Abstract
AUTHOR:e-mail address please Aim: To determine the incidence, regional variation in frequency, outcome, and risk factors for acanthamoeba keratitis (AK) in England and Wales.
Methods: AK cases presenting from 1 October 1997 to 30 September 1999 were identified by the British Ophthalmic Surveillance Unit active reporting system. Clinical and patient postal questionnaire data were analysed.
Results: 106 reported cases met study criteria. The annual incidence for the 2 years was 1.26 and 1.13 per million adults and, for contact lens (CL) wearers, 21.14 and 17.53 per million. There was marked regional variation in incidence (0 to 85.13 per million adult CL wearers), with CL wearers in the south having a ninefold increased risk of AK compared with those resident in the north (95% confidence limits: 2.2–38.9, p<0.0001), and a threefold increased risk with hard as opposed to soft domestic water (95% confidence limits: 1.73 to 6.58, p<0.001). Treatment and outcome data were similar to those previously reported. 93/106 (88%) patients were CL wearers. Among these, 46/77 (60%) were disinfecting irregularly, and 20/63 (32%) had been swimming in CLs. One step hydrogen peroxide and chlorine release soft CL (SCL) disinfection systems were significantly over-represented among the cases. Among SCL users, one or more previously established risk factors for AK were identified in 50/55 (91%) patients.
Conclusions: The incidence was considerably higher than most previous estimates, and was static. The geographical variation in incidence may be partly related to the increase in risk associated with hard water. The fact that water quality can have such an effect on the risk of AK suggests that many CL wearers must be letting tapwater come into contact with their lenses or storage cases. Improved education for CL wearers and practitioners about hygiene practice and the variable efficacy of contact lens systems could be expected to reduce the incidence of this disease.
Keywords: acanthamoeba, keratitis, England and Wales
A canthamoeba keratitis (AK) is a rare but severe corneal infection which, despite improvements in diagnosis and treatment, still culminates in prolonged morbidity and significant loss of visual acuity for up to 15% of patients.1,2
Since up to 93%2,3 of patients with AK are contact lens (CL) wearers, the incidence has been greatly influenced by changing trends in CL use and care. In the United States, a dramatic increase in cases paralleled the growing popularity of soft CL during the 1980s,3 attributable to the use of non-sterile CL solutions, swimming in lenses, and inadequate disinfection.4 In the United Kingdom, there was a marked rise in the number of cases in the first half of the 1990s, associated with the introduction and increasing popularity of disposable soft contact lenses (SCL)2,5,6 shown to be due to irregular and/or chlorine based disinfection.7 After late 1995 there was a decrease, perhaps resulting from an improvement in CL hygiene following the widespread dissemination of the results of a paper on AK,7,8 as well as the gaining penetrance of new CL hygiene systems.
The actual incidence of CL related AK (per million CL wearers) is still uncertain but has been estimated as 1.36 in the United States,3 and, more recently, 3.06 per million in Netherlands,9 and as high as 149 per million in the west of Scotland.10 In England, a 4 year multicentre survey of AK in 12 of the 14 regional health authorities showed an annualised incidence of 1.4 per million individuals and an estimated 19.5 per million CL wearers.2 However, this figure was probably underestimated as many smaller eye departments were unable to identify patients with AK retrospectively and growing awareness of the disease, and increased availability of the new antiamoebal drugs, may have reduced the referral of AK cases to specialist departments. Furthermore, the likelihood of enhanced detection by larger specialist referral centres prevented analysis of the apparently considerable regional variation in incidence.2 This variation may be related to differing levels of water hardness, since an association between the presence of limescale and amoebae has been shown.11
We conducted a 2 year prospective study of AK in England and Wales in order to monitor the incidence, investigate regional variation in frequency of cases and any association with differing water hardness, determine the proportion of cases attributable to previously established risk factors, and provide national data on treatment and outcome.
METHODS
Case ascertainment
The British Ophthalmic Surveillance Unit (BOSU),12 set up at the Royal College of Ophthalmologists in 1997, maintains a list of consultant ophthalmologists, associate specialists, and senior lecturers and runs an active reporting system using methods previously described.13 Following an introductory information pack, ophthalmologists were sent a monthly report card to indicate whether they had seen a new case of AK in the preceding month. Non-respondents received reminders. Each month, the study investigator sent reporting doctors in England and Wales a clinical data sheet as well as a questionnaire to post to their patient.
Case definition
Cases were defined as patients presenting during the study period with a diagnosis of acanthamoeba keratitis supported by positive tissue culture or histology, or diagnosed clinically by the presence of perineural infiltrates (pathognomonic for the disease)5 and/or multiple other typical features such as atypical keratitis with an indolent course, positive culture from CL paraphernalia, severe or disproportionate pain, epitheliopathy, limbitis, and ring ulcer.5 The inclusion of culture negative cases in surveys of AK has been validated by the finding that there is no significant difference in outcomes, as measured by final visual acuity2,5 and need for surgery,2 when confirmed and presumed cases are compared.2,5 Non-UK residents were excluded from the analysis of incidence.
Study period
Two years' data collection commenced on 1 October 1997 after an initial 3 month trial period to allow for the likelihood that participation would be reduced until familiarity with the reporting system had developed.13 Follow up data up until March 2000 were requested, allowing at least 5 months' data on patients presenting in the final month of the study.
Population data
The Office for National Statistics population estimates14,15 were used.
Domestic water hardness data
All 27 water supply companies in England and Wales16 gave data regarding the number of households supplied with soft, medium, and hard water (0–99, 100–199, and 200 or more mg/l as calcium carbonate respectively) and a map of water supply zones. Water hardness was determined for each patient, using the town and postcode district of the place where they had been staying at the time of infection.
RESULTS
BOSU response rate
The monthly card return rate averaged at 62%, 67%, 68%, and 71% during the four 6 month periods of the study (October 1997 to September 1998).
Reports of cases
There were 179 reports of AK from 97 ophthalmologists in England and Wales between October 1997 and October 1999. Of these cases, 106/179 (59%) met study criteria; 33 (18%) were duplicate reports; 16 (9%) had no clinical data available for verification, owing to ophthalmologists declining to send information or, more usually, failing to record the patient's details at the time and subsequently being unable to identify the patient; and 24 (13%) had been reported in error (8) or presented outside the study period (16). At both Moorfields Eye Hospital and Manchester Royal Eye Hospital, where it was possible to compare the cases reported via BOSU with those ascertained from pharmacy and microbiological records, there were reporting rates of 89% (33/37) and 100% (4/4) respectively. At both hospitals there were no confirmed cases reported by BOSU that were not verified by one of these other sources.
Incidence
Five non-UK residents were excluded from the analysis of incidence. The annual incidence for the two study years was 1.26 and 1.13 per million adults respectively, with no significant change between the 2 years (Table 1).
Table 1.
Incidence of acanthamoeba keratitis in England and Wales
| Year 1 (1 October 1997 to 30 September 1998) | |||
| Statistical region | Cases (excluding non-UK residents) | Adult (15 years+) population14 | Incidence per million |
| North | 4 | 2 499 985 | 1.60 |
| North West | 5 | 5 119 753 | 0.98 |
| Yorkshire and Humberside | 1 | 4 057 539 | 0.25 |
| East Midlands | 1 | 3 364 515 | 0.30 |
| West Midlands | 3 | 4 270 224 | 0.70 |
| South West | 8 | 3 993 160 | 2.00 |
| East Anglia | 7 | 1 761 986 | 4.00 |
| South East | 23 | 14 747 076 | 1.56 |
| Wales | 1 | 2 363 342 | 0.42 |
| Total | 53 | 42 177 580 | 1.26* |
| Year 2 (1 October 1998 to 30 September 1999) | |||
| Statistical region | Cases (excluding non-UK residents) | Adult (15 years+) population15 | Incidence per million |
| *No significant difference (Fisher's exact test p = 0.62). | |||
| North | 0 | 2 501 896 | 0.00 |
| North West | 2 | 5 133 295 | 0.39 |
| Yorkshire and Humberside | 1 | 4 066 254 | 0.25 |
| East Midlands | 2 | 3 379 177 | 0.59 |
| West Midlands | 3 | 4 284 816 | 0.70 |
| South West | 5 | 4 015 314 | 1.25 |
| East Anglia | 5 | 1 778 688 | 2.81 |
| South East | 25 | 14 855 254 | 1.68 |
| Wales | 3 | 2 371 724 | 1.26 |
| Other UK residents | 2 | – | |
| Total | 48 | 42 386 418 | 1.13* |
If a survey conducted by Market Opinion Research Int for the Eyecare Information Service (1998) is valid, the penetrance of CL wear in England and Wales was 5% at the middle point of the study period, but with regional variation of between 3% to 9%. This gives an overall incidence for CL wearers was 21.14 and 17.53 per million for years 1 and 2 respectively, with no significant change between the 2 years (Table 2).
Table 2.
Incidence of contact lens related acanthamoeba keratitis in England and Wales
| Statistical region | Cases (excluding non-UK residents) | % Population using CL (see text) | No of CL users | Incidence per million |
| Year 1 (1 October 1997 to 30 September 1998) | ||||
| North | 4 | 7% | 174 999 | 22.86 |
| North West | 4 | 3% | 153 593 | 26.04 |
| Yorkshire and Humberside | 1 | 9% | 365 179 | 2.74 |
| East Midlands | 0 | 4% | 134 581 | – |
| West Midlands | 3 | 6% | 256 213 | 11.71 |
| South West | 7 | 4% | 159 726 | 43.83 |
| East Anglia | 6 | 4% | 70 479 | 85.13 |
| South East | 23 | 6% | 884 825 | 25.99 |
| Wales | 0 | 3% | 70 900 | – |
| Total | 48 | 2 270 495 | 21.14* | |
| Year 2 (1 October 1998 to 30 September 1999) | ||||
| North | 0 | 7% | 175 133 | – |
| North West | 2 | 3% | 153 999 | 12.99 |
| Yorkshire and Humberside | 0 | 9% | 365 963 | – |
| East Midlands | 2 | 4% | 135 167 | 14.80 |
| West Midlands | 2 | 6% | 257 089 | 7.78 |
| South West | 4 | 4% | 160 613 | 24.90 |
| East Anglia | 3 | 4% | 71 147 | 42.17 |
| South East | 23 | 6% | 891 315 | 25.80 |
| Wales | 2 | 3% | 71 152 | 28.11 |
| Other UK residents | 2 | |||
| Total | 40 | 2 281 578 | 17.53* |
*No significant difference (Fisher's exact test p=0.40).
Regional variation in incidence
There was considerable regional variation in incidence both among the general population (0 to 4.00 per million) (Table 1) and among CL wearers (0 to 85.13 per million) (Table 2). Regional incidence analysis showed that the risk of AK was nine times greater among CL users in the south compared to the Midlands and north (Table 3).
Table 3.
Analysis of regional variation in incidence of AK (in year 2)
| General population | Cases (excluding non-UK residents) | Adult (15 years+) population15 | Incidence per million |
| North | 3 | 11 701 445 | 0.26 |
| Midlands | 5 | 7 663 993 | 0.65 |
| South | 38 | 23 020 980 | 1.65 |
| Relative risk | |||
| (confidence limits) | p Value | ||
| South v North | 6.40 (2.0 to 20.9) | 0.00 | |
| South v Midlands | 2.53 (1.0 to 6.4) | 0.05 | |
| Midlands v North | 2.50 (0.6 to 10.6) | 0.28 |
| CL wearing population | Cases (excluding non-UK residents) | Adult (15 years+) population15 | Incidence per million |
| Regions were grouped for analysis according to similarity of incidence: North = North + North West + Yorkshire and Humberside, Midlands = East Midlands + West Midlands, South = South West + East Anglia + South East + Wales. | |||
| North | 2 | 695 095 | 2.88 |
| Midlands | 4 | 392 256 | 10.20 |
| South | 32 | 1 194 227 | 26.80 |
| Relative risk | |||
| (confidence limits) | p Value | ||
| South v North | 9.30 (2.2 to 38.9) | 0.00 | |
| South v Midlands | 2.63 (0.9 to 7.4) | 0.08 | |
| Midlands v North | 3.50 (0.7 to 19.4) | 0.20 | |
Domestic water hardness
Domestic water hardness could be classified for 80/106 (76%) patients. The remainder were either out of the country at the onset of infection (22) or had insufficient data (4) and were excluded from analysis of water hardness as a risk factor. There appeared to be a significant trend towards an increased incidence of AK with increasing hardness of water supply, with hard water being significantly associated with a threefold increase in risk of AK when compared with soft water (Table 4).
Table 4.
Analysis of hardness of water supply as a risk factor for AK
| Domestic water supply | |||
| Soft | Medium | Hard | |
| AK cases | 10 | 10 | 60 |
| Non-cases | 16 439 752 | 8 976 160 | 29 265 203 |
| Risk per million people* | 0.61 | 1.11 | 2.05 |
| Relative risk | 1.0 (referent) | 1.83 | 3.37 |
| (95% confidence limits) | 0.76 to 4.40 | 1.73 to 6.58 | |
| p Value | 0.2406 | 0.00 | |
Test of trend in risk:χ2 = 15.62, p = 0.00.
Diagnosis
Bilateral disease occurred in 8/106 (7.5%) cases.
A positive tissue diagnosis of acanthamoeba was made for 46/106 (43%) patients, and for a further 30 (28%) diagnosis was confirmed by the presence of perineural infiltrates. The remaining 30 cases had a diagnosis of presumed AK, each having atypical keratitis with an array of characteristic signs and symptoms such as epitheliopathy (25), severe or disproportionate pain (17), limbitis (9), ring infiltrate (6), and culture of Acanthamoeba from contact lens, lens storage case, or solution bottle (5).
The proportion of patients diagnosed early (receiving antiamoebal therapy within 30 days of presentation) was 82/104 (79%), data were unavailable for 2/106. The most common initial diagnoses were acanthamoeba keratitis (33/106, 31%), herpes simplex virus keratitis (26/106, 25%), contact lens abrasion or hypoxia (19/106, 18%), and bacterial keratitis (18/106, 17%).
Treatment and outcome
Antiamoebal medical therapy, duration of treatment, surgery, and final acuities are shown in Table 5.
Table 5.
Treatment and outcome among the 106 AK patients
| No (%) | |
| Antiamoebal medical therapy: | |
| Propamidine/hexamidine | 86 (81) |
| PHMB | 72 (68) |
| Chlorhexidine | 40 (38) |
| Neomycin | 25 (24) |
| Oral/topical antifungal | 7 (7) |
| Duration of medical therapy | |
| (excluding 39 patients still on medication/lost to follow up) | |
| Mean: | 5.2 months |
| Median: | 4.3 months |
| Range: | 25–503 months |
| Surgery (14 patients) | |
| Penetrating keratoplasty | 9 |
| Superficial keratectomy | 6 |
| Cataract extraction | 5 |
| Amniotic membrane transplant | 2 |
| Trabeculectomy/cyclodiode | 2 |
| Lid surgery | 2 |
| Lamellar keratoplasty (41 patients still under review) | 1 |
| Final visual acuity: | |
| Discharged patients (54 eyes) | |
| 6/6 or better | 37 (68) |
| 6/9 | 14 (26) |
| 6/12 | 3 (6) |
| Patients under review or lost to follow up (60 eyes) | |
| 6/6 or better | 19 (32) |
| 6/9 | 12 (20) |
| 6/12 | 7 (12) |
| 6/18–6/60 | 9 (15) |
| 1/60–3/60 | 2 (3) |
| Hand movements/perception of light | 9 (15) |
| No recent acuity available | 2 (3) |
Patient characteristics
Patient ages ranged from 16 to 75 years, with a mean age of 36.9 years. At least 56/106 (53%) were female (the sex for two patients was unknown). At least 93/106 (88%) patients were CL wearers (for three patients contact lens wear before infection was uncertain).
Risk factors among non-contact lens wearers
Questionnaires were completed by 9/10 patients who had not been wearing CLs. Seven of these patients had a history of minor ocular trauma and/or eyes having been splashed by stagnant water in a rural (4) or other outdoor environment (3). One patient regularly splashed water into his eyes every morning, and another, with concurrent uveitis and glaucoma, used an eye bath after receiving a corneal abrasion during gonioscopy. Data were unavailable for one patient.
Risk factors among contact lens wearers
Only 3/93 (3%) patients had a medical indication for CL wear (aphakia (1), keratoconus (1) post graft for keratoconus (1)) , 8/93 (8%) had a history of trauma, minor in all but one case, or concurrent eye disease (6/93, 6%, all conjunctivitis or blepharitis).
All but one of the 22 patients who had been abroad at the onset of symptoms (or during the week before) were CL wearers. Fifty six per cent (9/16) had been swimming in sea (n=9), pool (n=3), or fresh water (n=2) while wearing their CL (for five patient data were unavailable).
Questionnaires were completed by 63/93 (68%) of patients known to be CL wearers. At least 66/67 (99%) had more than 6 months' experience of contact lens wear. Eighty of 93 (86%) were wearing soft CLs, in a variety of replacement schedules (Table 6). Data on penetrance of CL types and modalities are not available, but data on prescribing habits17–19 (which probably overestimate the proportion of the population wearing newer modalities) suggest that, among soft lens wearers, daily disposal lenses appeared to carry a significantly decreased risk, while 1–4 weekly disposal lenses were associated with a significantly increased risk.
Table 6.
Distribution of contact lens (CL) types among AK patients
| % CL types in population: Year: Sample size: | |||||
| AK cases CL use: (1 October 1997 to 30 September 1999) | 199717 | 199818 | 199919 | ||
| (n=93) | (n=1382) | (n=1947) | (n=1419) | Total (n=4748) | |
| SCL: | |||||
| Daily disposable | 5 (5%)* | 12% | 23% | 24% | 954 (20%)* |
| Disposable 1–4 weeks | 46 (60%)† | 37% | 36% | 37% | 1737 (37%)† |
| Disposable >4 weeks | 15 (19%) | 34% | 24% | 19% | 1207 (25%) |
| Unknown disposable | 14 (15%) | 0% | 0% | 0% | 0 |
| Soft-rigid | 2 (2%) | 0% | 0% | 0% | 0 |
| Rigid CL | 11 (12%)‡ | 17% | 17% | 20% | 850 (18%)‡ |
*Daily disposable v other SCL schedules (excluding unknown): χ2 = 10.122, p = 0.00; †1–4 weekly disposal v other SCL schedules (excluding unknown): χ2 = 16.569, p = 0.00; ‡rigid CL v SCL (including unknown SCL schedules): χ2 = 2.064, p = 0.14.
Among the 11 patients who had been wearing rigid CLs, all but one (a non-UK resident for whom data were insufficient) had either failed to carry out regular disinfection (n = 7) and/or developed AK following trauma (n = 3) or swimming with their CLs in (n = 3).
Among SCL wearers for whom disinfection data were sufficient there were 22/66 (33%) who were disinfecting irregularly and 17/66 (26%) who never disinfected their CL. The 24 SCL wearers carrying out regular disinfection had been using multipurpose solutions (8), chlorine based solutions (7), one step hydrogen peroxide systems (7), or a two step hydrogen peroxide system (1) (the solution type for one non-European patient was unknown). Three patients had been using daily disposable CLs, theoretically obviating the need for solutions. Among patients on monthly CL replacement who were disinfecting their lenses (n = 29), multipurpose solutions were significantly under-represented, while one step hydrogen peroxide systems and, in particular, chlorine release systems, were significantly over-represented when compared to their use in the population17–19 (Table 7).
Table 7.
Distribution of soft contact lens (CL) disinfection types among AK patients on a monthly disposal schedule
| % Disinfection types for monthly disposal SCL in population: Year: Sample size: | |||||
| Disinfection types among AK patients with monthly disposal SCL (1 October 1997 to 30 September 1999) | 199717 | 199818 | 199919 | Total | |
| (n=29) | (n=518) | (n=796) | (n=621) | (n=1935) | |
| Multipurpose | 7 (24%)* | 73% | 73% | 81% | 1462 (76%)* |
| H2O2 1 Step | 8 (28%)† | 12% | 17% | 11% | 265 (14%)† |
| H2O2 2 Step | 1 (3%) | 9% | 5% | 4% | 112 (6%) |
| Other | 4 (14%) | 3% | 5% | 3% | 74 (4%) |
| Chlorine release | 9 (31%)‡ | 3% | 0% | 1% | 21 (1%)‡ |
*Multipurpose solution v the rest of the SCL solutions used: χ2 = 40.070, p=0.00; †1 Step hydrogen peroxide v the rest of the SCL solutions used (excluding unknown): χ2 = 4.607, p = 0.00; ‡chlorine-release v the rest of the SCL solutions (including unknown SCL schedules): χ2 = 167.339, p=0.00.
Among the SCL wearers who had completed a questionnaire, the most common risk factors—determined by previous studies2,4,20—were swimming with CL in situ, irregular or omitted disinfection, and poor basic CL storage case hygiene. One or more of these risk factors were identified for 50/55 (91%) of these patients (Table 8).
Table 8.
Frequency of pre-established risk factors for soft contact lens (CL) related AK among questionnaire respondents
| No (%) (n=55) | |
| Swimming with CL in4 | |
| Chlorinated water | 12 (22) |
| Lakes, rivers, etc | 3 (5) |
| Sea water | 9 (16) |
| Swimming in one or more of the above | 18 (33) |
| Omitted/irregular disinfection (without CL disposal)4,7,20 | 24 (44) |
| Chlorine disinfection7,20 | 9 (16) |
| Rinsing/storing CL with non-sterile water20 | 3 (5) |
| Rinsing CL storage case with non-sterile water20 | 20 (36) |
| Failure to leave storage case to dry20 | 25 (45) |
| At least one of the above | 50 (91) |
DISCUSSION
Incidence of acanthamoeba keratitis
A limitation of the study with respect to incidence analysis was the use of a newly set up surveillance system with a mean response (monthly report card return) rate of 62% rising to 71%. Furthermore, despite initial instructions and subsequent reminders in 6 monthly newsletters, a potential 9% loss of cases occurred because ophthalmologists failed to record the name or hospital number of patients they had reported and subsequently being unable to identify them when clinical data were requested a few weeks later. Ethics committee guidelines, for the protection of patient confidentiality, prevented recording of the hospital number on the report card, which would have overcome this problem. Despite these difficulties, case ascertainment was found to be between 89% and 100% for two hospitals where alternative sources of case identification were available.
The incidence 1:1 000 000 of AK among adults in England and Wales confirms the result of a retrospective multicentre study of cases during 1992–6 in England.2 Among CL wearers, the 21 and 17 per million incidence for the 2 years respectively is also in keeping with the 19.5 annualised incidence previously found.2 The likelihood of marked under-reporting in the earlier study, however, suggests that the actual incidence may have decreased since 1996. The substantially lower incidence of AK found for the Netherlands has been attributed to the greater proportion of Dutch CL wearers using rigid lenses.9 The 149 per million incidence reported for the west of Scotland,10 however, is irreconcilable with the findings of either study, and warrants further evaluation.
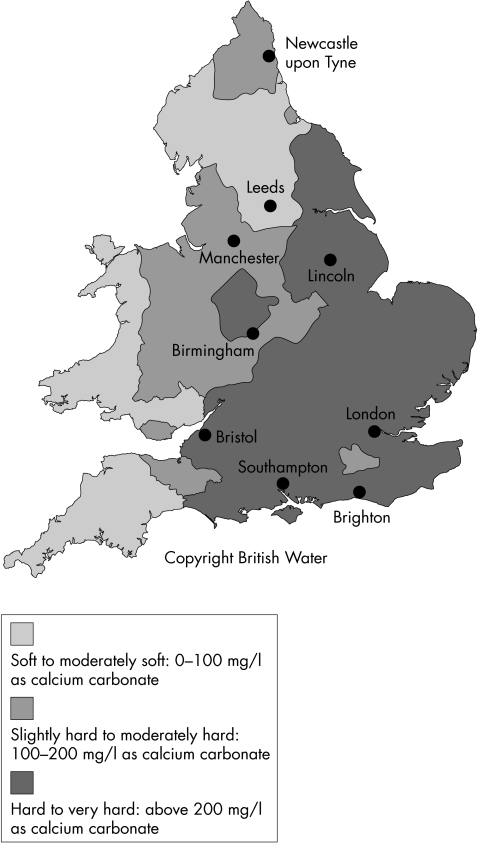
As in a previous study,2 there was considerable regional variation in incidence. Analysis now suggests that this may be independent of the varying proportions of CL wearers in each region, assuming the estimates of CL use for each region are valid, although inter-region variations in CL wear modality and CL care solutions, for which data are not available, may be a confounding factor. Possible explanations for the greatly increased incidence in the south include increased Acanthamoeba in the environment owing to higher average air temperature21 and, in particular, the much greater proportion of households supplied with hard domestic water16 (Fig 1). This suggests that many CL users are still contaminating their CLs with tap water either directly (showering, face washing, handling with wet hands) or by using water to rinse their storage case. It has been hypothesised that contact with tap water in hard water areas poses a greater risk than in soft water areas because of the presence of limescale, which may both provide an environment for amoebae and reduce the efficacy of some CL disinfection systems.11
Figure 1.
Hardness of distributed water supplies in England and Wales (with permission from British Water, London). (Owing to the complexity of water supply systems, the map can only give an indication of the likely hardness of the water in different parts of the country).
Diagnosis, treatment, and outcomes
A culture positive rate of 43% for acanthamoeba keratitis is similar to that reported in previous series.2,5 Comparison with the multicentre study2 conducted between 1992 and 1996 revealed that there has been no change in the proportion of patients receiving an early diagnosis; the proportion being treated with more effective antiamoebal therapy (PHMB and/or chlorhexidine); the mean duration of therapy; the frequency of needing surgical management or the proportion regaining good visual acuity.
Risk factors among contact lens wearers
As in previous studies,2,5 a very high proportion of patients were CL wearers, and less than 3% had a medical indication for CL wear or had less than 6 months' experience. As in a previous study2 AK was associated with irregular disinfection, trauma, and/or swimming in CLs for both rigid and SCL users; however, in SCL users the compliant use of some less effective disinfection systems was also associated with AK
Chlorine based disinfection, which is ineffective against Acanthamoeba, is well established as a highly significant risk factor for SCL related AK.7,20 This study suggests that there may also be a significant association between AK and one step hydrogen peroxide disinfection. Hydrogen peroxide is highly effective against acanthamoeba cysts, but the delayed release catalase tablet or platinum disc used for neutralising these one step systems prevents the minimum effective soaking time from being achieved.22 This observation is supported by a report of AK in patients using one step hydrogen peroxide systems appropriately.23
All SCL replacement schedules were represented among the patients with AK, including daily disposal. The latter, however, were significantly less common among the cases, probably because, if used correctly, they obviate the need for solutions. One to four weekly disposal, however, was markedly more common among patients with AK than in the population as a whole. This is probably a reflection of the differing patterns of disinfection among users of disposable as opposed to non-disposable lenses, in which the latter are significantly more likely to use a two step hydrogen peroxide system.17–19 Since silicon hydrogel lenses for 30 days' continuous wear were only introduced 4 months before the end of the study period, their effect on the risk of AK remains unknown. Although overnight wear has never been identified as a significant risk factor for acanthamoeba keratitis, the continuity of wear may increase the likelihood of the lenses being used while engaged in water sports.
On comparing established significant risk factors among the cases in this study with those reported for patients in the earlier multicentre survey2 it can be seen that while the declining availability and popularity of chlorine disinfection has reduced the number of cases associated with it, the proportion of cases associated with irregular or omitted disinfection has significantly increased. This may be due to the rapidly increasing preponderance of SCL issued on a disposable schedule, with which an increased tendency towards omitted or irregular disinfection has been reported.7 Swimming while wearing SCL, long established as a significant risk factor for AK,4 continues to be reported in nearly a third of the cases. As in the earlier study,2 there was a history of at least one previously established risk factor in over 90% of the SCL wearers who completed a questionnaire. These results emphasise the highly preventable nature of CL related AK.
The incidence of AK in England and Wales is considerably higher than previously deduced from most studies in other countries, and appeared to be static throughout the period of this study. A substantial minority of cases continue to be left with severely reduced acuity. Although a rare disease, an increasing proportion of the population are contact lens wearers and therefore at increased risk of developing AK. This is the first study to suggest a significant increase in risk associated with supply of hard domestic water and a significant variation in the distribution of cases which appeared to be (at least partly) related to this. The fact that water quality can have such an effect on the risk suggests that many CL wearers must be letting non-sterile water come into contact with their lenses or storage cases. Education of CL practitioners and wearers about the risks associated with tap water and swimming in lenses, variations in efficacy of disinfection systems and the risk reduction achieved with the disposal of soft lenses after a wearing period can be expected to reduce the incidence of AK.
Acknowledgments
We are grateful for funding from Moorfields Eye Hospital Special Trustees, Vistakon, Alcon, and Allergan, UK. We would also like to thank BOSU for the opportunity to conduct this study, the many ophthalmologists who contributed data, and Dr Ian Pallett (British Water) for technical advice.
REFERENCES
- 1.Duguid IGM, Morlet N, Allan BDS, et al. Outcome of Acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology 1997;104:1587–92. [DOI] [PubMed] [Google Scholar]
- 2.Radford CF, Lehmann OJ, Dart JKG. Acanthamoeba keratitis:multi-centre survey in England 1992–1996. Br J Ophthalmol 1998;82:1387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stehr-Green JK, Bailey TM, Visvesvara GS. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol 1989;107:331–6. [DOI] [PubMed] [Google Scholar]
- 4.Stehr-Green JK, Bailey TM, Brandt FH, et al. Acanthamoeba keratitis in soft contact lens wearers: a case-control study. JAMA 1987;258:57–60. [PubMed] [Google Scholar]
- 5.Bacon AS, Frazer DG, Dart JKG, et al. A review of 72 cases of Acanthamoeba keratitis 1984–1992. Eye 1993;7:719–25. [DOI] [PubMed] [Google Scholar]
- 6.Illingworth CD, Cook SD, Karabatsas CH, et al. Acanthamoeba keratitis:risk factors and outcome. Br J Ophthalmol 1995;79:1078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radford CF, Bacon AS, Dart JKG, et al. Risk factors for Acanthamoeba keratitis in contact lens wearers: a case-control study. BMJ 1995;310:1567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morlet N, Duguid G, Radford C, et al. Incidence of Acanthamoeba keratitis associated with contact lens wear. Lancet 1997;350:414. [DOI] [PubMed] [Google Scholar]
- 9.Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact-lens-related microbial keratitis and its related morbidity. Lancet 1999;354:181–5. [DOI] [PubMed] [Google Scholar]
- 10.Seal DV, Kirkness CM, Bennett HGB, et al and Keratitis Study Group. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Contact Lens and Anterior Eye 1999;22:49–57. [DOI] [PubMed] [Google Scholar]
- 11.Seal D, Stapleton F, Dart J. Possible environmental sources of Acanthamoeba spp in contact lens wearers. Br J Ophthalmol 1992;76:424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahi JS, Edelsten C. The British Ophthalmological Surveillance Unit. Eye 1998;11:766–7. [DOI] [PubMed] [Google Scholar]
- 13.Hall SM, Glickman M. The British Paediatric Surveillance Unit. Arch Dis Child 1988;63:344–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Office for National Statistics. Population and health monitor. London: Stationery Office, 1998.
- 15.Office for National Statistics. Population estimates.London: Stationery Office, 1999.
- 16.Water UK. Who's who in the water industry 1998. London: Water UK, 1998.
- 17.Morgan PB, Efron N. Trends in UK contact lens prescribing 1997. Optician 1997;214(5630):32–3. [Google Scholar]
- 18.Morgan PB, Efron N. Trends in UK contact lens prescribing 1998. Optician 1998;216(5679):18–19. [Google Scholar]
- 19.Morgan PB, Efron N. Trends in UK contact lens prescribing 1999. Optician 1999;217(5700):43–4. [Google Scholar]
- 20.Seal DV, Kirkness CM, Bennett HGB, et al and Keratitis Study Group. Acanthamoeba keratitis in Scotland: risk factors for contact lens wearers. Contact Lens and Anterior Eye 1999;22:58–68. [DOI] [PubMed] [Google Scholar]
- 21.Mathers WD, Sutphin JE, Lane JA, et al. Correlation between surface water contamination with amoeba and the onset of symptoms and diagnosis of amoeba-like keratitis. Br J Ophthalmol 1998;82:1143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niszl IA, Markus MB. Anti-Acanthamoeba activity of contact lens solutions. Br J Ophthalmol 1998;82:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radford CF, Moodaley LCM, Porter R. Acanthamoeba keratitis in compliant users of a one-step hydrogen peroxide contact lens disinfection system. Optometry Today 1999;39:21–4. [Google Scholar]