Abstract
Aims: The expression of the adhesion molecules ICAM-1, VCAM-1, and P-selectin, and the distribution and number of polymorphonuclear leucocytes (PMNs) were investigated in sickle cell retinopathy (SCR) and compared to the normal retina.
Methods: Postmortem ocular tissue was obtained from five subjects (16, 21, 28, 40, and 41 years of age) with sickle haemoglobinopathies and from one control subject. Tissue was cryopreserved, and streptavidin peroxidase immunohistochemistry was performed with antibodies against ICAM-1, VCAM-1, and P-selectin. Immunohistochemical reaction product was scored, and PMN numbers were counted in sections stained with non-specific esterase.
Results: Increased ICAM-1, VCAM-1, and P-selectin immunoreactivities were observed in sickle cell subjects compared to the control subject. The highest ICAM and P-selectin immunoreactivity was associated with intraretinal vessels adjacent to the preretinal neovascular formation in subjects with proliferative retinopathy. This was not the case with VCAM-1 immunoreactivity, which was highest in intraretinal vessels adjacent to the sea fan when the sea fan was still “in statu nascendi.” Fully formed, “older” sea fans had the highest levels of VCAM-1. The increase in adhesion molecule immunoreactivity was paralleled by an increase in intraretinal PMNs. The number of intraretinal PMNs increased with progression of the disease and the numbers surpassed those in control subjects by threefold. In the sea fan with the greatest VCAM-1 immunoreactivity, there were 20 times more PMNs were observed than in the rest of the retina in the same subject.
Conclusion: These data suggest that adhesion molecule mediated leucocyte adhesion might play an important part in the vaso-occlusive phase of sickle cell retinopathy and in autoinfarction of sea fan formations.
Keywords: ICAM-1, neutrophils, P-selectin, sickle cell, vaso-occlusions, VCAM-1
Sickle cell disease has the highest incidence for a population at risk of any genetically derived disease. Three in every 1000 African Americans born have sickle cell anaemia (SS disease).1 Sickle cell disease is caused by a point mutation in the β globin gene. Impairment of blood flow accounts for nearly all clinical manifestations of the sickling syndrome. Vaso-occlusion occurs in most organs, often causing morbidity, including splenomegaly, liver necrosis, painful crisis (largely marrow infarcts), acute chest syndrome, and retinopathy.
The initial retinal occlusions occur in periphery and have been observed in children with SS disease less than 2 years of age.2 The peripheral occlusions in children are primarily in capillaries but, with age, the occlusions occur in major blood vessels as well, and the peripheral retina becomes avascular.2–4 At the sites of occlusion, hairpin loops and arteriovenous anastomoses may form, resulting in abnormal blood flow. Preretinal neovascularisation, called sea fans, also forms adjacent to non-perfused peripheral retina.3,5,6 Proliferative sickle cell retinopathy occurs in 11–45% of sickle cell patients depending on genotype.7 There is a high incidence of autoinfarction of sea fans, perhaps occurring by the same vaso-occlusive processes that occur in retinal vasculature.8,9
It can be assumed that sickle erythrocytes (sRBCs) contribute to the vaso-occlusive processes that account for nearly all clinical manifestations of sickle cell disease. However, there is recent evidence that white blood cells contribute to the vaso-occlusive process as well. Polymorphonuclear leucocytes (PMNs) or neutrophils from sickle cell patients are less deformable and, therefore, less filterable (more rigid) than PMNs from non-sickle cell patients.10 Therefore, once adherent, they could potentially obstruct narrow capillary lumens. Increased numbers of PMNs are in the activated state in sickle cell patients.10,11 Recent evidence suggests that PMNs can bind to sRBCs, and this association activates the PMN.12
Leucocyte adhesion to vascular endothelium is modulated by adhesion molecules like intercellular adhesion molecule 1 (ICAM-1), vascular adhesion molecule 1 (VCAM-1), E-selectin, and P-selectin.13 The three major steps in leucocyte adherence and activation are: (1) rolling along the surface of the endothelium, (2) activation of the white blood cells (WBC), and (3) firm adhesion.13 Rolling, which is considered a prerequisite for firm adhesion, is modulated by both E and P-selectins. VCAM-1 and ICAM-1 are responsible for firm adherence of PMNs, and increased levels of soluble ICAM-1 have been reported in sera from sickle cell patients.14 Increased serum levels of soluble VCAM-1 have been found in steady state sickle cell subjects 15 as well as in subjects experiencing sickle cell acute chest syndrome.16 It has recently been demonstrated that VCAM-1 mediates sickle reticulocyte binding to endothelial cells in vitro.17–20
Cytokines like tumour necrosis factor α (TNFα) and interleukin α (IL-1α) upregulate leucocyte adhesion molecule expression by endothelial cells. Both TNFα and IL-1α are elevated in sera of steady state sickle cell subjects,21,22 perhaps due to low level inflammation caused by abnormal adhesion of sRBCs to endothelial cells in the microvasculature producing ischaemia and resultant tissue damage.23 The potent neutrophil chemokine IL-8 is also elevated in sera of sickle cell subjects during crisis.24
The purpose of the current study was to investigate the prevalence and localisation of the leucocyte adhesion molecules ICAM-1, VCAM-1, and P-selectin in sickle cell retina and in preretinal neovascular formations. The numbers of PMNs in retina and sea fan formations were also determined. The study demonstrates that all three adhesion molecules are upregulated and the number of PMNs elevated in sickle cell retina and further elevated in sea fans. The increased presence of PMNs and their adhesion molecules suggests that these leucocytes may participate in retinal vascular vaso-occlusive processes and autoinfarction of preretinal neovascularisation.
MATERIALS AND METHODS
Human postmortem eyes were obtained from Alabama Eye Bank (Mobile, AL, USA), Mid-America Eye and Tissue Bank (St Louis, MO, USA), and Medical Eye Bank of Maryland (Baltimore, MD, USA) and used with the approval of the Johns Hopkins Hospital joint committee on clinical investigations. One preretinal membrane (case 1) was obtained from a vitrectomy sample taken at the Johns Hopkins Hospital. In addition, cadaver eyes from five subjects with a history of sickle cell disease and one normal subject were examined. The normal subject was used previously in a study of leucocyte adhesion molecules and PMNs in diabetic retinopathy and was found to be representative of the six control, non-diabetic subjects used in that previous study.25 In the sickle cell group, the average age was 29 years (range 16–41 years), the average postmortem (or postoperative in case 1) time was 11 hours (range 2–31 hours), and the average death to enucleation time 3.75 hours (range 2–5.5 hours). In total, nine samples were examined from five sickle cell subjects (Table 1). Five of the eight sickle cell samples were obtained from the superior region of retina, and two samples from the inferior region of retina. All samples were serial sectioned, obtaining sections from three different preretinal neovascular formations (sea fans) and their surrounding regions of retina, and from four areas of sickle cell retina without preretinal neovascularisation. Thus, we could examine normal versus sickle cell retina, proliferative versus non-proliferative areas of sickle cell retina, and superior versus inferior region of sickle cell retina since retinopathy is said to be more prevalent in superior retina.26
Table 1.
Patient information
| Case No | Age (years), race, sex | Genotype | Retinopathy | PMT/DET | History |
| 1 | 16, BF | SC | Proliferative | 2 hours postop | Vitrectomy sample after haemorrhage, steroid therapy |
| 2 | 21, FB | SC | Proliferative | 31 hours/4.5 hours | Cardiac arrest |
| 3 | 28, BM | SS | Non-proliferative | 22 hours/3 hours | Sickle cell complications |
| 4 | 40, BF | SS | Proliferative | 24 hours/2 hours | Cardiac arrest |
| 5 | 41, BM | SC | Non-proliferative | 24 hours/5.5 hours | Pulmonary embolism, hypertensive |
| 6 | 72, WM | AA | No | 23.5 hours/3 hours | Cardiac arrest |
SC = heterozygote for haemoglobin S and C; SS = homozygote for haemoglobin S or sickle cell anaemia; AA, normal β globin; BF = black female; BM = black male; PMT = postmortem time; DET = death to enucleation time.
Immunohistochemistry was performed on serial 8 μm sections of cryopreserved tissue as previously described.27 In brief, the sections (two sections per slide) were incubated with primary antibodies for 20 hours at 4°C with the antibodies applied in the following order to serial sections: rabbit anti-von Willebrand factor (vWf, Dako Co, Carpintera, CA, USA) at 0.57 μg/ml; mouse anti-VCAM-1 (Genosys Inc, Woodlands, TX, USA) at 0.002 μg/ml; mouse anti-ICAM-1 (kindly provided by Robert Rothlein, Boehringer Ingelheim Inc, Ridgefield, CT, USA) at 2.6 μg/ml; mouse anti-P-selectin (Cytel Inc, San Diego, CA, USA) at 12.6 μg/ml; and rat anti-perlecan diluted 1:5000 (graciously provided by Alex Ljubimov, PhD, Cedar-Sinai Medical Center, Los Angeles, CA, USA). Each primary antibody was applied to duplicate slides in each experiment. Second step antibodies were biotinylated and streptavidin peroxidase was used as the third step (Kirkegaard and Perry Laboratories Inc, Gaithersburg, MD, USA). Peroxidase was developed with aminoethylcarbazol yielding a red reaction product. One of the two sections on each slide was counterstained with haematoxylin. Control sections were incubated with non-immune IgG (Jackson ImmunoResearch, Westgrove, PA, USA) from the same species and at the same protein concentration as the primary antibody. Sections from the control subject and at least three sickle cell specimens were included in each immunohistochemical experiment.
Qualitative analysis was performed by three masked investigators on a Zeiss light microscope with a 16× objective. The relative amount of immunoreactivity was evaluated using the seven point grading system of Page et al that we have previously reported for adhesion molecules.25,28 The average (SD) grades for all three investigators were then determined.
A slide in each series was stained with non-specific esterase (NSE) that stains granulocytes, as reported previously.25 PMN numbers were counted manually on all sections stained with NSE by two investigators and the average PMN numbers (SD)/mm2 of retina were then determined.
As a measure of quality control for our scoring system and a more accurate semiquantitative measure of immunoreactivity levels, we performed densitometric analysis of ICAM-1, VCAM-1, and P-selectin immunoreactivities in cases 6 and 4 as follows. We captured images of the same fields in serial sections on the non-counterstained sections using our image analysis system consisting of a Zeiss Photomicroscope II, a Hamamatsu Video Camera (model No C2400–77, Hamamatsu City, Japan), and the NIH-Image 1.47 software on a Macintosh IIci computer (Apple, Cupertino, CA, USA). On each section examined, we measured relative grey scale values of all vessels in retina and in the sea fans as we have described previously.29,30
Statistical evaluation of the immunohistochemical data (scores from three observers of triplicate slides from each subject incubated with each antibody) and PMN numbers was performed using the Student's t tests. p Values equal to or less than 0.05 were considered significant.
RESULTS
A brief description of the subjects used in this study is presented in Table 1. In the sickle cell group, two patients had sickle haemoglobin type SS (sickle cell anaemia) and three subjects had SC haemoglobin (SC disease) (Table 1). Three subjects had proliferative retinopathy while two had non-proliferative retinopathy. The sea fans in the specimens had different morphological features (Fig 1). One sea fan was composed primarily of large dilated lumens (Fig 1A), one was composed primarily of viable capillary-like lumens (Fig 1B), and one was partially infarcted (Fig 1C), as demonstrated by immunohistochemical localisation of perlecan, which we have previously demonstrated is present only in viable blood vessels.31
Figure 1.
Sections through portions of retina containing sea fans immunolabelled with anti-perlecan antibody, a marker for viable blood vessels.31 Sea fans varied in morphology from one featuring primarily large caliper dilated vessels (A) (from case 4), one consisting of small diameter capillary-like vessels (B) (from case 4), and one containing both viable and infarcted blood vessels (C) (from case 2). Infarcted, perlecan-negative blood vessels are indicated by the arrowheads in (C). Perlecan immunoreactivity and haematoxylin counterstain; magnification, (A) and (B) 120×, (C) 80×.
VCAM-1 immunoreactivity was generally elevated in proliferative sickle cell specimens when compared to control and was associated mostly with blood vessels in retina (Fig 2). The greatest difference in VCAM-1 immunoreactivity between control and sickle cell retina was noted for case 4 (p<0.0001) (Fig 3A). VCAM-1 immunoreactivity was highest in sea fans (Fig 4). Comparing scores in retina and in the sea fans of each subject, we found a significant increase in VCAM-1 immunoreactivity in sea fans from case 4 (sea fan in Fig 1A p<0.0001, sea fan in Fig 1B p=0.017) (Fig 3A). The vitrectomy specimen (case 1) and cases 2 and 3 also had elevated VCAM-1 immunoreactivity when compared with control, but the difference was not statistically significant. When superior and inferior regions of retina were compared, we found a statistically significant increase in VCAM-1 immunoreactivity for case 4 between superior and inferior (proliferative sickle cell retinopathy (SCR)) (p<0.0001), but not for case 5 (non-proliferative SCR) whose VCAM-1 scores were comparable to control in both areas of retina (Fig 5A).
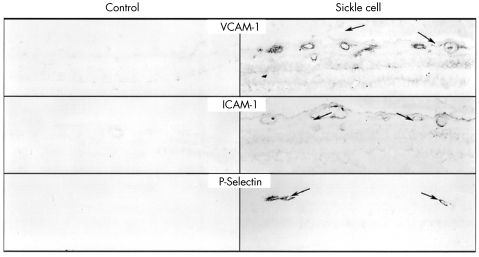
Figure 2.
Localisation of VCAM-1 , ICAM-1, and P-selectin in the retina from a control subject (case 6) (left) and from a sickle cell subject (case 4) (right). Upregulation of all three molecules was observed in blood vessels of sickle cell retinas. Magnification 85×.
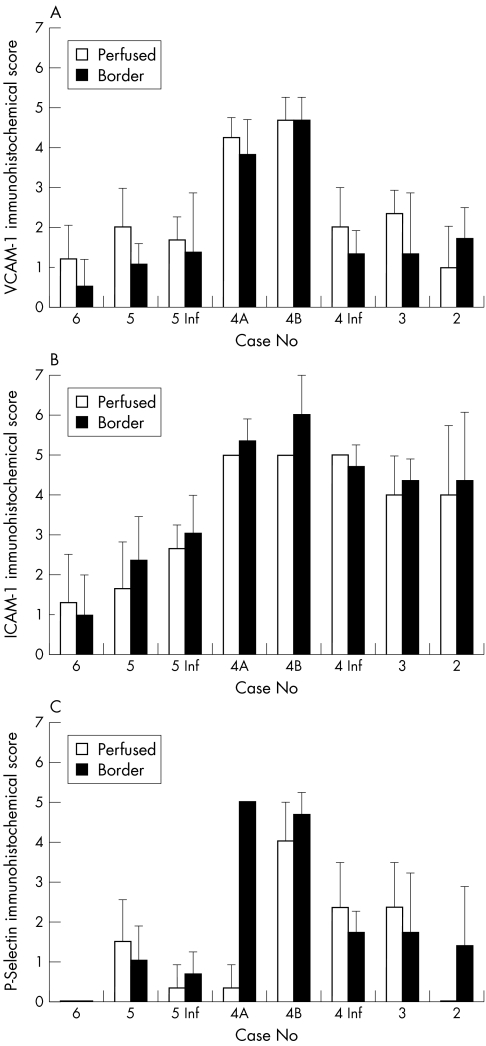
Figure 3.
Comparison between immunohistochemical scores in subjects with proliferative sickle cell retinopathy (cases 1, 2, 4) and the control subject (case 6). Two areas in the retina of case 4 are represented (4A and 4B) and scores for sea fan formations and two areas within retina (perfused central retina and border of perfused and non-perfused peripheral retina) are represented for the sickle cell subjects. (A) Mean (SEM) score for VCAM-1 immunoreactivity. (B) Mean immunohistochemical scores for ICAM-1. (C) Mean immunohistochemical scores for P-selectin.
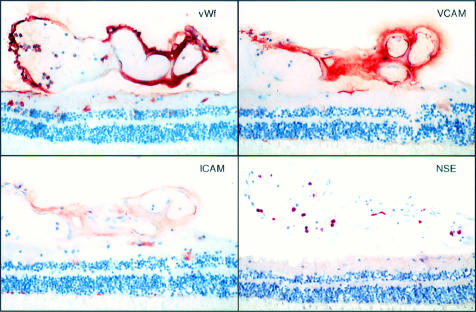
Figure 4.
Immunolocalisation of von Willebrand factor (vWf) (top left), VCAM-1 (top right), ICAM-1 (bottom left), and enzyme histochemical localisation of non-specific esterase (NSE) (bottom right) in the large vessel sea fan shown in Figure 1A. VCAM-1 showed marked immunoreactivity. ICAM-1 immunostaining was relatively weak in sea fans compared to that observed in retinal vessels, but was elevated compared to controls. Numerous PMNs (NSE positive cells were located within the lumen of sea fans. Magnification 120×.
Figure 5.
Immunohistochemistry scores for perfused and non-perfused retina for all sickle cell subjects and control subject (case 6). Scores for three areas of subjects 4's eye are presented, inferior and two areas with sea fans (4A and 4B), and a superior (5) and inferior block (5 Inf) from case 5 which had non-proliferative retinopathy. Case 1 was excluded from this analysis because retina was not included in the sample. (A) Mean (SEM) score for VCAM-1 immunoreactivity. (B) Mean immunohistochemical scores for ICAM-1. (C) Mean immunohistochemical scores for P-selectin.
The densitometry results confirmed our scoring data (results not shown). Relative intraretinal grey scale values in the sickle cell subject examined (case 4) were significantly higher than in control (case 6, p<0.0001). The relative grey scale values in both sea fans were also higher than the intraretinal grey scale values from case 4 (sea fans in Fig 1A and B), but only the value for the sea fan in Figure 1A was statistically significant (p<0.0001).
P-selectin immunoreactivity was not observed in the control subject. In all sickle cell subjects, we found a statistically significant elevation of P-selectin immunoreactivity when compared to control. The greatest difference in immunoreactivity between control and sickle cell retina was again noted for case 4 (p<0.0001). P-selectin immunoreactivity was also elevated in preretinal formations. However, there was no statistically significant difference between sickle cell retina and sea fan in the same subject. There was significantly more P-selectin immunoreactivity in superior than inferior regions for the subject with proliferative SCR (case 4, p=0.04) as well as for the subject with non-proliferative SCR (case 5, p=0.05).
ICAM-1 immunoreactivity was generally elevated in sickle cell specimens in all areas of retina and in sea fans, whether the subject had proliferative or non-proliferative retinopathy. Unlike VCAM-1, the differences between intraretinal and preretinal ICAM-1 scores were very small. In two samples (case 4, sea fan in Fig 1B, and case 2), the preretinal ICAM-1 immunoreactivity was even lower than in retina, but it was still considerably elevated when compared to control. In the vitrectomy specimen (case 1), ICAM-1 immunoreactivity was comparable to control. ICAM-1 scores in the inferior region of retina paralleled ICAM-1 immunoreactivity findings in the superior region of case 4. In case 5, ICAM-1 immunoreactivity was significantly increased in the inferior retinal region (pinf= 0.005) when compared with control, but the difference between superior and inferior regions was not statistically significant. ICAM-1 immunoreactivity in the preretinal formations of the same subject was also significantly increased when compared to intraretinal values when measured with densitometry (p= 0.003 for specimen in Fig 1A and 0.0002 for specimen in Fig 1B).
We observed an increase in neutrophil numbers per mm2 of retina in sickle cell retina in general, when compared with control, which was statistically significant for cases 3, 4 (area B), and 5 (p = 0.0002, = 0.007, and <0.02 respectively). The largest number of intraretinal neutrophils was seen in case 3. In the sea fans from case 4 (Figs 1A, B) and case 2 (Fig 1C), neutrophil numbers were increased 305-fold, 81-fold, and 67-fold respectively when compared with intraretinal control PMN numbers. In the steroid treated case 1, no increase in neutrophil numbers was noted. When superior and inferior areas of retina were compared, there were fewer neutrophils in inferior retina in both cases, which was statistically significant for case 5 (p<0.02), but not for case 4.
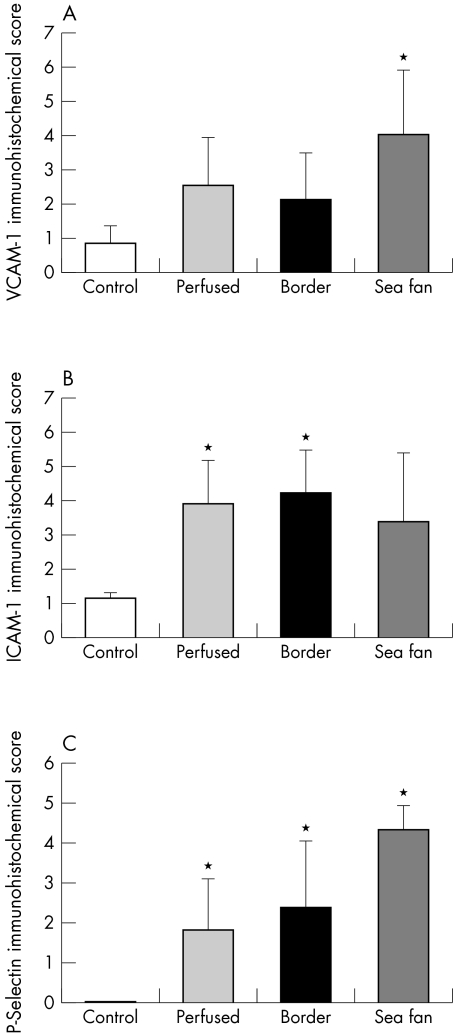
In summary, VCAM-1 was elevated in all sickle cell subjects, but the increase in reaction product was only significant when sea fans were compared with localisation in control subject's retina (Fig 7A). ICAM-1 immunoreactivity was also elevated in sickle cell group compared to control subject, but the difference was only significant for areas of retina sectioned and not for sea fans (Fig 7B). P-selectin immunoreactivity was significantly elevated in all areas of retina and in sea fans compared to control subjects (Fig 7C). Neutrophil numbers were significantly elevated in all areas of retina and in sea fans compared to control subjects (Fig 6) (p <0.007 and <0.0001 respectively). The number of PMNs in sea fan formations was significantly greater than the number in sickle cell retina (p <0.0001).
Figure 7.
Summary of immunohistochemistry scores for sickle cell subjects and control subject. The values for sickle cell subjects represent all of the scores in Figure 5. (A) When all scores for VCAM-1 in sickle cell subjects were combined, there was a significant difference between the control subject's scores and the sea fan scores (*p<0.01), but the difference was not significant for the two areas of sickle cell retina. (B) When ICAM-1 scores for all subjects were combined, there was a significant difference between ICAM-1 in perfused and border areas of the sickle cell retinas compared to control subjects retina (*p<0.05) but the difference between sea fans and control subjects retina was not significant (p=0.1). (C) The P-selectin scores for all areas of sickle cell retina and the sea fans was significantly greater than the score in the control subject (p<0.05).
Figure 6.
Mean number of polymorphonuclear leucocytes (PMNs) per mm2 of retina and sea fans. PMNs were elevated in all sickle cell retinas compared to the control subject (p <0.02 for cases 3, 4B, and 5) and further elevated in sea fan formations compared with control subject, case 6.
DISCUSSION
Vaso-occlusion is the initial event in sickle cell retinopathy. Although it is intuitive that sickle RBCs would be the initiating cell type in the vaso-occlusive process, the current study suggests that neutrophils may contribute as well. This seems likely since sickle cell disease subjects have high circulating cytokine levels, high white cell counts, and frequent infections.23 In the current study, the number of PMNs was elevated in the sickle cell retina and in sea fan formations. Sickle PMNs are more adherent to fibronectin than normal PMNs,32 and sickle cell subjects have more activated PMNs than non-sickle cell subjects.11 RBCs adhere to PMNs in sickle cell disease and may initiate the PMN's respiratory burst.12 We have observed PMNs in RBC cell plugs at sites of occlusions in sickle cell retinopathy.2
Not only were increased numbers of PMNs observed in the current study, but the adhesion molecules responsible for PMN rolling and firm adherence, P-selectin and ICAM-1 respectively, were elevated as well in sickle cell retina and in sea fan formations. ICAM-1 production can be stimulated by VEGF.33 Cao et al observed greatly elevated VEGF in the same sea fan formations studied in the current paper.31 ICAM-1 expression is also shear stress regulated,34 and shear stress may be elevated in sickle cell subjects. In a previous study of adhesion molecules in diabetic retina, ICAM-1 was elevated in diabetic retina but P-selectin was rarely observed,35 yet in the sickle cell subjects studied, both P-selectin and ICAM-1 were significantly elevated in retina.
Of the adhesion molecules studied and found to be elevated in this study, VCAM-1 may be the most significant for progression of vaso-occlusive processes. In binding its counter-receptor VLA-4 (α4β1), it can initiate the adherence of B and T lymphocytes, and monocytes. Several groups have recently demonstrated that some reticulocytes from sickle cell subjects have VLA-4 on their surface as well.17,18,20 These cells could be stress reticulocytes, which are prematurely released from marrow because of the rapid destruction of circulating RBCs in sickle cell subjects. VCAM-1 has been reported to mediate sickle reticulocyte binding to human endothelium in vitro via VLA-4.17,18,20 Setty and Stuart have found that VCAM-1/VLA-4 interaction is responsible for the in vitro adherence to both macrovascular and retinal microvascular endothelial cells.19 Elevated serum levels of soluble VCAM-1 have been observed in sickle cell subjects with acute chest syndrome, another vaso-occlusion initiated event in the sickle cell subject,16 suggesting that VCAM-1 may be overexpressed in other organs during pathological events.
Using a rat model,36 we have observed adherence of reticulocytes via VLA-4 after administration of TNFα to the rats.37 Blocking VLA-4 with a cyclic peptide or monoclonal antibody has been shown to prevent sickle RBC adherence in our rat model of sickle cell mediated retinal vascular occlusion.37 However, in that model, fibronectin appeared to be the counter-receptor for VLA-4, not VCAM-1. Kaul's work in mesocaecum and mesoappendix suggest that adherence of lighter density cells initiates the vaso-occlusive process and dense cells (ISC) may be trapped upstream at sites of reticulocyte adherence.38–40 Adhesion of reticulocytes to endothelial cells would also cause leucostasis owing to changes in flow patterns, which increases the opportunity for leucocytes to firmly adhere to their endothelial adhesion molecules like ICAM-1.
Cytokines like TNFα and IL-1α upregulate leucocyte adhesion molecule expression by endothelial cells and activated PMNs. Both TNFα and IL-1α are elevated in steady state sickle cell subjects,21,22 perhaps because of a low level of inflammation caused by abnormal adhesion of sRBCs to endothelial cells in the microvasculature.23 The potent neutrophil chemokine IL-8 is also elevated in sickle cell subjects during crisis.24 IL-6 is also elevated in the plasma of sickle cell subjects and it stimulates adherence of sickle PMNs and RBCs to fibronectin.32 Alternatively, it may be the sickle erythrocytes themselves that cause the upregulation of the leucocyte adhesion molecules observed in this study. Shiu et al recently demonstrated in vitro, using endothelial cells in flow chambers, that ICAM-1 and VCAM-1 gene expression increased in endothelial cells after perfusion with sickle RBCs.41
The localisation and relative levels of adhesion molecules were assessed in several locations in the sickle cell retina. Inferior retina was compared with superior because retinopathy occurs less in inferior retina.26 No significant difference or trend in adhesion molecule levels were observed between superior and inferior retina in the two cases used for that analysis. We also did not see a difference in adhesion molecule localisation between SS and SC subjects (Table 1), although case 4, an SS subject, had the highest levels of all adhesion molecules. We compared localisation and relative levels of reaction product between perfused areas and the border of perfused and non-perfused retina, as determined by vWf and perlecan localisation in serial sections. The border area could potentially be hypoxic because the continuum of vaso-occlusive events in sickle cell retina occurs in the border area most often. Furthermore, ICAM-1 can be induced by hypoxia.42 The border area had the highest level of ICAM-1 in almost all specimens, but the difference between border and perfused retina was not significant. For VCAM-1, the perfused area tended to have greater levels than the border areas but, again, the difference was not significant. Sea fan formations had the highest VCAM-1 levels of all areas. The sea fan from case 1, the vitrectomy specimen, had the lowest ICAM-1 and VCAM-1 scores of any of the preretinal membranes. This may be related to the steroid treatment the patient received before surgery or, alternatively, could be related to this specimen having the shortest postmortem (postoperative) time of any specimen.
Sea fan formations also had the greatest number of PMNs. This suggests that PMNs may contribute to the autoinfarction of these neovascular formations that has previously been reported.8,9 Poor flow properties of these pathological vasculatures could also contribute to the retention of PMNs in sea fans. None of the eyes used in this study was perfused before preparation for freezing, so we assume that the numbers of PMNs in the sections represent those present in retina at the death of the subject.
In summary, the current study has demonstrated that leucocyte adhesion molecules are elevated in sickle cell retina. VCAM-1 was elevated in sickle cell retina and especially in preretinal neovascular formations. Expression of VCAM-1 in sickle cell retina can modulate the adherence of B and T lymphocytes, monocytes, and sickle reticulocytes. ICAM-1 and P-selectin were elevated in sickle cell retina and sea fan formations, which can result in increased adhesion of PMNs to vascular endothelium. The number of PMNs in these sickle cell retinas was greatly elevated. If PMNs do not initiate the vaso-occlusions, they may contribute to finalising the occlusive event. They can destroy endothelial cells with their oxidative burst, exposing basement membrane, and allowing platelet adhesion. We have observed PMNs in platelet fibrin thrombi at sites of vaso-occlusions in sickle cell subjects.2 Increase in PMNs in the sickle cell retina is one of the many pleiotropic effects of this disease caused by a point mutation in the haemoglobin gene. The pleiotropic effects are further modulated by the epistatic effects like upregulation of the adhesion molecule expression.43 The positive effects of hydroxyurea treatment, one of the few therapies available to most sickle cell subjects, may in part be due to inactivation of PMNs15 and reduction in production of endothelial cell VCAM-1, although soluble VCAM-1 levels were not significantly reduced during hydroxyurea therapy in a recent study.44 In conclusion, adhesion molecule mediated leucocyte adhesion might have an important role in retinal vaso-occlusive processes and autoinfarction of sea fans.
Acknowledgments
This study was supported by NIH grants HL45922 (GL) and EY01765 (Wilmer Institute). G Lutty is an American Heart Association established investigator and the recipient of a Research to Prevent Blindness Lew Wasserman Merit Award. The authors thank Albert M. Maguire, MD, and Donald Rucknagel, MD, for their assistance in tissue donations and Alex Ljubimov, PhD, and Robert Rothlein, PhD, for graciously providing anti-perlecan and anti-ICAM-1 respectively. We also would like to thank the organ donors and their families for their generosity.
Abbreviations
ICAM-1, intercellular adhesion molecule 1
IL-1α, interleukin α
NSE, non-specific esterase
PMN, polymorphonuclear leucocytes
SCR, sickle cell retinopathy
sRBCs, sickle erythrocytes
TNFα, tumour necrosis factor α
VCAM-1, vascular adhesion molecule 1
WBC, white blood cells
vWf, von Willebrand factor
REFERENCES
- 1.Sickle Cell Disease Group Panel. Sickle cell disease: screening, diagnosis, management, and counseling in newborns and infants. Clinical practice guideline no 6. Rockville, MD: Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, 1993:7–10.
- 2.McLeod D, Goldberg M, Lutty G. Dual perspective analysis of vascular formations in sickle cell retinopathy. Arch Ophthalmol 1993;111:1234–45. [DOI] [PubMed] [Google Scholar]
- 3.McLeod DS, Merges C, Fukushima A, et al. Histopathological features of neovascularization in sickle cell retinopathy. Am J Ophthalmol 1997;124:473–87. [DOI] [PubMed] [Google Scholar]
- 4.Harlan JB, Fekrat S, Lutty GA, et al. Hemoglobinopathies. In: Ryan SJ, ed. Retina. St Louis: Mosby, 2001:1454–71.
- 5.Goldberg MF. Retinal neovascularization in sickle cell retinopathy. Trans Am Acad Ophthalmol Otolaryngol 1977;83:409–31. [PubMed] [Google Scholar]
- 6.Romayananda N, Goldberg MF, Green WR. Histopathology of sickle cell retinopathy. Trans Am Acad Ophthalmol Otolaryngol 1973;77:652–76. [PubMed] [Google Scholar]
- 7.Clarkson JG. The ocular manifestations of sickle-cell disease: a prevalence and natural history study. Trans Am Ophthalmol Soc 1992;90:481–504. [PMC free article] [PubMed] [Google Scholar]
- 8.Nagpal KC, Patrianakos D, Asdourian GK, et al. Spontaneous regression (autoinfarction) of proliferative sickle retinopathy. Am J Ophthalmol 1975;80:885–92. [DOI] [PubMed] [Google Scholar]
- 9.Condon PI, Serjeant GR. Behaviour of untreated proliferative sickle retinopathy. Br J Ophthalmol 1980;64:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates TD, Fisher TC, Pecsvarady Z, et al. Neutrophil deformability and FcRIII expression are decreased in sickle cell disease patients with clinically severe b globin haplotypes. 18th Annual Meeting—National Sickle Cell Disease Program 93. Philadelphia: 1993:63a.
- 11.Lard LR, Mul FP, de Haas M, et al. Neutrophil activation in sickle cell disease. J Leukoc Biol 1999;66:411–15. [DOI] [PubMed] [Google Scholar]
- 12.Hofstra T, Kaira V, Meiselman H, et al. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood 1996;87:4440–7. [PubMed] [Google Scholar]
- 13.Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science 1992;258:964–9. [DOI] [PubMed] [Google Scholar]
- 14.Blei F, Fancher T, Guarini L. Elevated levels of circulating molecule of potential endothelial cell origins in sickle cell disease. Blood 1994;84(suppl):409a. [Google Scholar]
- 15.Saleh AW, Hillen HF, Duits AJ. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol 1999;102:31–7. [DOI] [PubMed] [Google Scholar]
- 16.Stuart MJ, Settty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for therapy. Blood 1999;95:1555–60. [PubMed] [Google Scholar]
- 17.Gee B, Platt O. Sickle reticulocytes adhere to VCAM-1. Blood 1995;85:268–74. [PubMed] [Google Scholar]
- 18.Joneckis C, Ackley R, Orringer E, et al. Integrin α4β1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemia. Blood 1993;82:3548–55. [PubMed] [Google Scholar]
- 19.Setty BN, Stuart MJ. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood 1996;88:2311–20. [PubMed] [Google Scholar]
- 20.Swerlick RA, Eckman JR, Kumar A, et al. α4β1-integrin expression on sickle reticulocytes: vascular cell adhesion molecule-1-dependent binding to endothelium. Blood 1993;82:1891–9. [PubMed] [Google Scholar]
- 21.Francis R Jr, Haywood LJ. Elevated immunoreactive tumor necrosis factor and interleukin-1 in sickle cell disease. J Natl Med Assoc 1992;84:611–15. [PMC free article] [PubMed] [Google Scholar]
- 22.Malave I, Perdomo Y, Escalona E, et al. Levels of tumor necrosis factor α/cachectin (TNFa) in sera from patients with sickle cell disease. Acta Haematol 1993;90:172–6. [DOI] [PubMed] [Google Scholar]
- 23.Moore C, Ehlayel M, Leiva L, et al. New concepts in the immunology of sickle cell disease. Ann Allergy Asthma Immunol 1996;76:385–403. [DOI] [PubMed] [Google Scholar]
- 24.Duits AL, Schnog JB, Lard LR, et al. Elevated IL-8 levels during sickle cell crisis. Eur J Haematol 1998;61:302–5. [DOI] [PubMed] [Google Scholar]
- 25.McLeod DS, Lefer DJ, Merges C, et al. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol 1995;147:642–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg MF, ed. Sickle cell retinopathy. In: Clinical ophthalmology. Hagerstown, MD: Harper and Row, 1976:1–44.
- 27.Lutty GA, Merges C, Threlkeld AB, et al. Heterogeneity in localization of isoforms of TGF-β in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci 1993;34:477–87. [PubMed] [Google Scholar]
- 28.Page C, Rose M, Yacoub M, et al. Antigenic heterogeneity of vascular endothelium. Am J Pathol 1992;141:673–83. [PMC free article] [PubMed] [Google Scholar]
- 29.Lutty GA, Merges C, McLeod DS. 5'-Nucleotidase and adenosine during retinal vasculogenesis and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2000;41:218–29. [PubMed] [Google Scholar]
- 30.Taomoto M, McLeod DS, Merges C, et al. Localization of adenosine A2a receptor in retinal development and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2000;41:230–43. [PubMed] [Google Scholar]
- 31.Cao J, Kunz Mathews M, McLeod DS, et al. Angiogenic factors in human proliferative sickle cell retinopathy. Br J Ophthalmol 1999;83:838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasschau M, Barabino G, Bridges K, et al. Adhesion of sickle neutrophils and erythrocytes to fibronectin. Blood 1996;87:771–80. [PubMed] [Google Scholar]
- 33.Lu M, Perez V, Ma N, et al. VEGF increases retinal ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci 1999;40:1808–12. [PubMed] [Google Scholar]
- 34.Nagel T, Resnick N, Atkinson WJ, et al. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest 1994;94:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLeod D, Lefer D, Merges C, et al. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic retina and choroid. Am J Pathol 1995;147:642–53. [PMC free article] [PubMed] [Google Scholar]
- 36.Lutty GA, Phelan A, McLeod DS, et al. A rat model for sickle cell-mediated vaso-occlusion in retina. Microvasc Res 1996;52:270–80. [DOI] [PubMed] [Google Scholar]
- 37.Lutty GA, Taomoto M, Cao J, et al. Inhibition of TNFα-induced sickle RBC retention in retina by a VLA-4 antagonist. Invest Ophthalmol Vis Sci 2001;42:1349–55. [PubMed] [Google Scholar]
- 38.Kaul DK, Chen D, Zhan J. Adhesion of sickle cells to vascular endothelium is critically dependent on changes in density and shape of the cells. Blood 1994;83:3006–17. [PubMed] [Google Scholar]
- 39.Kaul DK, Fabry ME, Nagel RL. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc Natl Acad Sci USA 1989;86:3356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaul DK, Fabry ME, Nagel RL. Erythrocytic and vascular factors influencing the microcirculatory behavior of blood in sickle cell anemia. Ann NY Acad Sci 1989;565:316–26. [DOI] [PubMed] [Google Scholar]
- 41.Shiu Y-T, Udden MM, McIntire LV. Perfusion with sickle erythrocytes up-regulates ICAM-1 and VCAM-1 gene expression in cultured human endothelail cells. Blood 2000;95:3232–41. [PubMed] [Google Scholar]
- 42.Arnould T, Michiels C, Remacle J. Increased PMN adherence on endothelial cells after hypoxia: involvement of PAF, CD18/CD11b, and ICAM-1. Am J Physiol 1993;264:C1102–10. [DOI] [PubMed] [Google Scholar]
- 43.Kaul DK, Fabry ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev 1996;10:29–44. [DOI] [PubMed] [Google Scholar]
- 44.Saleh AW, Duits AJ, Gerbers A, et al. Cytokines and soluble adhesion molecules in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol 1998;100:26–31. [DOI] [PubMed] [Google Scholar]