Abstract
Aims: To assess the vitreous penetration of oral levofloxacin (a new fluoroquinolone antibiotic with improved Gram positive activity) in uninflamed phakic eyes.
Methods: 15 patients for macula hole surgery were recruited to the study. 10 received a single 500 mg dose of levofloxacin by mouth preoperatively. Five acted as controls. Serum and undiluted vitreous samples were obtained at surgery and analysed by HPLC.
Results: Levofloxacin was detectable 2.5 hours after administration in the vitreous. A peak concentration of 1.6 μg/ml (or mg/l) was measured between 2.5 and 4 hours post-dose.
Conclusion: Oral levofloxacin reaches the vitreous rapidly in the uninflamed phakic eye. Levels did not reach MIC90 for the commonest infecting organisms. Nevertheless, levofloxacin would be expected to be active against a higher proportion of infecting organisms than either ciprofloxacin or ofloxacin.
Keywords: vitreous penetration, levofloxacin, phakic human eye
The blood-ocular barrier effectively excludes many toxic substances from the eye, but also limits entry of many therapeutic agents including antibiotics. The fluoroquinolone antibiotics have been shown to produce therapeutic levels in both aqueous and vitreous after systemic administration.1–3 Fluoroquinolones have a unique mechanism of action, inhibiting bacterial DNA gyrase, leading to cell death. The concentration required for killing susceptible organisms is little different from that required for inhibition of growth.
Unfortunately, their spectrum of antimicrobial activity does not coincide with the organisms frequently found in endophthalmitis. Following cataract surgery 94% of culture positive infections are caused by Gram positive organisms, including 70% coagulase negative staphylococci.4 The activity of fluoroquinolones against Gram positive organisms has limited their usefulness in other specialties—for example, in the treatment of pneumonia. This has led to the development of fluoroquinolones with an improved bactericidal spectrum. Levofloxacin (the active S-isomer of ofloxacin) is the first of these to be licensed in the United Kingdom. Its minimum inhibitory concentrations are approximately half those for racemic ofloxacin.
We aimed to evaluate how quickly levofloxacin reached therapeutic levels in the vitreous after oral administration. Previous studies assessing vitreous penetration of antibiotics have included heterogeneous patient groups with a whole variety of underlying conditions that would be expected to severely compromise the blood-ocular barrier—for example, proliferative diabetic retinopathy, trauma, retinal detachment, dropped nuclei.1–3,5 We selected patients having vitrectomy for idiopathic macula hole only, so that penetration was studied in as near as possible the normal uninflamed eye.
METHODS
The study was approved by the Liverpool research and ethics committee and informed consent obtained from all participants. Patients undergoing vitrectomy to treat macula hole were recruited between June and December 2000. Exclusion criteria were any previous eye surgery, diabetic retinopathy, epilepsy, sensitivity to quinolones, renal impairment, pregnancy, and age less than 18 years. Ten patients received a single 500 mg tablet of levofloxacin a variable time before surgery. Five patients acted as controls receiving no levofloxacin. A serum sample was obtained immediately before commencement of surgery, and an undiluted vitreous sample obtained with the ocutome before commencing the infusion as previously described.6 Specimens were anonymised and stored at −70°C until analysed by high performance liquid chromatography (HPLC).7 Assays were performed in a masked fashion at the Bristol Centre for Antimicrobial Research and Evaluation. Each sample was tested once. The system was flushed between each test to eliminate the risk of carryover. Positive controls made to a known concentration were used to confirm the accuracy of the assay. Samples were deemed negative at a level of 0.1 μg/ml (or mg/l) or less. Repeat sampling of vitreous from a single patient is not possible, therefore single samples from each patient were combined to construct a model of the pharmacokinetic profile of levofloxacin.
RESULTS
Fifteen patients were recruited. Of the 10 receiving levofloxacin seven were female, three male. Mean age was 65 (range 46–76) and mean weight was 70 kg (range 57–100 kg). All were phakic. Seven had surgery under local anaesthesia with no preoperative fasting; three had a general anaesthetic with 6 hours' fasting. Vitreous samples were collected, on average, 20 minutes (range 15–40 minutes) after the serum samples.
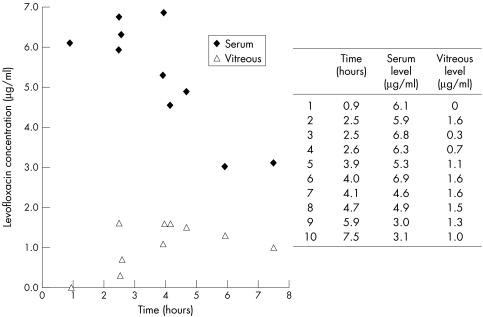
Levels of levofloxacin achieved are shown in Figure 1. Levofloxacin was detectable in the serum of all treated patients, the earliest being taken only 55 minutes after administration (range 3.0–6.9 μg/ml). The range of vitreous levels was 0.0–1.6 μg/ml. No levofloxacin was found in the serum or vitreous of control cases. No adverse drug reactions were noted.
Figure 1.
Levofloxacin levels in vitreous and serum after oral administration.
DISCUSSION
The fluoroquinolones have high oral availability, approximately 99% for levofloxacin, and are absorbed rapidly. Food does not significantly affect absorption. Maximum serum concentrations are achieved after 1 hour when fasted or 2 hours unfasted.8 Our results are consistent with this rapid availability in serum. We have found that levofloxacin reaches the vitreous of uninflamed phakic eyes within 2.5 hours of a single oral dose. The peak concentration measured (1.6 μg/ml) was less than half the highest reported MIC90 for Staphylococcus epidermidis, the commonest organism isolated in post-cataract endophthalmitis (Table 1). It is well recognised that sensitivities to most antibiotics for S epidermidis are very variable (as seen by the large range in Table 1) and resistance widespread. The MIC90 values quoted may not be representative of organisms encountered in endophthalmitis as they include isolates obtained from blood cultures in cancer patients, many of whom had received recent antimicrobial therapy.9 Susceptibility data obtained from ocular isolates would provide the most relevant reference point, but none is presently published for levofloxacin. The small number of isolates available makes calculation of meaningful MIC90 values difficult.
Table 1.
MIC90 values for organisms commonly isolated in bacterial endophthalmitis. Values in μg/ml
| % isolates in EVS4 | Highest MIC908,9,11,12 | Ref 8, 1999 | Ref 9, 1997 | Ref 11, 1994 | Ref 12, 1998 (MIC90 range) | |
| Staphylococcus epidermidis | 70 | 4.0 (16.0) | 4.0 | 4.0 (16.0) | 0.42 | 0.25–0.41 |
| Staphylococcus aureus | 9.9 | 0.5 (16.0) | 0.25 | 0.25 (8.0) | 0.41 (0.52) | 0.25–0.5 (0.05–16) |
| Streptococcus viridans | 3.7 | 2.0 | 2.0 | 2.0 | ||
| Streptococcus pneumoniae* | 2.2 | 2.0 | 2.0 | 1.0 | 1.91 | 0.06–2.0 |
| Enterococcus faecalis | 1.2 | 3.13 | 2.0 | 2.0 | 1.89 | 1.0–3.13 |
| Bacillus cereus† | 0.3 | 2.0 | 0.25 | 2 | ||
| Proteus mirabilis | 1.9 | 0.25 | 0.25 | 0.18 | 0.06–0.25 | |
| Haemophilus influenzae* | 0.05 | 0.05 | 0.03 | 0.02 | 0.015–0.05 |
MIC90 is the minimum concentration of antibiotic required to inhibit replication in 90% of clinical isolates in vitro.
Figures in parentheses are for methicillin resistant organisms.
*More common in late post-trabeculectomy endophthalmitis; †more common in post-traumatic endophthalmitis.
Higher vitreous levels may be achieved when treating endophthalmitis or following trauma due to a breakdown in the blood-ocular barrier. In a rabbit model inflammation increased both the penetration of systemic ofloxacin in to vitreous and prolonged its elimination half life.10 Fiscella et al5 found a repeat 500 mg dose of levofloxacin 12 hours after the first produced higher vitreous levels compared to a single dose alone (mean level of 2.48 μg/ml v 0.59 μg/ml sampled greater than 2 hours after last dose), exceeding most of the MIC90 values they quoted. Our peak concentration of 1.6 μg/ml also exceeds the MIC90 values they chose. However our own review of the literature found higher MIC values and therefore fewer grounds for optimism. Levofloxacin would nevertheless be expected to be effective against a higher proportion of organisms than either ciprofloxacin or ofloxacin as it generally demonstrates lower MIC90 values9,11,12 and equivalent or better vitreous penetration (El Baba et al report a peak vitreous level of 0.49 μg/ml 5.5 hours after a single dose of 750 mg of ciprofloxacin3).
In vitro testing of antibiotic susceptibilities does not relate precisely to in vivo response. Infections due to apparently resistant organisms may respond and, conversely, treatment may fail in susceptible infections, yet it remains the most practical guide available. It is likely that MIC values will rise as resistance becomes more widespread. This has been seen for other fluoroquinolones tested on isolates from bacterial keratitis.13 Discretion should be used to reduce inappropriate use of these antibiotics and limit spread of resistance.
The role of systemic antibiotics in therapy or as prevention for endophthalmitis has not been established.14 Direct intravitreal treatment remains the mainstay of treatment. Systemic agents with adequate vitreous penetration and an appropriate antimicrobial spectrum may have a valuable role. The penetration of levofloxacin and newer fluoroquinolones into the infected eye and their effect on outcome require further investigation.
Acknowledgments
We thank Aventis (formerly Hoechst Marion Roussel) for provision of levofloxacin and covering assay costs; Dr L White and Dr A MacGowan at Bristol Centre for Antimicrobial Research and Evaluation for performing assays; and Professor CA Hart Department of Medical Microbiology, Royal Liverpool University Hospital, for advice on the manuscript.
REFERENCES
- 1.Keren G, Alhalel A, Bartov E, et al. The intravitreal penetration of orally administered ciprofloxacin in humans. Invest Ophthalmol Vis Sci 1991;32:2388–92. [PubMed] [Google Scholar]
- 2.Donnenfeld ED, Perry HD, Snyder RW, et al. Intracorneal, aqueous humor, and vitreous humor penetration of topical and oral ofloxacin. Arch Ophthalmol 1997;115:173–6. [DOI] [PubMed] [Google Scholar]
- 3.El Baba FZ, Trousdale MD, Gauderman WJ, et al. Intravitreal penetration of oral ciprofloxacin in humans. Ophthalmology 1992;99:483–6. [DOI] [PubMed] [Google Scholar]
- 4.Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol 1996;122:1–17. [DOI] [PubMed] [Google Scholar]
- 5.Fiscella RG, Nguyen TKP, Cwik MJ, et al. Aqueous and vitreous penetration of levofloxacin after oral administration. Ophthalmology 1999;106:2286–90. [DOI] [PubMed] [Google Scholar]
- 6.Briggs MC, Mc Donald P, Bourke R, et al. Intravitreal penetration of teicoplanin. Eye 1998;12:252–5. [DOI] [PubMed] [Google Scholar]
- 7.Tobin CM, Sunderland J, White LO, et al. A reverse phase, isocratic high-performance liquid chromatography assay for levofloxacin. J Antimicrob Chemother 1999;43:434–5. [DOI] [PubMed] [Google Scholar]
- 8.Hoechst Marion Roussel. Levofloxacin. Scientific Product Monograph, 1999.
- 9.Rolston KVI, Ho DH, LeBlanc B, et al. In-vitro activity of trovafloxacin against clinical bacterial isolates from patients with cancer. J Antimicrob Chemother 1997;39:Suppl B, 15–22. [DOI] [PubMed] [Google Scholar]
- 10.Gatti G, Panozzo G. Effect of inflammation on intraocular penetration of intravenous ofloxacin in albino rabbits. Antimicrob Agents Chemother 1995;39:549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis R, Bryson HM. Levofloxacin: a review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs 1994;47:677–700. [DOI] [PubMed] [Google Scholar]
- 12.Wimer SM, Schoonover L, Garrison MW. Levofloxacin: a therapeutic review. Clin Ther 1998;20:1049–70. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis. Ophthalmology 1999;106:1313–18. [PubMed] [Google Scholar]
- 14.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study: a randomised trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 1995;113:1479–96. [PubMed] [Google Scholar]