transwell system), and allowed to attach and grow in support medium for at least 24 hours before co-culture experimentation. For co-culture, endothelial cells were plated in the lower compartment of the CoStar Transwell system. Twenty four hours before co-culture, the support medium was replaced with assay medium. Assay medium consisted of support medium without additives such as BBE, but including 1% bovine or human serum. Attempts to grow HUVEC or GPNT in the complete absence of serum were unsuccessful; enzyme linked immunosorbent assay (ELISA) analysis of the culture medium was performed to determine baseline cytokine concentrations. On initiating co-culture experiments, transwell chambers were inserted into the lower wells and incubated at 37°C in 99% humidity in standard incubator conditions. The cells were monitored by direct microscopy before ATP bioassay.
Figure 1.
Transwell system for co-culture of endothelial cells and melanoma derived cells. Melanoma cells are placed in the filter chamber, while endothelial cells are present in the wells of the plate allowing diffusion of secreted molecules between the two cell types. Cells in the wells and in the transwells are viewed via the base of the well using an inverted microscope with a long focal length lens.
Endothelial ATP bioassay
Endothelial cell growth was measured using an assay of adenine triphosphate (ATP) as previously described.43,44 The ATP assay provides a sensitive and reproducible measure of biomass and was ideal for this purpose as it allowed us to use fewer cells in each experiment than alternative methods.43,44 It has been used previously to assess the chemosensitivity of uveal melanoma.45,46 After 48–120 hours of co-culture, endothelial cells were lysed by addition of 75 μl ATP extractant (TCER; DCS Innovative Diagnostik Systeme), and intracellular ATP measured by adding 50 μl from each well to 55 μl of luciferin-luciferase reagent (DCS Innovative Diagnostik Systeme). Luminescence was then measured using relative light units (RLU) in a Dynatech ML1000 luminometer (Dynex Inc, Chantilly, USA) set to measure over a 20 second period.43,44 Results were compared against ATP standard curves performed before and after each luminesence assay to confirm the linearity and stability of the luciferase reagent. A maximum inhibitor (Maximum Inhibitor, DCS Innovative Diagnostik Systeme GmbH, Hamburg, Germany) was added to each experimental plate to provide negative control for ATP production. The degree of growth or suppression obtained was assessed as a percentage using the formula D = [(RLUTest − RLUMI)/(RLUMO − RLUMI) × 100].43,44
Cytokines and antibodies used for cell culture, their abbreviations, concentrations, and sources are shown in Table 2. Cytokines and antibodies were diluted to the concentrations required (Table 2) and added to triplicate wells for each experiment.
Table 2.
Cytokines and antibodies used for cell culture experiments
| Antibody or cytokine | Supplier | Catalogue number | Concentration used |
| VEGF | R&D Systems | 293-VE-050 | 4 ng/ml |
| bFGF | Sigma | F0291 | 2 ng/ml |
| Anti-VEGF | R&D Systems | AB-293-NA | 1 mg/ml |
| Anti-bFGF | Calbiochem | PC194L | 1 mg/ml |
ELISA
Undiluted cell culture media were assessed for the presence of VEGF and bFGF by ELISA using commercially available kits (R&D Systems) according to the manufacturer's instructions. The limit of detection for VEGF was 5 pg/ml and bFGF 4 pg/ml.
Data analysis
Data were collected in an Excel spreadsheet (Microsoft) and analysed using non-parametric statistics (Statgraphics ver 7.0, Manugistics, CA, USA). Grouped data were analysed by analysis of variance (ANOVA). Owing to small numbers, differences between individual tumours in cell culture experiments could not be assessed by rigorous statistical methods.
RESULTS
Immunohistochemistry
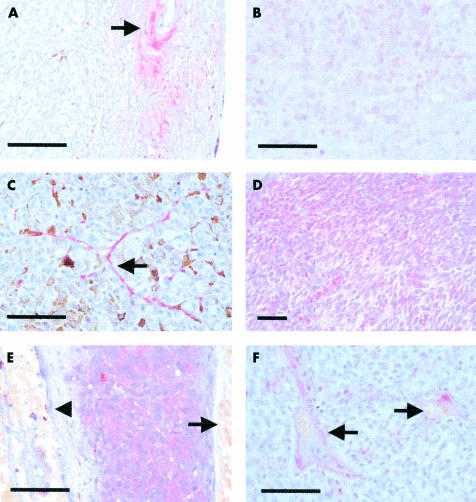
VEGF staining was largely absent within the tumours stained with antibodies 1, 2, and 3 (Table 3, Fig 2A). However, weak staining within uveal melanoma cells was occasionally present around blood vessels towards the centre of the largest tumours (Fig 2B, 2/36) or adjacent to small discrete areas of necrosis (a rare finding in uveal melanoma). However, using antibody 3 (SC-152) with microwave followed by trypsin antigen retrieval,39 low but definite staining of uveal melanoma cells was noted in 11 of 49 tumours (22%). This was also seen in the von Willebrand positive cells of small blood vessels within the tumour (Fig 2C). There was more staining with all three antibodies within fibrous tissue overlying many of the tumours (Fig 2A), often in a patchy distribution. VEGF positive cells, morphologically macrophages or fibroblasts, were occasionally seen. VEGF was found quite consistently in the RPE, and often in retina (14/50, 28%), and occurred primarily where there was retinal detachment. Negative controls (antibody omission or non-immune similar antibody subclass) were uniformly negative. Furthermore, all staining was abolished for VEGF antibodies 1, 2, and 3 in slides in which the primary antibody was neutralised with excess recombinant VEGF (R&D Systems).
Table 3.
Summary of immunostaining results in 50 eyes
| Tumour | Retina | |
| VEGF: | ||
| Negative | 45 | 37 |
| Weak | 10 | 11 |
| Moderate | 1 | 3 |
| Strong | 0 | 0 |
| Total | 50 | 51 |
| bFGF: | ||
| Negative | 6 | 8 |
| Weak | 14 | 16 |
| Moderate | 16 | 13 |
| Strong | 12 | 14 |
| Total | 48 | 51 |
Figure 2.
Representative immunohistochemisty results. (All at ×400 original magnification, unless otherwise stated.) The bar on each graph represents 100 μm. (A) VEGF negative tumour with positivity of the overlying fibrovascular scar tissue (arrow) (×100 original magnification). (B) VEGF positivity within the cytoplasm of uveal melanoma cells in one of the three positive tumours. (C) VEGF positivity in blood vessel endothelium (arrow) within the tumour. (D) Low power view showing bFGF throughout the tumour (×2100 original magnification). (E) High power view of bFGF in tumour cells with absence of staining in blood vessels (arrowhead) and retinal pigment epithelium (arrow). (F) bFGF negative tumour with positivity of the overlying retinal blood vessels (arrows).
Basic FGF staining localised strongly to the cytoplasm of uveal melanoma cells in 42/47 tumours (89%) as shown in Figure 2D and E. It was also present in the perivascular area, possibly in pericytes, fibroblasts, or extracellular matrix, and was not as clearly associated with von Willebrand positive endothelial cells as was the VEGF staining (Fig 2F). Mild to moderate bFGF staining was also seen within the retina (Fig 2E) in 43/50 eyes (86%), and often in the detached retina overlying the tumour. Negative controls (antibody omission or similar antibody subclass) were uniformly negative.
Microvessel densities varied from 17 to 148 vessels per mm2 (median 61). There was no statistical relation between vessel counts and bFGF scores (ANOVA, NS). There was also no correlation between tumour size, mitotic index, tumour site, and bFGF score. VEGF staining was detected in too few tumours to allow statistical analysis with any other pathological parameters. There was no correlation between predominant tumour cell type and vessel parameters.
Dual cell culture
Initial experiments growing the GPNT endothelial cell line or primary uveal melanoma cells separately in CAM media with 1% AB serum demonstrated that over 6 days, intracellular ATP of these cells was unchanged. The cells flattened and became adherent to the well or transwell surface as expected, and we noted no evidence of cell death or detachment during the assays. Addition of exogenous recombinant bFGF did not enhance GPNT ATP. Anti-bFGF antibodies had no appreciable effect (106% of control), while anti-VEGF antibodies, and anti-VEGF/anti-bFGF together (anti-V/F), led to reduction of ATP (84% and 77% of control respectively). Similar single culture endothelia served as controls for dual culture experiments (Fig 3A).
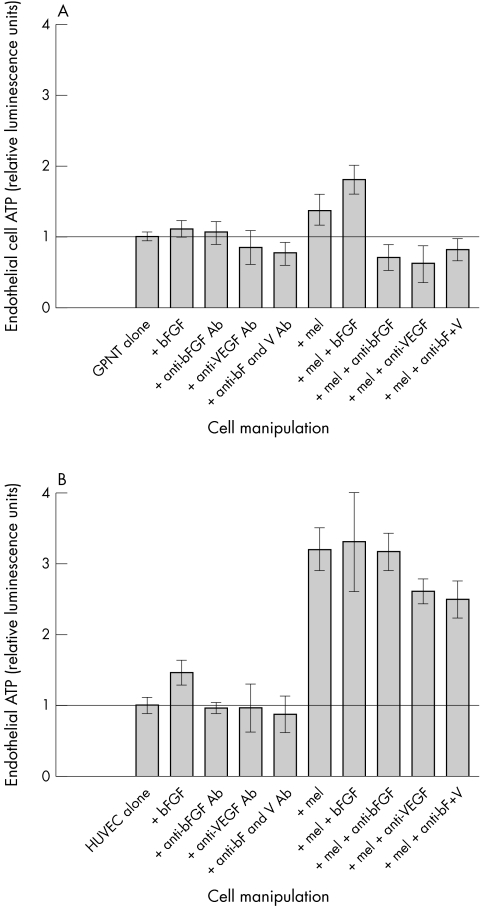
Figure 3.
Dual culture transwell experiments showing the effects of cytokines, antibodies, and co-culture with primary human uveal melanoma: each panel represents a single experiment with triplicate wells for each manipulation. Effects of cytokines, antibodies, and co-culture with primary human uveal melanoma. (A) GPNT rat brain endothelial cell line, showing enhancement of endothelial cell growth by co-culture with melanoma cells. In this instance, addition of bFGF to the co-culture produces greater growth. (B) HUVEC, showing much greater sensitivity to co-culture with melanoma cells. In this instance, addition of further bFGF does not produce further growth, and the melanoma effect is only reduced 23% by the simultaneous addition of blocking antibodies to bFGF and VEGF. x-axis: experimental manipulation, y-axis: endothelial cell ATP, expressed in relative luminescence units.
In co-culture experiments when GPNT were grown in the presence of melanoma derived cells, cell growth was increased to 136% of control. Addition of bFGF to this dual culture further increased GPNT ATP production to 182% of control. When antibodies against bFGF, VEGF or both were added to this co-culture, endothelial cell growth was reduced to 70%, 66%, and 80% of single culture control levels, respectively. Repetition of these experiments using a further passage with the same primary melanoma cells gave results within 10% of those previously observed.
In single culture experiments performed using HUVEC (Fig 3B), bFGF increased endothelial cell ATP to 148% of control, while anti-VEGF and anti-bFGF antibodies and antibodies against both cytokines reduced the ATP levels to 96%, 95%, and 87%, respectively. Growth of HUVEC was increased to 320% of control on co-culture with melanoma cells. In this co-culture, anti-VEGF antibodies were able to reduce melanoma stimulated growth by 20%—that is, to 260% of single culture control, while addition of anti-bFGF antibodies reduced the enhanced growth due to melanoma by only 8%. Antibodies against both cytokines reduced endothelial cell ATP by 23%, to 248% single culture control.
ELISA
VEGF and bFGF could not be detected in cell culture medium even after addition of 5% heat inactivated AB serum. Basic FGF was detectable (65 pg/ml) in the EBM support medium when prepared using supplemental bovine brain extract (used in support medium before experimentation, not in assay medium). ELISA analyses of supernatants from a single HUVEC co-culture experiment demonstrated that the primary melanoma culture produced both bFGF (26 pg/ml) and VEGF (500 pg/ml). This tumour stained positively for both cytokines by immunohistochemistry using sections from the block reserved for histopathology. HUVEC alone produced very low levels of bFGF (4.8 pg/ml).
RT-PCR
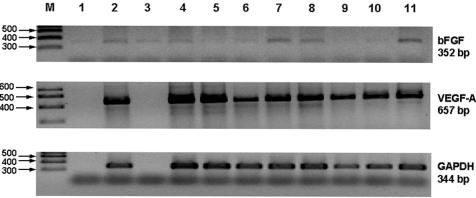
RT-PCR of primary uveal melanomas included in the series showed mRNA for both VEGF (35 cycles) and bFGF (30 cycles) in all 20 tumours tested (Fig 4). Quantification of these messages was not performed.
Figure 4.
RT-PCR gels stained with ethidium bromide. The results are non-quantitative but there is variable intensity for bFGF, which was amplified for 30 cycles compared with VEGF, which was amplified for 35 cycles, as this was not present in immunostained sections from these tumours. Lane M contains size markers: no sample was present in lanes 1 and 3 for the VEGF-A and GADPH gels shown in this figure.
DISCUSSION
This study demonstrates strong immunostaining for bFGF diffusely throughout most uveal melanomas within the tumour cell cytoplasm, and also occasionally around small blood vessels within the tumour. RT-PCR confirms the presence of bFGF mRNA. Basic FGF is well described as a direct acting pro-angiogenic cytokine and a potent mitogen for cutaneous melanoma cells.26,27 Our data suggest that it could have the same potential for uveal melanoma.
VEGF, the best characterised pro-angiogenic molecule, has been variably described in studies of uveal melanoma. Several studies using immunohistochemistry or in situ hybridisation were unable to identify VEGF,35–37 while two recent studies report its presence.38,39 Using several commercially available antibodies we found VEGF to be largely absent within tumour cells, but present centrally within the tumour or adjacent to areas of necrosis. This may represent a response to local hypoxia. VEGF was also found in the immediate perivascular area, corresponding to the vascular endothelium, adjacent cells and extracellular matrix, and in rare areas of fibrosis. This stromal or perivascular localisation is not surprising given that VEGF can be produced by fibroblasts in tumour formation and wound healing,47 and that it is induced by hypoxia.48–52 Both locations are consistent with a putative role for VEGF in uveal melanoma angiogenesis.
The controversy regarding the presence or absence of VEGF protein in uveal melanoma may reflect differences in the specificities of the polyclonal antibodies used for immunohistochemistry and/or the techniques employed by various investigators. Although all three antibodies used in this study gave similar patterns of staining, one (SC-152), demonstrated somewhat more staining overall, while the use of prolonged microwave antigen retrieval gave even greater enhanced staining not consistent with our other three analyses. It is likely that prolonged denaturation of the tissue provided epitopes which were detected by the polyclonal sera used. Whether these represent newly exposed native epitopes or production of an artefactual epitope is not known. Competition assays using this technique were undertaken by another group and suggest that the staining is specific.39 Monoclonal antibodies against VEGF are now available, but were not available to us at the time that this work was performed. Another explanation for variable VEGF staining may relate to the different techniques by which these tumours are fixed following enucleation. Standard techniques rely on the diffusion of formalin through the entire, unopened globe following immersion. By contrast, our technique ensures rapid fixation of the tumour by opening the globe, dissection of the tumour as needed for analysis, and immersion of these smaller pieces in formalin. Prolonged time to fixation using the standard technique could potentially allow for production of detectable amounts of VEGF post mortem as has been found for several other cytokines in whole blood samples.53,54 However, the results of Sheidlow et al 39 are similar to ours as many of their more weakly stained sections would have been regarded as negative on our grading system. Differences of interpretation may therefore affect the results of these different studies.
VEGF mRNA has been described in transformed uveal melanoma cells lines,40 but little information is available concerning its presence in primary melanoma. Radioactive in situ hybridisation has been unable to detect its presence.37 Using the sensitive technique of RT-PCR we have now demonstrated that VEGF mRNA is present in 100% of tumours examined. In addition, and in keeping with the observed bFGF immunostaining, bFGF mRNA is also found. As performed in this study, whole tumour RT-PCR is unable to determine the cellular origin of these mRNA, which could arise from tumour cells, from cells of the extracellular matrix, vascular support cells, or from inflammatory cells within the tumour.49–52,55,56 In situ hybridisation or single cell RT-PCR is necessary to answer this question. It must be stated however that the presence of mRNA does not necessarily reflect protein production, and it may be that the identified transcripts give rise to low levels or non-functional protein. By extension, the demonstration of protein by immunohistochemistry similarly does not necessarily indicate functional protein.
Having found both bFGF and VEGF to be present in uveal melanoma, we asked whether uveal melanoma cells could promote or support the growth of endothelial cells using a co-culture system. These pilot studies were aimed to develop a reproducible method for co-culture of uveal melanoma cells and endothelial cells, and to examine the effects of exogenous growth factors and antibodies against these factors on the cross talk between these cells. After determining the suitability of several culture media for the support of both endothelia and melanoma, four transwell experiments were performed in triplicate using a rat brain microvascular cell line, GPNT, and HUVEC. Cell lines often behave differently from tissue derived cells57 and we were not surprised to find some differences in the growth characteristics of these target cells. We chose to use the dual chamber transwell system, as this configuration permits the diffusion of soluble molecules between isolated cells and prevents direct cell-cell contact. Unfortunately we were not able to utilise a serum free defined medium as cell viability was compromised. We therefore chose to use media with minimal supplementation (1% human AB serum) and devoid of recommended supplements such as total brain extract (as per EBM media, Clonetics). We were unable to detect VEGF or bFGF in our media by ELISA before initiation of the co-culture period.
The co-culture studies demonstrate that uveal melanoma can support and stimulate the growth of both a transformed rat brain endothelial cell line and primary HUVEC, the latter being especially responsive. Melanoma stimulated endothelial cell growth was reduced, but not eliminated by anti-bFGF and anti-VEGF antibodies and supports a role of these cytokines in uveal melanoma endothelial cell proliferation. The observed inability of antigrowth factor antibodies to completely block melanoma stimulated endothelial cell proliferation may reflect inadequate molar ratios of antibody to antigen, or could point to a multiplicity of growth factors which together play a part in angiogenesis. It is interesting to note that occasional tumours were immunohistochemically negative for both bFGF and VEGF, suggesting the importance of other angiogenic factors. There was also no apparent relation between the degree of immunohistochemical staining for VEGF, bFGF, and microvessel count, further suggesting that these two growth factors are unlikely to be sole determinants of uveal melanoma angiogenesis.
In this study we observed that exogenous bFGF is not necessary for autonomous cell division of uveal melanoma, but where assayed, low levels of this cytokine were found in cell culture supernatants indicating endogenous production. Previous cytokine studies in this laboratory (Neale et al, unpublished) also determined that exogenous bFGF had inconsistent effects on uveal melanoma growth in culture. However, addition of bFGF to endothelial cell cultures led to enhanced growth, but generally had no supplemental effect when added in addition to the melanoma cells in co-culture. This suggests either that the melanoma cells had already maximally stimulated the endothelial cell response, or that the tumour cells in some way interfere with endothelial cell utilisation of exogenous bFGF. A potential role for bFGF in endothelial cell growth is also supported by the functional interruption of bFGF by neutralising antibodies in the presence of melanoma.
In summary, the data reported here show that bFGF and VEGF can be found in uveal melanoma, though VEGF staining was not common and was associated with areas of fibrosis and necrosis. Basic FGF was found diffusely through the tumour, while both cytokines could be found associated with the microvasculature. Cultured primary human uveal melanoma cells were shown to support the growth of both a transformed endothelial cell line and primary human endothelial cells. That this interaction could be modified with pro-cytokine and anti-cytokine stimulation suggests the existence of precise cross talk between a tumour and its vasculature. Further studies are under way to determine more precisely the nature of the complex interaction of different cytokines and cell types within uveal melanomas.
Acknowledgments
We wish to thank Ms Chrysanthopoulou for her invaluable assistance, and Dr I Zachary for provision of HUVEC. This research was supported by the Guide Dogs for the Blind Association and Schering-Plough Ltd.
Abbreviations
bFGF, basic fibroblast growth factor
BBE, bovine brain extract
BSA, bovine serum albumin
ELISA, enzyme linked immunosorbent assay
HUVEC, human umbilical vein endothelial cells
PCR, polymerase chain reaction
RLU, relative light units
RT-PCR, reverse transcriptase polymerase chain reaction
TBS, TRIS buffered saline
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Albert DM, Niffenegger AS, Wilson JK. Treatment of metastatic uveal melanoma: review and recommendations. Surv Ophthalmol 1992;36:429–38. [DOI] [PubMed] [Google Scholar]
- 2.Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the MD Anderson Cancer Center experience and prognostic factors. Cancer 1995;76:1665–70. [DOI] [PubMed] [Google Scholar]
- 3.Folberg R, Pe'er J, Gruman LM, et al. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma:a matched case-control study. Hum Pathol 1992;23:1298–305. [DOI] [PubMed] [Google Scholar]
- 4.Foss AJ, Alexander RA, Jefferies LW, et al. Microvessel count predicts survival in uveal melanoma. Cancer Res 1996;56:2900–3. [PubMed] [Google Scholar]
- 5.Folberg R, Mehaffey M, Gardner LM, et al. The microcirculation of choroidal and ciliary body melanomas. Eye 1997;11:227–38. [DOI] [PubMed] [Google Scholar]
- 6.Daniels KJ, Boldt HC, Martin JA, et al. Expression of type VI collagen in uveal melanoma: its role in pattern formation and tumor progression. Lab Invest 1996;75:55–66. [PubMed] [Google Scholar]
- 7.Foss AJE, Alexander RA, Hungerford JL, et al. Reassessment of the PAS patterns in uveal melanoma. Br J Ophthalmol 1997;81:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mäkitie T, Summanen P, Tarkkanen A, et al. Microvascular loops and networks as prognostic indicators in choroidal and ciliary body melanomas. J Natl Cancer Inst 1999;91:359–67. [DOI] [PubMed] [Google Scholar]
- 9.Tapper D, Langer R, Bellows AR, et al. Angiogenesis capacity as a diagnostic marker for human eye tumors. Surgery 1979;86:36–40. [PubMed] [Google Scholar]
- 10.Folkman J. What is the evidence that tumors are angiogenesis dependent. J Natl Cancer Inst 1990;82:4–6. [DOI] [PubMed] [Google Scholar]
- 11.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis B correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Weidner N. Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol 1998;184:119–22. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- 14.Fallowfield ME, Cook MG. The vascularity of primary cutaneous melanoma. J Pathol 1991;164:241–4. [DOI] [PubMed] [Google Scholar]
- 15.Fox SB. Tumour angiogenesis and prognosis. Histopathology 1997;30:294–301. [DOI] [PubMed] [Google Scholar]
- 16.Macchiarini P, Fontanini G, Hardin MJ, et al. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 1992;340:145–6. [DOI] [PubMed] [Google Scholar]
- 17.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995;1:149–53. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Christofori G, Naik P, et al. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer 1996;32A:2386–93. [DOI] [PubMed] [Google Scholar]
- 19.McCulloch P, Choy A, Martin L. Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet 1995;346:1334–5. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–64. [DOI] [PubMed] [Google Scholar]
- 21.Parangi S, O'Reilly M, Christofori G et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci 1996;93:2002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergers G, Javaherian K, Lo KM, et al. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 1999;284:808–82. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 1985;315:115–22. [DOI] [PubMed] [Google Scholar]
- 24.Pepper MS, Mandriota SJ, Jeltsch M, et al. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 1998;177:439–52. [DOI] [PubMed] [Google Scholar]
- 25.Hata Y, Rook SL, Aiello LP. Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes 1999;48:1145–55 [DOI] [PubMed] [Google Scholar]
- 26.Dotto GP, Moellmann G, Ghosh S, et al. Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotypes in a reconstituted cutaneous environment. J Cell Biol 1989;109:3115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halaban R. Growth factors and melanomas. Sem Oncol 1996;23:673–81. [PubMed] [Google Scholar]
- 28.Richmond A. The pathogenic role of growth factors in melanoma. Sem Dermatol 1991;10:246–55. [PubMed] [Google Scholar]
- 29.Rodeck U, Melber K, Kath R, et al. Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J Invest Dermatol 1991;97:20–6. [DOI] [PubMed] [Google Scholar]
- 30.Reed JA, McNutt NS, Albino AP. Differential expression of basic fibroblast growth factor (bFGF) in melanocytic lesions demonstrated by in situ hybridization. Implications for tumor progression. Am J Pathol 1994;144:329–36. [PMC free article] [PubMed] [Google Scholar]
- 31.Albino AP, Davis BM, Nanus DM. Induction of growth factor RNA expression in human malignant melanoma:markers of transformation. Cancer Res 1991;51:4815–20. [PubMed] [Google Scholar]
- 32.Szebenyi G, Fallon JF. Fibroblast growth factors as multifunctional signalling factors. Int Rev Cytol 1999;185:45–106. [DOI] [PubMed] [Google Scholar]
- 33.Czubayko F, Liaudet-Coopman ED, Aigner A, et al. A secreted FGF-binding protein can serve as the angiogenic switch in human cancer. Nat Med 1997;3:1137–40. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Becker D. Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 1997;3:887–93. [DOI] [PubMed] [Google Scholar]
- 35.Vinores SA, Küchle M, Mahlow J, et al. Blood-ocular barrier breakdown in eyes with ocular melanoma. A potential role for vascular endothelial growth factor/vascular permeability factor. Am J Pathol 1995;147:1289–97. [PMC free article] [PubMed] [Google Scholar]
- 36.Kvanta A, Steen B, Seregard S. Expression of vascular endothelial growth factor (VEGF) in retinoblastoma but not in posterior uveal melanoma. Exp Eye Res 1996;63:511–8. [DOI] [PubMed] [Google Scholar]
- 37.Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998;105:412–16 [DOI] [PubMed] [Google Scholar]
- 38.Stitt AW, Simpson DA, Boocock C, et al. Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumours. J Pathol 1998;186:306–12. [DOI] [PubMed] [Google Scholar]
- 39.Sheidlow TG, Hooper PL, Cruckley C, et al. Expression of vascular endothelial growth factor in uveal melanoma and its correlation with metastasis. Br J Ophthalmol (in press). [DOI] [PMC free article] [PubMed]
- 40.Ijland SAJ, Jager MJ, Heijdra BM, et al. Expression of angiogenic and immunosuppressive factors by uveal melanoma cell lines. Melanoma Res 1999;9:445–50. [DOI] [PubMed] [Google Scholar]
- 41.Cree IA, McDougall AC, Coghill G, et al. Quantitation of the granuloma fraction in leprosy skin biopsies by planimetry. Int J Lepr 1985;53:582–6. [PubMed] [Google Scholar]
- 42.Beck JS, Nordin BEC. Histological assessment of osteoporosis by iliac crest biopsy. J Pathol Bacteriol 1960;80:391–7. [DOI] [PubMed] [Google Scholar]
- 43.Andreotti PE, Cree IA, Kurbacher CM, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res 1995;55:5276–82. [PubMed] [Google Scholar]
- 44.Cree IA. Luminescence-based cell viability testing. In: LaRossa RA ed. Bioluminescence methods and protocols. Methods in Molecular Biology 1998;102:169–77. [DOI] [PubMed] [Google Scholar]
- 45.Myatt N, Cree IA, Kurbacher CM, et al. The ex vivo chemosensitivity profile of choroidal melanoma. Anti-Cancer Drugs 1997;8:756–62. [DOI] [PubMed] [Google Scholar]
- 46.Neale MH, Myatt N, Cree IA, et al. Combination chemotherapy for choroidal melanoma:ex vivo sensitivity to treosulfan with gemcitabine or cytosine arabinoside. Br J Cancer 1999;79:1487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukumura D, Xavier R, Sugiura T, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell 1998;94:715–25. [DOI] [PubMed] [Google Scholar]
- 48.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA 1998;95:15809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinbrech DS, Mehrara BJ, Chau D, et al. Hypoxia upregulates VEGF production in keloid fibroblasts. Ann Plast Surg 1999;42:514–19. [DOI] [PubMed] [Google Scholar]
- 50.Simpson DA, Murphy GM, Bhaduri T, et al. Expression of the VEGF gene family during retinal vaso-obliteration and hypoxia. Biochem Biophys Res Commun 1999;262:333–40. [DOI] [PubMed] [Google Scholar]
- 51.Dibbens JA, Miller DL, Damert A, et al. Hypoxic regulation of vascular endothelial growth factor mRNA stability requires the cooperation of multiple RNA elements. Mol Biol Cell 1999;10:907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozaki H, Yu AY, Della N, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina:temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 1999;40:182–9. [PubMed] [Google Scholar]
- 53.Engelberts I, Möller A, Schoen GJ, et al. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res 1991;10:69–76. [PubMed] [Google Scholar]
- 54.Moussa K, Michie HJ, Cree IA, et al. Phagocyte function and cytokine production in community acquired pneumonia. Thorax 1994;49:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iijima K, Yoshikawa N, Nakamura H. Activation-induced expression of vascular permeability factor by human peripheral T cells: a non-radioisotopic semiquantitative reverse transcription-polymerase chain reaction assay. J Immunol Methods 1996;196:199–209. [DOI] [PubMed] [Google Scholar]
- 56.Tolnay E, Kuhnen C, Voss B, et al. Expression and localization of vascular endothelial growth factor and its receptor flt in pulmonary sarcoidosis. Virchows Arch 1998;432:61–5. [DOI] [PubMed] [Google Scholar]
- 57.Andreotti PE, Linder D, Hartmann DM, et al. TCA-100 tumor chemosensitivity assay: differences in sensitivity between cultured tumor cell lines and clinical studies. J Biolum Chemilum 1994;9:373–8. [DOI] [PubMed] [Google Scholar]