Abstract
Aims: To evaluate a new delivery system of 5-fluorouracil (5-FU) using 5-fluorocytosine (5-FC) as a prodrug and cytosine deaminase induced in vitro and in vivo.
Methods: Fibroblastic cells from rabbit Tenon's capsule were cultured. The cells were exposed to 5-FU and 5-FC with or without cytosine deaminase induced by recombinant adenovirus. In the in vitro study, cell proliferation and DNA synthesis were assessed by MTS, BrdU assay. The effect of 5-FC removal after the treatment of 5-FC and cytosine deaminase induction was also assayed. In the in vivo study cells with or without cytosine deaminase induction were transplanted into the subconjunctival space of mice, followed by eye drops of 1000 μg/ml of 5-FC three times a day. The mice were sacrificed at days 1, 5, and 10, then the cells transplanted were evaluated.
Results: Cell proliferation was inhibited by exposure to 5-FU in a dose dependent manner; however, up to 1000 μg/ml of 5-FC did not affect cell proliferation. Cell proliferation was inhibited by exposure to 5-FC in a time dependent manner with induction of cytosine deaminase following infection of recombinant adenovirus. When 5-FC was removed 3 or 6 days after the treatment, the cells grew again. The effect was reproduced in the in vivo model of subconjunctival cellular proliferation although 5-FC was administrated as eye drops. There were no cases with corneal erosion.
Conclusion: Cell proliferation was inhibited by co-exposure of 5-FC and cytosine deaminase. This new delivery system may merit controlled delivery of 5-FU after filtering surgery.
Keywords: 5-fluorouracil, 5-fluorocytosine, cytosine deaminase, adenoviral vector, gene therapy, filtering surgery
A ntimetabolites have been shown to enhance the success rate of filtering surgery, although overapplication of these drugs may result in failure of surgery and severe complications. Heuer et al1 reported the effects of 5-fluorouracil (5-FU) after filtering surgery, and noted that 5-FU should be applied to the eye for several days after surgery. Although the treatment improves the results of filtering surgery, the daily administration is inconvenient and the patient experiences discomfort with each treatment. Therefore, intraoperative application of mitomycin C (MMC),2–5 became the standard adjunctive treatment. Only a few minutes of exposure to MMC improves the success rate more than do multiple injections of 5-FU.6
This difference between 5-FU and MMC depends on the pharmacological nature of these antimetabolites. The antiproliferative effect of 5-FU arises from its metabolites acting as metabolic blockers that inhibit thymidylate synthetase, which converts ribonucleotides to deoxyribonucleotides, thus inhibiting DNA synthesis. It acts selectively on the growth phases corresponding to DNA and RNA synthesis, respectively, in the cell cycle. Therefore, only those cells in the synthesis phase are affected, thus allowing the remaining cells to continue to proliferate after exposure to 5-FU.7
MMC, on the other hand, has a direct cytotoxic effect, and acts by reducing fibroblast collagen synthesis through inhibition of DNA dependent RNA synthesis. This is independent of the cell cycle and is directly proportional to the concentration applied. Its other modes of action are the result of sustained tissue binding by alkylation and the formation of DNA interstrand crosslinks, and its effects on other components of wound healing including cell migration and extracellular matrix production.8,9
It was thought best to inject 5-FU over several days after surgery because the static cells are not influenced by 5-FU, but recent studies suggest a beneficial effect of a single application of 5-FU during surgery.10–19 Of course, the appropriate dosages for these disparate applications are different. Furthermore, the intraoperative single application is effective only for low to moderate risk cases.11,13,16 In addition, a controllable drug delivery system is needed.
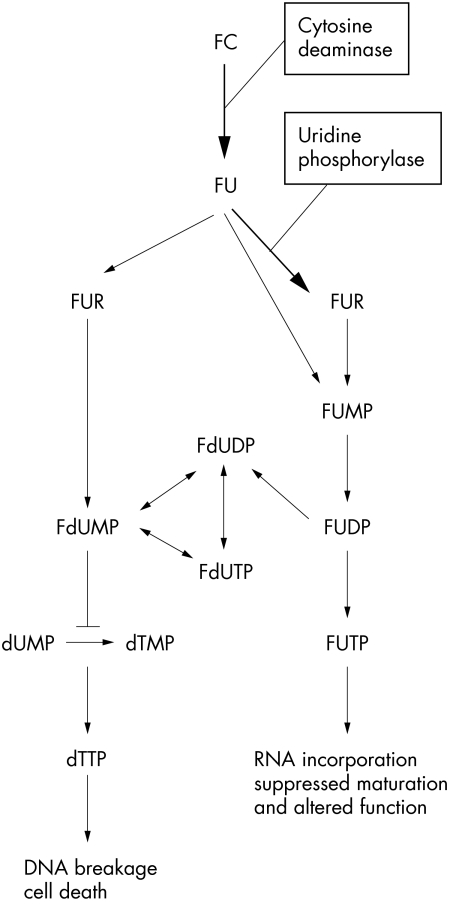
Indeed, when it comes to drug delivery, 5-FU might be safer than MMC because delivery can be controlled for several days with 5-FU but for only a few minutes with MMC. When we focused on the development of a safe and effective method to deliver some antimetabolites, we discovered the system that converts 5-fluoro cytosine (5-FC) to 5-fluorouracil. Certain bacteria and fungi contain the enzyme cytosine deaminase, which metabolises the innocuous compound 5-FC to the highly toxic compound 5-FU (Fig 1), whereas mammalian cells ordinarily do not.20 In this study we evaluated the effect of this drug delivery in vitro and in vivo, using 5-FC eye drops instead of 5-FU injections.
Figure 1.
Schematic representation of the biological mechanism of action of fluoropyrimidine. FC = 5-fluorocytosine; FU = 5-fluorouracil; FUR = 5-fluorouridine; FUMP = 5-fluoro-5 monophosphate; FdUMP = 5-fluoro-2 deoxy-5 monophosphate; FUDP = 5-fluorouridine-5 diphosphate; FUTP = 5-fluorouridine-5 triphosphate; FdUDP = 5-fluorodeoxyuridine-5 diphosphate; FdUTP = 5-fluorodeoxyuridine-5 triphosphate; dUMP = deoxyuridin-5 monophosphate; dTMP = deoxythymidine-5 monophosphate; dTTP = deoxythymidine-5 triphosphate. Modified from the report of Lee et al.42
MATERIALS AND METHODS
Cell culture
Albino rabbit Tenon's capsule fibroblasts were established in culture as described previously.21 The cells were grown in Dulbecco's modified Eagle's medium (DMEM, Wako, Osaka, Japan) supplemented with 10 % fetal bovine serum (FBS, MA Bioproducts, Walkersville, MD, USA), penicillin G (100 IU/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 mg/ml), then cultured in an incubator at 37°C in a humidified atmosphere of 5% carbon dioxide and 95% air. Cells from the 4th to the 10th passage were used.
Construction of adenovirus vector
The replication deficient recombinant adenovirus vector containing cDNA of cytosine deaminase was constructed according to the COS/TPC method, as previously described.22–24 Briefly, a 3664 bp fragment containing the coding region of Escherichia coli cytosine deaminase was obtained from E coli genomic DNA and blunt ended. After purification by gel electrophoresis, this fragment was subcloned into the expression unit of pAxCAwt: a cassette cosmid bearing an expression unit and full length adenovirus type 5 genome with deletions of the E1 and E3 region (a generous gift from Drs I Saito and Y Kanegae).25 The expression unit consists of a CA promoter (chicken, b-actin promoter and cytomegalovirus enhancer), a DNA cloning site, and rabbit β globin poly A.26 The constructed cosmid was then co-transfected to 293 cells (American Type Culture Collection, Rockville, MD, USA) together with the EcoT22I digested adenovirus DNA terminal protein complex (from Dr I Saito) using the calcium phosphate precipitation method. The viruses generated through homologous recombination were purified by the limiting dilution technique, and the virus with the desired gene construction was confirmed by restriction analysis. The clone was then propagated in 293 cells, and purified by sequential centrifugation in CsCl step gradients, and then stored in aliquots at −80°C (AxCACD).27 The control vector was AxCAlacZ (from Dr I Saito), which carries the E coli β galactosidase (LacZ) cDNA in the same expression unit. The same virus stocks were used in all experiments.
In vitro study
Cell proliferation
After growing to confluence, the cells were enzymatically detached with 0.05% trypsin (Sigma, St Louis, MO, USA) at 37°C for 3 minutes, and trypsinisation was stopped by the addition of growth medium with 10 % FBS (GM). The suspended cells were centrifuged at 1000 rpm for 3 minutes, after which the pellets were resuspended, diluted to 104 cells/ml with GM, and seeded into 96 well plates as 103 cells in 100 μl GM. The virus and drug solutions were diluted with GM to reach the final concentrations desired. Of the diluted virus solution or GM, 50 μl were added to each well. The plate was then incubated at 37°C for 30 minutes.
Finally, 50 μl of GM with or without 5-FC (Wako, Osaka, Japan) or 5-FU (Wako, Osaka, Japan) was added to each well. GM, with or without 5-FU or 5-FC, was exchanged every 3 days. The number of cells was evaluated with colorimetric assay using the CellTiter 96 AQ One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA) at days 0, 1, 3, 6, 9, 12, and 15, according to the manufacturer's instructions. Briefly, cells were cultured on 96 well plates. The cell counting solution was made by mixing 100 μl of GM with 20 μl of the reagent for each well. The GM was removed using multichannel micropipettes, after which 120 μl of cell counting solution was added. The plate was placed at 37°C for 45 minutes, and the absorbance at 490 nm was then measured.
DNA synthesis was also evaluated with a 5-bromo-2'-deoxyuridine (BrdU) Labelling and Detection kit III (Roche, Mannheim, Germany) at day 1. Cells were cultured on 96 well plates. Serum free medium with BrdU (50 μl) was added into each well 4 hours before assay. The cells were fixed in 70% ethanol with 0.5 M HCl at −20°C for 30 minutes, then the DNA was fragmented with nuclease at 37°C for 30 minutes. Monoclonal antibody for BrdU conjugated with peroxidase was applied at 37°C for 30 minutes. The cells were then incubated with substrate solution at room temperature for 10 minutes, and absorbance was measured at 405 nm.
In vivo study
To evaluate the effect of cytosine deaminase induction in situ followed by eye drops of 5-FC, the method of Khaw et al28,29 was modified. Fibroblastic cells were infected at multiplicity of infection (MOI) of 10 with AxCAlacZ, or with AxCACD and AxCAlacZ. AxCAlacZ carrying β galactosidase was used as a marker of the cells. Cells were washed with PBS, then harvested, and diluted at a concentration of 106 cells/ml GM.
Eight week old mice were anaesthetised by an intraperitoneal injection of diluted sodium pentobarbitone. Subconjunctival injections were performed under an ophthalmic surgical microscope. A Hamilton syringe with a 30 gauge needle was inserted into the superior lid until the needle could be observed through the tarsal conjunctiva. A pinhole at the thin bulbar conjunctiva may alter the efficiency of drug delivery, so we used the tarsal conjunctiva although the ultimate target is the bulbar conjunctiva. A total of 103 cells in 10 μl of GM were injected. Eye drops of 10 mg/ml of 5-FC were administered three times a day beginning immediately after the treatment. The mice were sacrificed at days 1, 5, or 10, and the lids dissected and prefixed by immersion in 4% paraformaldehyde/PBS for 2 hours. The lids were then washed in cold PBS overnight and incubated in PBS containing 1 mg/ml of X-gal (5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside), 0.01% sodium deoxycholate, 0.02% NP40, 2 mM MgCl2, 5 mM K3Fe(CN)6, and 5 mM K4Fe(CN)6 overnight at room temperature. Each sample was observed by microscopy. All animals were cared for in accordance with the ARVO statement for the use of animals in ophthalmic and vision research and with federal and local regulations.
Statistical analysis
All values are reported as mean (SD). Statistical significance was determined with an analysis of variance followed by Dunnet's procedure in multiple comparisons. The group without treatment was used as the control. The group without removal of 5-FC, however, was used as the toxic control when the effect of removal of 5-FC was assessed. All experiments in vitro were repeated twice with quadruplicates. Non-parametric analysis was performed using the Kruskal-Wallis test followed by the Mann-Whitney U test. A difference at a level of p <0.05 was considered to be statistically significant.
RESULTS
In vitro study
Cytotoxicity of 5-FU, 5-FC, and adenovirus infection
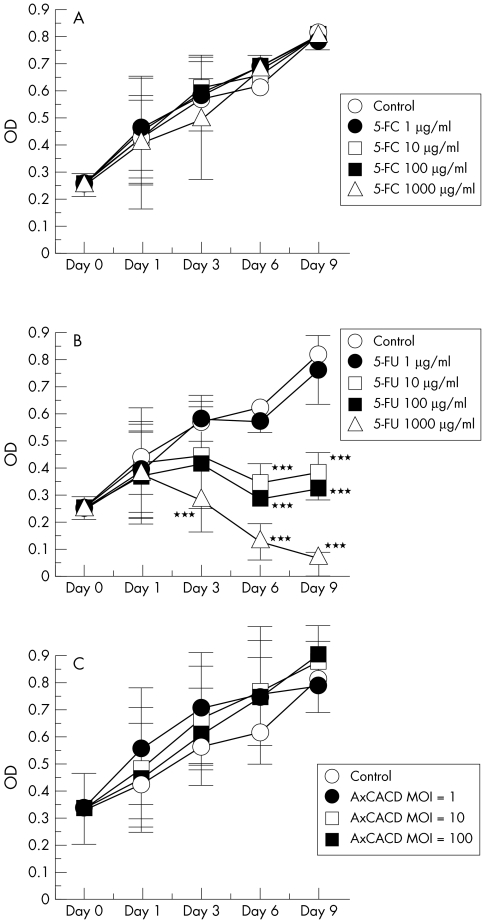
Firstly, the cytotoxicity of each drug or virus was assessed independently. Various final concentrations from 1 μg/ml to 1000 μg/ml of 5-FU and 5-FC were tried. As shown in Figure 2A, up to 1000 μl/ml of 5-FC does not affect cell growth, although 5-FU inhibits cell growth in a time dependent and dose dependent manner (Fig 2B). The toxic effect of 5-FU was not observed on day 1, but was observed on days 3, 6, and 9. Cell growth was completely inhibited by 1000 μg/ml of 5-FU. Next, the cytotoxicity of adenovirus infection was assessed. Infection of AxCACD at various concentrations, up to an MOI of 100, did not produce a cytotoxic effect (Fig 2C).
Figure 2.
Cytotoxic effect of various concentrations of 5-FC (A), 5-FU (B), and induction of cytosine deaminase (AxCACD) (C) on rabbit Tenon's capsule fibroblast growth at different incubation times. 5-FC does not affect cell growth (A), although 5-FU inhibits cell growth in a time dependent and dose dependent manner (B). Infection of AxCACD did not produce a cytotoxic effect (C). Each point represents mean (SD). (***p <0.001, ANOVA) MOI = multiplicity of infection.
Cytotoxicity of 5-FC after adenovirus infection
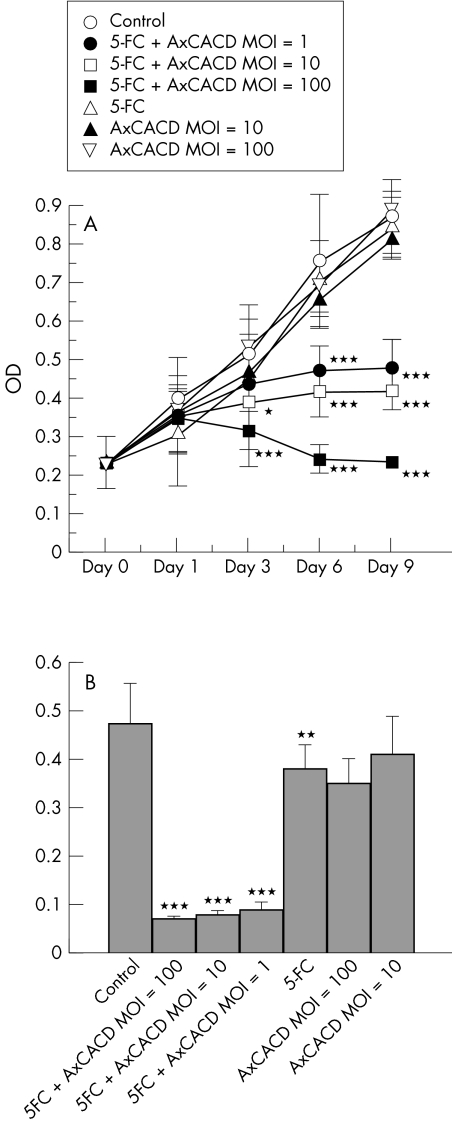
The cytotoxic effect of a combination of 5-FC and AxCACD was assessed. The concentration of 5-FC was 1000 μg/ml because this concentration did not exert a cytotoxic effect. Virus concentration was varied from MOI 1 to 100. A cytotoxic effect was not observed with either 5-FC or infection of AxCACD, although the combination treatment resulted in a cytotoxic effect in a time dependent and dose dependent manner (Fig 3A). The effect on DNA synthesis was also evaluated. The DNA synthetic activity was reduced by 1000 μg/ml with 5-FC (p <0.01) or high titres of AxCACD (p <0.001), whereas moderate titres of AxCACD had no effect. DNA synthetic activity was markedly suppressed by the combination treatment (p <0.001) (Fig 3B).
Figure 3.
Cytotoxic effect of 1000 μg/ml of 5-FC and various concentrations of cytosine deaminase (AxCACD) induction on rabbit Tenon's capsule fibroblast growth at different incubation times using the MTS assay (A) and BrdU assay (B). The combination treatment resulted in a cytotoxic effect in a time dependent and dose dependent manner. Each point represents mean (SD). (*p <0.05, **p <0.01, ***p <0.001, ANOVA).
Reversal of cell viability with treatment of 5-FC and adenovirus infection followed by removal of 5-FC
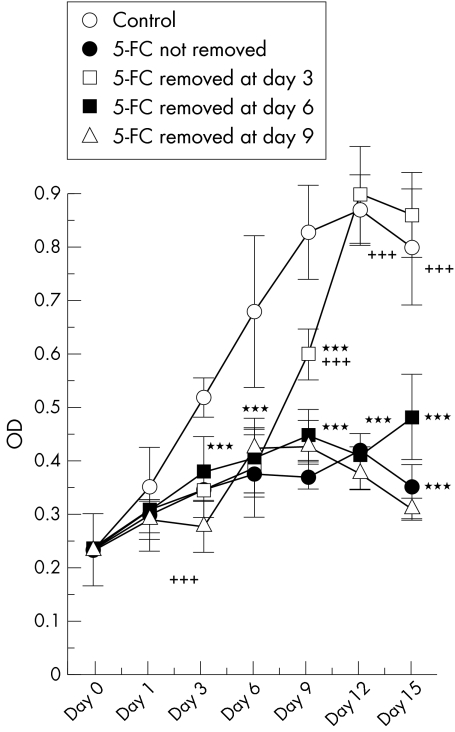
A cytotoxic effect was observed following treatment with 5-FC and adenovirus infection. However, it is not known whether cell viability is reversible after removal of 5-FC. Accordingly, 5-FC was removed on days 3, 6, or 9, after which the cells were washed three times with GM. Cell viability was then determined after every 3 days. Cell growth was observed at day 9, 6 days after 5-FC removal on day 3, and at day 15, 9 days after 5-FC removal on day 6. Cellular viability did not reverse after 5-FC removal on day 9 in this observation (Fig 4).
Figure 4.
Cytotoxic effects and reversibility of 1000 μg/ml of 5-FC and cytosine deaminase induction at MOI 10 on rabbit Tenon's capsule fibroblast growth at different incubation times. 5-FC was removed at day 3, 6, or 9. Cell viability was reversed after some period since 5-FC was removed. Each point represents mean (SD). (***p <0.001, ANOVA) Data under the asterisks showed significant difference compared to the control as the asterisks indicated. After 5-FC removal, the data were also compared to the toxic control without 5-FC removal (++p <0.01, +++p <0.001, ANOVA).
In vivo study
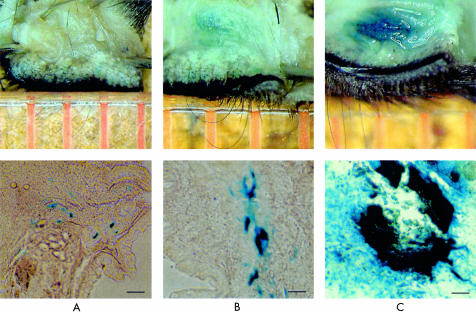
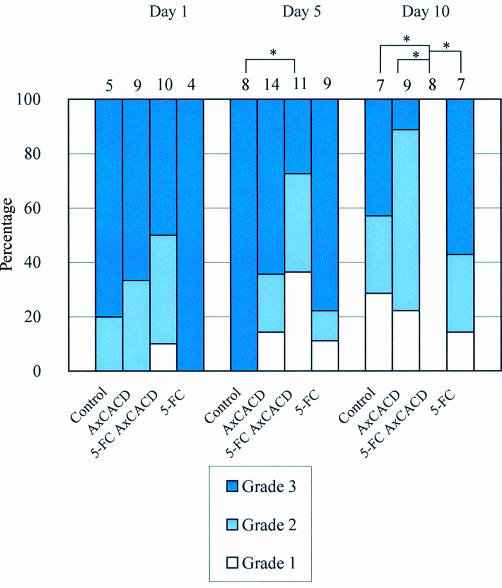
Each sample was observed by microscopy and the tissue staining graded as follows: grade 1, no staining; grade 2, patchy staining; grade 3, marked staining. Blue staining means the cells injected remain. Representative photographs of each grade are shown in Figure 5. In vivo cytotoxic effects of gene induction followed by instillation of 5-FC eye drops were observed with a mouse model (Fig 6). Most treated animals showed marked staining of X-gal on day 1, and there were no significant differences among the groups, with almost all of the control cases showing marked staining on days 1 and 5. Many cases that underwent only cytosine deaminase induction or 5-FC application showed staining up to day 10, whereas no cases showed staining of tarsal conjunctiva at day 10 after cytosine deaminase induction followed by 5-FC application. No animals exhibited corneal erosion.
Figure 5.
Representative photographs of each grade. Grade 1, no staining (A); grade 2, patchy staining (B); grade 3, marked staining (C). Bar = 100 μm.
Figure 6.
In vivo effect of cytosine deaminase induction followed by of 5-FC eye drops. Staining of the cells was evaluated on days 1, 5, and 10. Each number above the bar is the number included. There were significant differences among the groups on day 5 (p <0.05) and day 10 (p <0.01). In each subgroup comparison, significant differences are indicated by asterisks. (*p <0.05) Kruskal-Wallis test was used for statistics.
DISCUSSION
Both 5-FU and MMC have been used as an adjunctive treatment in glaucoma filtering surgery,3–6,30,31 although MMC is preferred because of its more convenient application. It is easy to apply and is not unpleasant for patients, although application must be limited to a few minutes during surgery and it may be difficult to select the appropriate dosage for each individual eye. In contrast, 5-FU can be administered over a course of several days after surgery. Thus, with the use of 5-FU it is easier to avoid the problems of overdosage, although its administration is less inconvenient for both patients and surgeons. Based on results of previous studies, various factors that are released gradually after administration were studied,32–37 which may reduce the inconvenience of 5-FU administration. However, the material should be removed surgically when the drug effect exceeds optimal conditions. Recent studies revealed a possible beneficial effect of a single application of 5-FU during surgery.10–19 However, the appropriate dosage for each case appears to be different. Intraoperative single application is presently effective only for low to moderate risk cases,11,13,16 and a more controllable drug delivery system is needed.
In this study, we showed that cytosine deaminase induction and 5-FC administration leads to production of 5-FU in vitro and in vivo. Vector administration and 5-FC eye drops induce lesser toxicity to other cells, such as those of the corneal epithelium, and the produced 5-FU will maintain an ischaemic bleb.
We used the cytosine deaminase gene, which converts 5-FC to 5-FU and enhances drug sensitivity of the cells involved. This type of application is referred to as suicide gene therapy.38 Sakamoto et al39 reported the effectiveness of herpes simplex virus thymidine kinase and ganciclovir in a model of proliferative vitreoretinopathy. While this information is applicable, we have the great advantage of previous experience in the use of 5-FU after filtering surgery. With regard to cancer gene therapy, the effect of drug removal has not been described, but our data showed that removal of 5-FC resulted in return of cell viability after several days of treatment. It would be advantageous of course to have a gradual release delivery system. Additionally, the switching system of Cre-loxP may be beneficial in cases of overdosage.25,40 Using this system, production of cytosine deaminase can be halted by means of another viral infection. We also tried to enhance the effects of 5-FC by accelerating the toxic conversion with co-induction of cytosine deaminase and uridine phosphorylase, which enhance conversion from 5-fluorouracil to 5-fluorouridine (Fig 1). However, no enhancement of cellular toxicity was observed (data not shown). It seemed cytosine deaminase is critical in this cascade.
One problem is how to deliver cytosine deaminase into the subconjunctival space. In an attempt to do this, we used an adenovirus vector to deliver cytosine deaminase. By its nature, adenovirus is not incorporated into genomic DNA, although retrovirus is. As most adults have an acquired immunity to adenovirus, the vector is avoided within a few months. However, rather than being a problem, this seems to increase safety. The various safety levels needed to be examined closely before clinical use, but the adenovirus vector appears to be powerful and safe for in vivo use compared to others available, such as retrovirus and liposome based vectors.41
Further investigation is needed in rabbit and monkey models mimicking the clinical situation, although we have shown the feasibility of a new drug delivery system.
Acknowledgments
We thank Drs I Saito and Y Kanegae for their generous gifts of materials. Supported in part by grants from the Japanese Ministry of Education, Science and Culture.
REFERENCES
- 1.Heuer DK, Parrish RK2d, Gressel MG, et al. 5-Fluorouracil and glaucoma filtering surgery. II. A pilot study. Ophthalmology 1984;91:384–94. [DOI] [PubMed] [Google Scholar]
- 2.Chen CW, Huang HT, Bair JS, et al. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J Ocul Pharmacol 1990;6:175–82. [DOI] [PubMed] [Google Scholar]
- 3.Robin AL, Ramakrishnan R, Krishnadas R, et al. A long-term dose-response study of mitomycin in glaucoma filtration surgery [see comments]. Arch Ophthalmol 1997;115:969–74. [DOI] [PubMed] [Google Scholar]
- 4.Kitazawa Y, Suemori-Matsushita H, Yamamoto T, et al. Low-dose and high-dose mitomycin trabeculectomy as an initial surgery in primary open-angle glaucoma. Ophthalmology 1993;100:1624–8. [DOI] [PubMed] [Google Scholar]
- 5.Kitazawa Y, Kawase K, Matsushita H, et al. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Arch Ophthalmol 1991;109:1693–8. [DOI] [PubMed] [Google Scholar]
- 6.Nakano Y, Araie M, Shirato S. Effect of postoperative subconjunctival 5-fluorouracil injections on the surgical outcome of trabeculectomy in the Japanese. Graefes Arch Clin Exp Ophthalmol 1989;227:569–74. [DOI] [PubMed] [Google Scholar]
- 7.Hartman KU, Heidelberger C. Studies on fluorinate pyrimidines. XIII. Inhibition of thymidyrlate synthetase. J Biol Chem 1961;236:3006–13. [PubMed] [Google Scholar]
- 8.Jampel HD. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon's capsule fibroblasts. Ophthalmology 1992;99:1471–6. [DOI] [PubMed] [Google Scholar]
- 9.Khaw PT, Doyle JW, Sherwood MB, et al. Prolonged localized tissue effects from 5-minute exposures to fluorouracil and mitomycin C. Arch Ophthalmol 1993;111:263–7. [DOI] [PubMed] [Google Scholar]
- 10.Anand N, Sahni K, Menage MJ. Modification of trabeculectomy with single-dose intraoperative 5-fluorouracil application. Acta Ophthalmol Scand 1998;76:83–9. [DOI] [PubMed] [Google Scholar]
- 11.Singh K, Egbert PR, Byrd S, et al. Trabeculectomy with intraoperative 5-fluorouracil vs mitomycin C. Am J Ophthalmol 1997;123:48–53. [DOI] [PubMed] [Google Scholar]
- 12.Khaw PT, Doyle JW, Sherwood MB, et al. Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtration surgery in the rabbit. Ophthalmology 1993;100:367–72. [DOI] [PubMed] [Google Scholar]
- 13.Watts P, Karia N, McAllister J. Is the single use of intraoperative 5-fluorouracil in filtering surgery for high-risk cases enough? Eye 1998;12(Pt 3a):374–8. [DOI] [PubMed] [Google Scholar]
- 14.Bell RW, Habib NE, O'Brien C. Long-term results and complications after trabeculectomy with a single per-operative application of 5-fluorouracil. Eye 1997;11(Pt 5):663–71. [DOI] [PubMed] [Google Scholar]
- 15.Smith MF, Doyle JW, Nguyen QH, et al. Results of intraoperative 5-fluorouracil or lower dose mitomycin-C administration on initial trabeculectomy surgery. J Glaucoma 1997;6:104–10. [PubMed] [Google Scholar]
- 16.Katz GJ, Higginbotham EJ, Lichter PR, et al. Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended follow-up. Ophthalmology 1995;102:1263–9. [DOI] [PubMed] [Google Scholar]
- 17.Lanigan L, Sturmer J, Baez KA, et al. Single intraoperative applications of 5-fluorouracil during filtration surgery: early results. Br J Ophthalmol 1994;78:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egbert PR, Williams AS, Singh K, et al. A prospective trial of intraoperative fluorouracil during trabeculectomy in a black population. Am J Ophthalmol 1993;116:612–6. [DOI] [PubMed] [Google Scholar]
- 19.Doyle JW, Sherwood MB, Khaw PT, et al. Intraoperative 5-fluorouracil for filtration surgery in the rabbit. Invest Ophthalmol Vis Sci 1993;34:3313–9. [PubMed] [Google Scholar]
- 20.Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci USA 1992;89:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong VK, Shapourifar-Tehrani S, Kitada S, et al. Inhibition of rabbit ocular fibroblast proliferation by 5-fluorouracil and cytosine arabinoside. J Ocul Pharmacol 1991;7:27–39. [DOI] [PubMed] [Google Scholar]
- 22.Miyake S, Makimura M, Kanegae Y, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA 1996;93:1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akimoto M, Miyatake S, Kogishi J, et al. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci 1999;40:273–9. [PubMed] [Google Scholar]
- 24.Cao X, Ju DW, Tao Q, et al. Adenovirus-mediated GM-CSF gene and cytosine deaminase gene transfer followed by 5-fluorocytosine administration elicit more potent antitumor response in tumor-bearing mice. Gene Ther 1998;5:1130–6. [DOI] [PubMed] [Google Scholar]
- 25.Kanegae Y, Lee G, Sato Y et al. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res 1995;23:3816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa H, Yamamura K, Miyazaki J Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991;108:193–9. [DOI] [PubMed] [Google Scholar]
- 27.Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn J Med Sci Biol 1994;47:157–66. [DOI] [PubMed] [Google Scholar]
- 28.Reichel MB, Cordeiro MF, Alexander RA et al. New model of conjunctival scarring in the mouse eye. Br J Ophthalmol 1998;82:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordeiro MF, Schultz GS, Ali RR, et al. Molecular therapy in ocular wound healing. Br J Ophthalmol 1999;83:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldenfeld M, Krupin T, Ruderman JM, et al. 5-Fluorouracil in initial trabeculectomy. A prospective, randomized, multicenter study. Ophthalmology 1994;101:1024–9. [DOI] [PubMed] [Google Scholar]
- 31.Ruderman JM, Welch DB, Smith MF, et al. A prospective, randomized study of 5-fluorouracil and filtration surgery. Trans Am Ophthalmol Soc 1987;85:238–53. [PMC free article] [PubMed] [Google Scholar]
- 32.Sintzel MB, Heller J, Ng SY, et al. In vitro drug release from self-catalyzed poly(ortho ester): case study of 5-fluorouracil. J Controlled Release 1998;55:213–8. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Tucker IG, Roberts MS, et al. In vitro and in vivo evaluation in rabbits of a controlled release 5-fluorouracil subconjunctival implant based on poly(D,L-lactide-co-glycolide). Pharm Res 1996;13:1059–64. [DOI] [PubMed] [Google Scholar]
- 34.Gokce M, Akata RF, Kiremitci-Gumusderelioglu M. 5-FU loaded pHEMA drainage implants for glaucoma-filtering surgery: device design and in vitro release kinetics. Biomaterials. 1996;17:941–9. [DOI] [PubMed] [Google Scholar]
- 35.Merkli A, Heller J, Tabatabay C, et al. Purity and stability assessment of a semi-solid poly(ortho ester) used in drug delivery systems. Biomaterials. 1996;17:897–902. [DOI] [PubMed] [Google Scholar]
- 36.Hostyn P, Villain F, Malek-Chehire N, et al. [Biodegradable controlled-release 5-FU implant in the surgery for glaucoma. Experimental study] J Fr Ophtalmol 1996;19:133–9. [PubMed] [Google Scholar]
- 37.Lee DA, Leong KW, Panek WC et al. The use of bioerodible polymers and 5-fluorouracil in glaucoma filtration surgery. Invest Ophthalmol Vis Sci 1988;29:1692–7. [PubMed] [Google Scholar]
- 38.Moolten FL. Drug sensitivity (“suicide”) genes for selective cancer chemotherapy. Cancer Gene Ther 1994;1:279–87. [PubMed] [Google Scholar]
- 39.Sakamoto T, Kimura H, Scuric Z et al. Inhibition of experimental proliferative vitreoretinopathy by retroviral vector-mediated transfer of suicide gene. Can proliferative vitreoretinopathy be a target of gene therapy? [see comments] Ophthalmology. 1995;102:1417–24. [DOI] [PubMed] [Google Scholar]
- 40.Kanegae Y, Miyake S, Sato Y, et al. Adenovirus vector technology: an efficient method for constructing recombinant adenovirus and on/off switching of gene expression. Acta Paediatr Jpn 1996;38:182–8. [DOI] [PubMed] [Google Scholar]
- 41.Weichselbaum RR, Kufe D. Gene therapy of cancer. Lancet 1997;349(Suppl 2):SII10–2. [DOI] [PubMed] [Google Scholar]
- 42.Lee DA, Shapourifar-Tehrani S, Stephenson TR, et al. The effects of the fluorinated pyrimidines FUR, FUdR, FUMP, and FdUMP on human Tenon's fibroblasts. Invest Ophthalmol Vis Sci 1991;32:2599–609. [PubMed] [Google Scholar]