Abstract
Background/aims: In diabetics, cataract is associated with higher risk of death. In non-diabetics the data are conflicting, but some indicate an association between one type of cataract (nuclear) and increased mortality. The aim of this study was to estimate and compare age and sex specific mortality for elderly people with and without cataract in a population based cohort.
Methods: A random sample drawn from a defined population of elderly people (age 65 and older) registered with 17 general practice groups in north London formed the study cohort and were followed up for 4 years. The age and sex specific mortality from various causes was estimated and compared in those with and without cataract.
Results: In non-diabetics (n=1318), cataract (lens opacity at baseline) was significantly associated with higher mortality in women. The age standardised death rate per 1000 was 39.8 and 24.8 in women with and without cataract, respectively (age adjusted hazard ratio 1.7, confidence limits 1.1 to 2.7, p=0.032). This was not the case in non-diabetic men (hazard ratio 0.9, confidence limits 0.6 to 1.5, p=0.782). The excess mortality in women with cataract was consistent for cardiovascular, respiratory, and other non-cancer causes of death. There was no association between cataract and mortality from cancer.
Conclusions: This study has shown, for the first time, that cataract is associated with higher mortality in women but not in men, among the non-diabetic population. This sex effect suggests that women may be exposed to risk factors that increase both the risk of cataract and mortality, and that men may have little or no exposure to these “sex specific” factors. Possible risk factors that warrant further investigation may be those associated with some pregnancy and childbearing experience.
Keywords: mortality, women, cataract, London
Cataract as a cause of visual impairment is a major problem in the elderly. The World Heath Report in 1998 estimated that of the 44.9 million blind, 19.3 million (43%) were blind in both eyes from cataract. Policymakers at international level highlight the need for planning for prevention and related aetiological research, and the development of models for meeting the population need for surgical treatment.1,2 In England and Wales, among the 8.3 million people aged 65 and older, an estimated 2.4 million have vision impairing cataract, and more than one million new cases are expected over a 5 year period.3 A comprehensive approach to the modelling of the population dynamics of the problem and the level of services, requires reliable data on survival. There is some evidence that cataract may be associated with mortality. This comes from two main types of work within ophthalmic research. Most have been hospital based studies where the cases having cataract surgery are compared either with other hospital patients or with the general population for mortality. A few (five), have been population based studies where cohorts with and without lens opacities have been followed up and the death rates compared.4–8 The first of these (1985)4 from the Framingham Eye Study found an effect (increased mortality in cataract) in diabetics, but no such effect was found in the non-diabetic cohort. The second study (1995)5 in the United States found that poor survival in non-diabetics was associated with level of severity of nuclear sclerosis (one of three main types of cataract), but no such association was found in diabetics. In the only population based study in the United Kingdom (1993)6 there was a significant association between nuclear cataract and higher mortality among non-diabetics, but other types of cataract were not associated with mortality.
Our study was designed to provide reasonably precise estimates of mortality in men and in women with and without cataract, for two main purposes—firstly, to enable modelling of the population dynamics of cataract and its management in the United Kingdom for public health; secondly, to establish whether or not cataract is a marker for higher mortality in non-diabetics, giving due regard to the fact that men have higher mortality and lower prevalence of cataract than women.9–11
METHODS
The North London Eye Study was a population based cross sectional survey of the elderly (aged 65 and older) using a two stage cluster random sampling method, where the general practice groups constituted the primary sampling units. The lens status at the time of the survey was ascertained by ophthalmologists using a slit lamp microscope and the lens opacity classification system (LOCSII).12 The methodology details have been reported recently in the BMJ.10
Deaths occurring in the study cohort were monitored by the Office for National Statistics, and notified to the study coordinators. The monitoring covered a period from March 1995 (start of the survey) to December 1999 (3 years from the end of the survey). A lag period of 12 months beyond the monitoring period was allowed for the death notifications, so that late notices of death would not be lost. This, however, proved to be an unnecessary precaution. The mortality data included all recorded causes of death, place and date of death, and postmortem status.
At baseline, members of the study cohort were classified according to presence or absence of cataract, and also by type of lens opacity. The cataract cases were those with lens opacity (LOCSII grade 1 or higher) of any type in either eye, or those who had had cataract surgery (aphakic). For the survival analysis by type of cataract, three non-exclusive groups were defined: nuclear opacity, cortical, and posterior subcapsular opacity in either eye. Each group was compared to a common group who had no cataract in either eye, in separate regression models. Since the type of cataract was not known in aphakic subjects, these were excluded from the cataract type classification. Other personal data ascertained at baseline included date of birth, sex, ethnic group, history of smoking, and diabetes.
Three distinct areas of residence were defined, according to the Jarman underprivileged area scores. The Jarman scores were −0.88 and 0.35 for area 1; 18.51, 21.35, and 33.98 for area 2; and 36.06 for area 3, the most underprivileged area of the three.10
Statistical analysis
To compare the mortality in the whole study cohort with that in the general population, a standardised mortality ratio (SMR) for the study sample was computed using the age specific mortality data for England and Wales as the standard. Confidence limits for the SMR were calculated according to the method outlined by Armitage,13 when the purpose is to see whether the SMR differs significantly from 100%.
The main non-diabetic cohort was analysed separately from the diabetic group, in line with previous studies that found diabetes to be a significant effect modifier—that is, the “effect” of cataract on mortality was different in diabetics compared to non-diabetics.4
Age standardised death rates for people with and without cataract were computed using the age structure of the whole cohort as the standard. The Cox proportional hazard model was used to assess the association between cataract status at baseline and death rate. Factors included in the models to adjust for their confounding effects were age (as six age groups: 65–69, 70–74, 75–79, 80–84, 85–89, 90+); sex; ethnic group (white, Asian); smoking history (non-smokers, past and current smokers); and area of residence (areas 1, 2, and 3). The survival analyses included a detailed assessment of interaction between cataract and sex, to identify any significant differences in men and women in respect of the cataract mortality association (effect modification by sex). This included comparison of age adjusted death rate ratios in men and women, and the inclusion (or exclusion) of a “sex cataract” interaction term from the Cox regression models to assess the improvement (or otherwise) in the model fit. Age adjusted survival curves were constructed for men and for women with and without cataract, using the output from the Cox proportional hazards models. A similar analysis was also performed to assess any effect modification by age. The appropriateness of the proportional hazards model was checked by “log minus log” plots of hazard functions for levels of factors such as cataract status. The plots should show constant differences (parallel curves) for the levels.
RESULTS
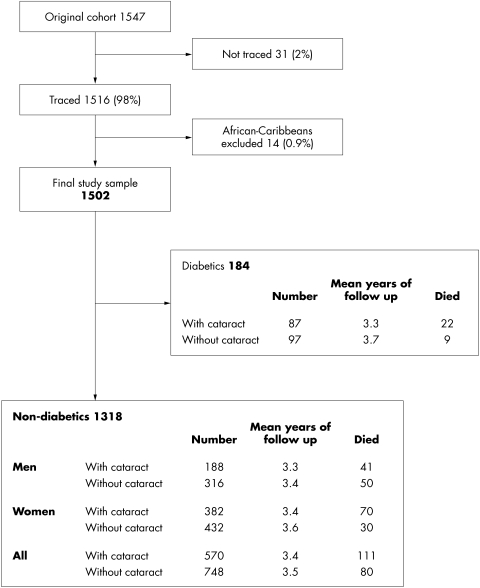
Of the original study sample of 1547 people aged 65 and older, 31 could not be traced. These, together with 14 African-Caribbean people, were excluded from the survival analysis, leaving a final study cohort of 1502. The follow up details are shown in Figure 1. The population from which the study sample was drawn is described elsewhere.10
Figure 1.
The study cohort and the follow up.
The baseline profile of the study cohort of 1502 is shown in Table 1. There were 222 deaths in the study cohort (191 in non-diabetics and 31 in diabetics). The 1 year cumulative death rate in the whole study cohort was about 79% of the annual death rate for the population of England and Wales (SMR 78.9% (95% confidence limits 67.8 to 90.1%). The details are shown in Table 2.
Table 1.
Profile of the study cohort at start of the follow up (n = 1502 aged 65 and older)
| Number (%) | |||
| Characteristic | Without cataract | With cataract | All |
| Age structure: | |||
| 65–69 | 296 (35) | 79 (12) | 375 (25) |
| 70–74 | 273 (32) | 132 (20) | 405 (27) |
| 75–79 | 164 (19) | 162 (25) | 326 (22) |
| 80–84 | 70 (8) | 157 (24) | 227 (15) |
| 85–89 | 34 (4) | 97 (15) | 131 (9) |
| 90+ | 8 (1) | 30 (5) | 38 (2) |
| Sex: females | 468 (55) | 426 (65) | 894 (60) |
| Ethnic group: | |||
| White | 821 (97) | 616 (94) | 1437 (96) |
| Asian | 24 (3) | 41 (6) | 65 (4) |
| Diabetics | 97 (11) | 87 (13) | 184 (12) |
| Smokers (past and current) | 132 (16) | 102 (16) | 234 (16) |
Table 2.
Mortality in the study cohort, compared to that in the elderly population (age 65 and older) of England and Wales
| Statistic* | North London Eye Study cohort | Population of England and Wales |
| a Death rate per 1000 PYAR | 42.6 | |
| b 1 year cumulative incidence (per 1000) | 41.7 | 56.3 |
| c Standardised cumulative incidence | 42.1 | |
| d Number of people aged 65 and older | 1502 | 8258800 |
| e Total PYAR | 5210.1 | |
| f Observed number of deaths | 222 | 465102 |
| g Expected deaths if population rates applied | 281.3 | |
| h Standardised mortality ratio: f/g (%) | 78.9 | |
| k 95% confidence limits for SMR | 67.8 to 90.1 |
*Calculations:
a, observed number of deaths/total person years at risk = (222/5210.1).
b, for NLES, derived from a using exp(−0.0427 × 1.0) × 103.
For population, derived from Office for National Statistics 1996 mortality statistics.
c, calculated by direct standardisation, using the study cohort sex and age specific cumulative incidence rates, and the population sex-age structure as the standard.
f, For population, taken from Office for National Statistics 1996 mortality statistics.
g, calculated by applying the population sex and age specific death rates (Pi) to the study cohort.
k, variance of the SMR taken as 104 × ΣniPiQi/(ΣniPi)2 as outlined by Armitage,12 when the purpose is to see whether the SMR differs significantly from 100% (ni is the number in sex-age group i in the study cohort, and Qi=1−Pi).
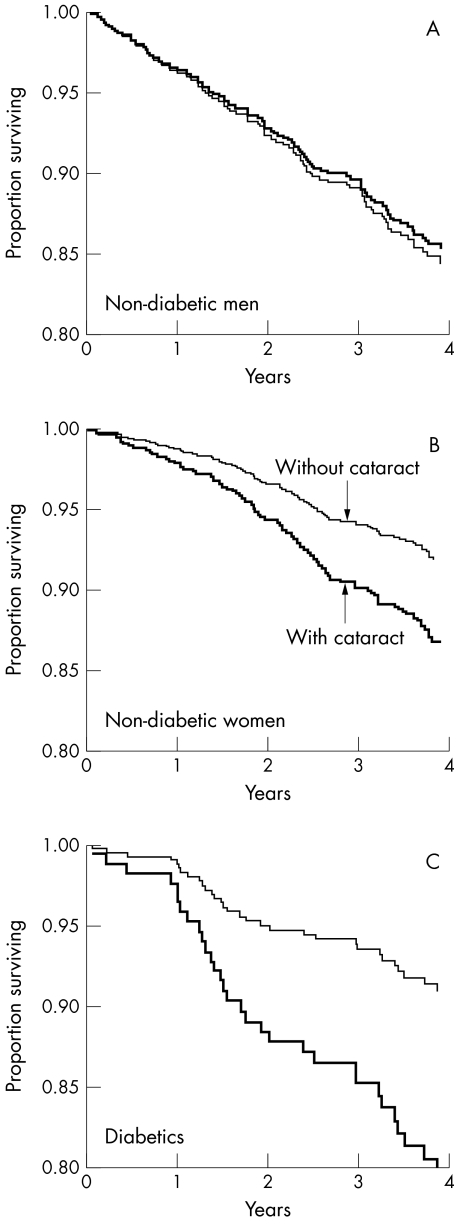
The main cohort of 1318 non-diabetics was analysed separately from the 184 diabetics. Table 3 and Figure 2 show the initial survival analysis results for the non-diabetics. These suggest a marked dependence of the cataract mortality association upon sex—that is, the increased mortality in cataract was apparent in women but not in men. This effect modification by sex was statistically significant, as indicated by formal tests (for the observed differential risk in women). These included stepwise forward inclusion and backwards elimination of the “sex cataract” interaction term in the regression models. The significant (p=0.035) interaction term could not be removed and had to be included in the resulting final models (critical p values for stepwise and backwards procedures were set at 0.05 and 0.15, respectively). If the effect modification was ignored, the hazard ratio for cataract in the whole sample was 1.2 (0.9 to 1.6, p=0.292), after adjustment for age, smoking, and sex. The “sex cataract” interaction was apparent within each of the three main types of cataract. There was no significant effect modification by age.
Table 3.
Age standardised death rates (per 1000) in non-diabetic people with and without cataract, stratified by sex. The age adjusted hazard ratios are from Cox proportional hazards regression models, values greater than 1.0 indicating excess mortality in people with cataract (that is, mortality in cataract cases = hazard ratio × mortality in people without cataract)
| Subgroup | Died | Age standardised death rate per 1000 | Age adjusted hazard ratio (95% CL) | p Value |
| Women | ||||
| with cataract | 70 | 39.8 | 1.7 (1.1 to 2.7) | 0.032 |
| without cataract | 30 | 24.8 | ||
| Men | ||||
| with cataract | 41 | 57.8 | 0.9 (0.6 to 1.5) | 0.782 |
| without cataract | 50 | 63.0 | ||
Figure 2.
Age adjusted survival curves for men and women with and without cataract.
As the initial results showed strong effect modification by sex, the subsequent multiple regression analyses were performed for men and women separately, and are reported in Table 3 as age adjusted hazard ratios from Cox regression models. In women, cataract (lens opacity at baseline) was significantly associated with higher mortality. The age standardised death rate per 1000 was 39.8 and 24.8 in women with and without cataract respectively (age adjusted hazard ratio 1.7, confidence limits 1.1 to 2.7, p=0.032). This was not the case in non-diabetic men, where the age standardised death rates were 57.8 and 63.0 per 1000 with and without cataract respectively (hazard ratio 0.9, confidence limits 0.6 to 1.5, p=0.782). The excess mortality in women with cataract was consistent for cardiovascular, respiratory, and other non-cancer causes of death (age adjusted hazard ratio for non-cancer deaths 2.0, confidence limits 1.1 to 3.4, p=0.015). There was no association between cataract and mortality from cancer. Further adjustment for effects of smoking, for area of residence, and for ethnic group did not materially change the findings.
Excluding the Asian ethnic group from the analysis did not materially change the findings. The age adjusted hazard ratio in non-diabetic women remained significant, only slightly reduced from 1.7 to 1.6 (confidence limits 1.0 to 2.6, p=0.038). Similarly, the age adjusted hazard ratio in men remained unchanged at 0.9 after excluding the Asian group.
All three types of cataract were significantly associated with mortality in non-diabetic women. The age adjusted mortality hazard ratios for nuclear opacity (1.8), cortical opacity (1.9), and posterior subcapsular opacity (2.1) were all significant (p<0.04 for each of the three cataract types). The corresponding hazard ratios in non-diabetic men were 0.8, 0.9, and 1.1 (p>0.3 for each cataract type). The distribution of the main types of cataract in women was similar to that in men (Table 4).
Table 4.
Distribution of the main types of cataract in non-diabetic men and women
| Type of cataract | Women (%) | Men (%) |
| Nuclear only | 124 (32) | 50 (27) |
| Cortical only | 38 (10) | 24 (13) |
| Posterior subcapsular only | 7 (2) | 4 (2) |
| Nuclear and cortical | 56 (15) | 26 (14) |
| Other mixed types | 68 (18) | 44 (23) |
| Unknown/aphakia | 89 (23) | 40 (21) |
| Totals | 382 (100) | 188 (100) |
Among diabetics (n=184), the mortality was significantly higher in both men and women with cataract (Fig 2). The age and sex adjusted hazard ratio was 2.6, with 95% confidence limits of 1.1 to 6.0 (p=0.023). Because of the small number of diabetics, the analysis was limited to mortality from all causes and all types of cataract combined.
DISCUSSION
Our study cohort was a representative random sample of a defined population rather than a selected special group, and the North London Eye Study survey achieved an overall response rate of 84% (1547/1840). The inferences, therefore, may be more readily generalised. The sample size was relatively large, allowing detailed analysis of non-diabetics, and estimates of mortality by sex and cataract status, with reasonable precision. The exclusion of the African-Caribbean group was based on anticipated problems that could arise from very small numbers in a stratum, when adjustments have to be made for the possible confounding effects of ethnic group in the multiple regression analysis. The ascertainment of death during the follow up period was exhaustive (as in the previous smaller UK study), with successful tracing of 98% of the cohort by the Office for National Statistics, whereas the verification of death in some of the US studies had to rely on non-official sources and was problematic.
The low SMR found in the study was not unexpected. Local mortality data from the district indicate that elderly stroke cases in the district have better survival compared to those in many other London districts, and that the mortality among the elderly in the district is generally lower than the regional and the national figures (the annual public health reports of the Director of Public Health, Barnet, 1993 and 1994).
The few recent studies in this area that have found an association between mortality and cataract, make no distinction between men and women, the focus being on the morphological type of cataract. Our findings suggest that in non-diabetics all types of cataract are markers for increased mortality in women, but none is associated with mortality in men. We note with interest a new report by Christen et al who found no association between cataract and mortality in a large cohort of 18 669 male physicians, participating in the US Physicians' Health Study, after a long average follow up period of 12.4 years.14
That cataract as a marker for increased mortality should depend on sex was not a declared explicit a priori hypothesis. It was, however, intended from the outset, to estimate age specific mortality for men and for women separately by cataract status, and to look for effect modification by age and by sex, according to the study protocol. The interaction was discovered during the analysis. This included multiple regression analysis using a strategy described by Kleinbaum et al,15 with particular care taken to identify effect modification by sex and by age (“sex cataract” and “age cataract” interactions) if any, giving priority to issues of validity over precision. The modelling process in such analysis demands deep familiarity with the clinical subject matter as well as understanding of the statistical procedures and their limitations. Failing to detect important interactions when they are present may lead to overall estimates that are misleading or inappropriate for one or more of the population strata. Equally important is the caution in accepting an observed interaction as a real phenomenon. Thus, in addition to the statistical evidence, careful considerations of the nature of the data, the groupings, the consistency, and the biological feasibility of an observed interaction, are important prerequisites to the final conclusion. In our study, the statistical evidence for the interaction was strong, the stratification of the data by sex was not arbitrary, and consideration of the biological feasibility of the phenomenon (the observed differential risk in women) raises interesting issues and questions.
A possible explanation for the observed differential risk in women is that there may be one or more “sex specific” risk factors (for cataract and mortality), to which men have little or no exposure. Possible risk factors that warrant further investigation may be those associated with some pregnancy and childbearing experience. Another possible explanation for our findings is that men have higher exposure to factors that increase mortality but not the risk of cataract. The repeated finding of higher cataract prevalence in women compared to age matched men is consistent with this idea. Detailed lifetime exposure data on factors that might cause systemic damage resulting in both higher risk of cataract and higher mortality could offer valuable pointers to risk factors for cataract in women.
Acknowledgments
Sources of support: Thames Regional Health Authority R&D Responsive Funding (chair Professor R Feldman and the committee), Moorfields Eye Hospital, and Barnet Health Authority.
We thank the members of the public and their families who participated in the study. We thank the chief executive of Moorfields Eye Hospital, Mr I A Balmer, for his interest and support.
Contributors
All authors were part of the study team. AR and DCM were the principal investigators. AR conceived the study, collected data and contributed to the analysis and writing of the paper. DCM helped with the study design, did the analysis, and contributed to the writing of the paper. PD was responsible for validation of the cataract status data and its classification. SF was the public health and demography adviser. GV and JJ were in charge of quality control for documentation of the clinical ophthalmic data. AC was responsible for collection and validation of the health status data.
REFERENCES
- 1.Thylefors B, Negrel AD, Pararajasegaram R, et al. Global data on blindness. Bull World Health Organ 1995;73:115–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Foster A. Cataract—a global perspective: output, outcome and outlay. Eye 1999;13:449–53. [DOI] [PubMed] [Google Scholar]
- 3.Minassian DC, Reidy A, Desai P, et al. The deficit in cataract surgery in England and Wales and the escalating problem of visual impairment: epidemiological modelling of the population dynamics of cataract. Br J Ophthalmol 2000;84:4–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podgor MJ, Cassel GH, Kannel WB. Lens changes and survival in a population-based study. N Engl J Med 1985;313:1438–43. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BEK, Moss SE. Age-related eye disease and survival. The Beaver Dam Study. Arch Ophthalmol 1995;113:333–9. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JR, Sparrow JM, Gibson JM, et al. Cataract and survival in an elderly nondiabetic population. Arch Ophthalmol 1993;111:675–9. [DOI] [PubMed] [Google Scholar]
- 7.Minassian DC, Mehra V, Johnson GJ. Mortality and cataract: findings from a population-based longitudinal study. Bull World Health Organ 1992;70:219–23. [PMC free article] [PubMed] [Google Scholar]
- 8.West SK, Munoz B, Istre J, et al. Mixed lens opacity and subsequent mortality. Arch Ophthalmol 2000;118:393–7 [DOI] [PubMed] [Google Scholar]
- 9.Gibson JM, Rosenthal AR, Lavery J. A study of the prevalence of eye disease in the elderly in an English community. Trans Ophthalmol Soc UK 1984;104:196–203. [PubMed] [Google Scholar]
- 10.Reidy A, Minassian DC, Vafidis G, et al. Prevalence of serious eye disease and visual impairment in a north London population: population based, cross sectional study. BMJ 1998;316:1643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell P, Cumming RG, Attebo K, et al. Prevalence of cataract in Australia. Ophthalmology 1997;104:581–8. [DOI] [PubMed] [Google Scholar]
- 12.Chylack LT, Leske MC, Khu P, et al. Lens opacities classification system II (LOCS II). Arch Ophthalmol 1989;107:991–7. [DOI] [PubMed] [Google Scholar]
- 13.Armitage P. Statistical methods in medical research. Oxford: Blackwell Scientific, 1977.
- 14.Christen WG, Glynn RJ, Ajani UA, et al. Baseline self-reported cataract and sibsequent mortality in Physicians' Health Study I. Ophthalmic Epidemiol 2000;7:115–25. [PubMed] [Google Scholar]
- 15.Kleinbaum DG, Kupper LL, Morgensten H. Epidemiologic research: principles and quantitative methods. New York: Van Nostrand Reinhold, 1982.