Abstract
Aim: To develop a reliable and objective non-touch technique for determination of tear volume and tear secretion by means of tear film fluorophotometry.
Methods: 10 defined concentrations of sodium fluorescein were given in an artificial chamber and measured by the Fluorotron Master (Coherent Radiation Inc). A calibration line was established and the calibration equation was programmed into the Fluorotron Master computer software. In 28 patients (56 eyes) with dry eye symptoms and 15 volunteers (30 eyes) tear volume and tear secretion were then measured directly without taking a tear sample, using the new technique.
Results: The values obtained by the Fluorotron Master measuring 10 defined fluorescein concentrations within an artificial chamber are approximately 100 times lower than the actual concentrations. The calibration line led to a calibration equation: y = 36 + 0.01 × X. The mean tear secretion in 56 eyes of patients with dry eye symptoms was 2.48 μl/min, compared to 3.4 μl/min in healthy volunteers. The difference was statistically significant. Average tear volume was 7.0 μl in patients and 7.2 μl in healthy volunteers with no statistically significant difference.
Conclusion: The method developed is a simple and reliable non-touch technique without the need for taking a tear sample, which makes the examination faster and more comfortable for the patient.
Keywords: fluorophotometry, tear film, tear volume, tear secretion
Fluorophotometry in tear film diagnostics was first described by Maurice1 and Mishima et al.2 They established fluorophotometric examinations of tear volume and tear secretion in healthy volunteers. Ever since, tear film fluorophotometry has been used to detect a circadian rhythm3 or age dependency of tear secretion4,5 and various dry eye symptoms have been assessed by tear film fluorophotometry.6–16 In 1985, a new, very sensitive completely automated fluorophotometer was developed (Fluorotron Master, Coherent Radiation Inc, Palo Alto, CA, USA).17,18 Using the Fluorotron Master, tear secretion and tear volume could be quantified with high reproducibility.10
With the Fluorotron Master, tear volume and tear secretion were determined by obtaining a tear specimen after applying an aqueous solution of sodium fluorescein (1 μl) to the lateral upper bulbar conjunctiva.10
Collecting tear samples for fluorophotometric determination of the fluorescein concentration was necessary because the Fluorotron Master's focus on the excited and detected light beam can only be adapted to a 0.2 mm signal (as anterior chamber measurements); however, the thickness of a normal tear film is only 7 μm. Thus, measuring tear film fluorescence in situ would lead to false negative results.
This study was performed to calibrate the measurement focus as given by the Fluorotron Master, so that direct non-touch determination of tear film fluorescence in situ and therefore calculation of tear volume and secretion can be done without the necessity of taking tear samples.
MATERIALS AND METHODS
Preparation of a 7 μm chamber
To determine fluorescein concentration in 7 μm of fluid film, an artificial anterior chamber of exactly 7 μm had to be built. This chamber needed to be easy to screen and easy to fill and approximately the size of the ocular surface. A metal ring measuring 7 μm in height and 24 mm in diameter was glued to a piece of glass, thus forming a trough. This assembly and a second piece of glass that served as a lid were held in place by metal clamps thus forming a stable artificial chamber with a depth of exactly 7 μm (Hellma, Müllheim, Germany). The chamber was suspended in front of the Fluorotron Master.
Fluorescein measurement within the artificial chamber
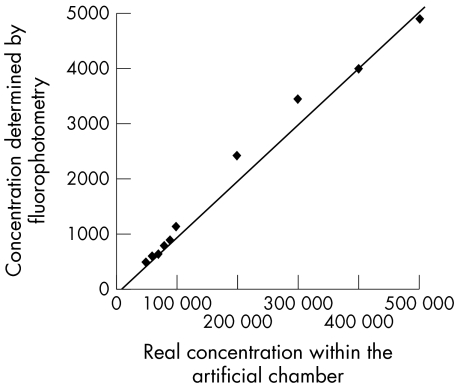
A dilution series of 10 known fluorescein concentrations between 0.005% and 0.05% (corresponding to 50 000 to 500 000 ng/ml) was established. Each fluorescein concentration was poured into the artificial chamber, which is mounted 2 cm in front of the anterior chamber adapter of the Fluorotron Master. The distance reflected the positioning of the patient's eye during anterior chamber measurements. The fluorescence of each dilution was determined five times by the Fluorotron Master with 1 minute intervals. Each dilution was created twice, so that in the end the mean of 2 × 5 fluorophotometric measurements per dilution concentration was calculated. The calculated mean of each dilution was then compared to the actual already known concentration of the fluorescein dilution. A calibration curve was drawn to acquire a calibration equation (Fig 1).
Figure 1.
Calibration line of known fluorescein concentrations within the artificial chamber versus the values obtained by the Fluorotron Master (ng/ml).
Measurement of tear volume and secretion
Tear film fluorophotometry was performed on 28 patients (56 eyes) with bilateral idiopathic keratoconjunctivitis sicca—that is, keratoconjunctivitis sicca not secondary to systemic or other ocular disease, and 15 healthy volunteers (30 eyes). Patients as well as volunteers had given their informed consent. All examinations were performed in a dark room at 20°C room temperature between 8 and 12 o'clock in the morning. The patients included 16 women and 12 men, aged 26–80 years (mean 50 years). Excluded were patients with other pathological findings, such as nasolacrimal duct obstruction, local infections or corneal epithelial lesions, and patients wearing contact lenses. The 15 healthy volunteers (seven women, eight men) were between 25 and 34 years of age (mean 28 years). Subjects with acute or chronic blepharitis, acute or chronic diseases of the anterior segment, lid abnormalities, or history of wearing contact lenses or using local ophthalmic medication or presumed allergic reactions were excluded from the study.
The patient/volunteer was positioned in front of the Fluorotron Master adapted with the anterior chamber adapter. Fluorescence was determined to exclude autofluorescence of the eye. A 0.1% fluorescein solution, 1 μl, was applied to the lateral upper bulbar conjunctiva and mixed with the tear film by having the patient/volunteer quickly move his/her eyes from side to side with closed lids.
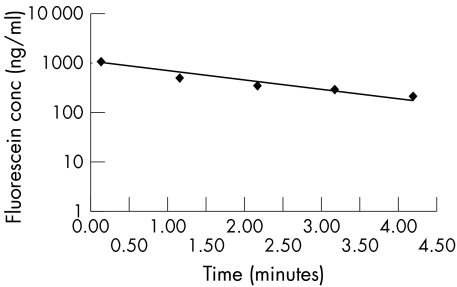
Ten seconds after application the clearance of tear film fluorescein was determined by measuring the decrease in tear film fluorescein five times with 1 minute intervals. A regression line for the clearance of the tear film fluorescein and the tear film turnover rate was calculated automatically. The semilog plot of concentration against time was extrapolated to time zero, the fluorescein concentration at the time of fluorescein installation (Fig 2).
Figure 2.
Example of a regression line of the measured fluorescein concentration (ng/ml) versus time (minutes).
The latter was then automatically corrected according to the correction factor obtained from the calibration line mentioned above. Tear volume at time zero was now calculated by the following equation:
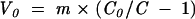 |
m = amount of fluorescein solution applied to the conjunctiva (1 μl)
C0 = applied concentration of the fluorescein solution (0.1%)
C = fluorescein concentration at time zero
V0 = tear volume at time zero
Tear secretion (flow) was then determined using the following formula:
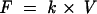 |
k = turnover rate (%/min)
V = volume (μl)
F = flow
Statistical analysis of tear secretion and tear volume of patients and volunteers was performed using the Mann-Whitney-Wilcoxon test.
RESULTS
Results of the calibration measurements
The fluorescein concentrations (ng/ml) of the 10 defined fluorescein concentrations as measured by the fluorophotometer within the artificial chamber are shown in Table 1. They are given as the average of two samples, each measured five times. Values obtained by the Master Fluorotron lay between 480 and 4900 ng/ml and were therefore approximately 100 times lower than the known concentrations within the samples.
Table 1.
Fluorescein concentrations used versus corresponding values obtained by the Fluorotron Master
| Concentration within the artificial chamber (%) | Concentration within the artificial chamber (ng/ml) | Concentration as measured by the Fluorotron Master (ng/ml) |
| 0.005 | 50 000 | 482 |
| 0.006 | 60 000 | 579 |
| 0.007 | 70 000 | 622 |
| 0.008 | 80 000 | 770 |
| 0.009 | 90 000 | 862 |
| 0.01 | 100 000 | 1115 |
| 0.02 | 200 000 | 2404 |
| 0.03 | 300 000 | 3441.5 |
| 0.04 | 400 000 | 3999.5 |
| 0.05 | 500 000 | 4907 |
Calibration line
Taking the 10 known concentrations and their corresponding fluorescein values measured by fluorophotometry into a coordinate system resulted in the following equation:
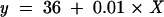 |
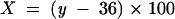 |
This equation was thereafter used in the computer program to correct values determined in tear film fluorophotometry.
Results of the fluorophotometric determination
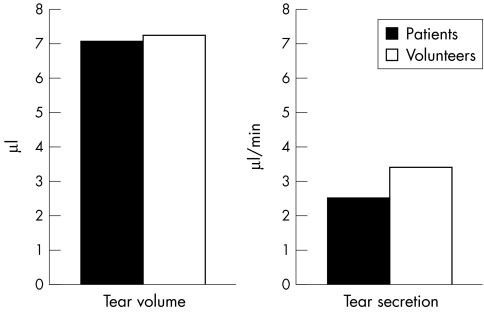
The mean tear secretion in 56 eyes of patients with dry eye symptoms was 2.48 μl/min (SD 1.33). The average tear secretion in 30 eyes of volunteers was 3.4 μl/min (1.97). Tear film secretions in the patients' eyes were significantly lower than in the volunteers (p<0.01). The average tear volume in patients was 7.0 μl (2.997); the average tear volume within the volunteers was 7.2 μl (2.9). There was no statistically significant difference between the two groups (p>0.8). Figure 3 compares the mean tear secretion and tear volume in patients and volunteers.
Figure 3.
Mean tear volume and tear secretion in patients and volunteers.
DISCUSSION
The determination of tear secretion or tear volume by tear film fluorophotometry in its current status requires the collection of a tear specimen by dipping the end of a microlitre capillary into the lower tear meniscus. The tear sample is then put in a cuvette, and fluorescein concentration is measured within the cuvette. Collecting a tear sample makes this method susceptible to errors owing to the mechanical irritation and reactive tear secretion of the eye, as well as volume or concentration errors by pouring the tear sample into another container and diluting it. It is a time consuming method that is highly dependent on the skills of the examiner.
With this present study we introduce a new method of tear film fluorophotometry that enables direct determination of fluorescein concentration in the precorneal tear film at time zero without taking a specimen. The above mentioned sources of errors in determining the fluorescein concentration at time zero are therefore extremely reduced. The patients have also confirmed that the eye is not irritated.
When preparing the fluorescein dilutions used in the calibration curve, one has to be aware of the fact that the most efficient fluorescein concentration to be determined is limited by the increase of extinction (self absorption) of the emitting light according to Lambert-Beer's law (extinction = absorption coefficient × concentration × thickness of the layer). Therefore, the self absorption is proportional to the concentration and/or the thickness of the layer. A fluorescein concentration of, for example, 10−6 g/ml with a layer thickness of 10 mm results in an extinction of 10%; the same extinction is achieved by a fluorescein concentration of 10−4 g/ml and a layer thickness of 100 μm. The in situ determination within 7 μm tear film fluorescein concentrations measured should not be above 10−3 ng/ml.19 The lower detection limit of fluorescein is about 10−9 g/ml. This is in part due to the extremely short time interval of the absorption emission conversion of 4 × 10−9 seconds, allowing a precise differentiation against the background even in low concentrations. Fluorescein concentrations lower than 10−9 g/ml lead to false values because of bleaching of the colour in strong light or interferences with the optical system.19 The fluorescein concentrations used were therefore chosen within a range not susceptible to falsification by extinction and well above the detection limit.
De Kruijf and coworkers20 also had to deal with the problem of limited spatial resolution of the Fluorotron Master (0.5 mm) when trying to determine corneal epithelial permeability in humans. They proposed a correction factor of 1.56 for the cornea and 460 for the tear film by measuring the peak fluorescence of a fluorescein solution of known concentration in layers of variable thickness, assuming a corneal thickness of 0.52 mm and a tear film thickness of 5.1 μm. Van Best and coworkers16 were facing the same problems when trying to establish a protocol for basal tear turnover measurement.
Whereas tear turnover is defined as the percentage of decrease of fluorescein concentration in tears during 1 minute, which makes it therefore independent of calibration factors, tear flow and tear volume are not. Van Best and coworkers16 therefore relied on the correction factors given by de Kruijf et al.20 They concluded that the values obtained for the fluorescein decay are about 400 times lower than the actual values as a result of the limited spatial resolution of the apparatus.16 In contrast, our results showed that the fluorescein concentration measured was approximately 1000 times lower than the actual concentration. There have been different proposals concerning the thickness of the tear film. The normal tear film is thought to be 7 μm.21 Therefore, we used an artificial chamber of exactly 7 μm to imitate the in vivo situation. De Kruijf and coworkers,20 however, used a fluorescein layer of 5.1 μm as tear film phantom. That might in part explain the differences found. Using different fluorescein concentrations within a stable chamber thickness enabled us to obtain a calibration line that turned out to be very reliable.
Different recommendations are published regarding the time interval between instillation of the fluorescein drop onto the conjunctiva and the first scan.2,10,16,22 Our preliminary results of determinations longer than 5 minutes (8 minutes) showed a steady decrease in fluorescein concentration. In our volunteers we did not find an initial rapid decay, followed by a slower decay after 4–5 minutes, as described by Jordan and Baum23 and Mishima et al,2 indicating initially increased tear flow because of irritation of the eye. In contrast with others we therefore determined tear flow by measuring the decay in fluorescein within the first 5 minutes after instillation. Evaluation of tear volume and tear secretion can be determined during the same measurement, reducing the examination time to 6 minutes per eye. Fluorescein uptake to the cornea is excluded because of the short measurement time. Problems with extinction because of a high fluorescein concentration within the tear film, leading to false negative values, were prevented by using low concentrations of the fluorescein solution.4,24
The results of our study confirm the mean values of tear volume of 7 μl in healthy volunteers, as proposed by others.2,23 The average tear volume of the volunteers and the distribution of values did not differ significantly from values previously determined in patients. Other studies showed, however, that patients with dry eye symptoms do have a significantly lower tear volume than healthy volunteers.10
The average tear secretion of healthy volunteers and patients with dry eye symptoms found in the study were higher than in other studies.2,4,10,23 One possible explanation might be that former studies measured the clearance of fluorescein, starting 4–5 minutes after application because of the presumed technically related rapid decay within the first 4 minutes.
In conclusion, the advantages of our new method are: (1) it is an objective quantitative method; (2) it is not necessary to take a tear sample, which makes the examination faster, simpler, and more reliable.
REFERENCES
- 1.Maurice DM. A new objective fluorophotometer. Exp Eye Res 1963;2:33–8. [DOI] [PubMed] [Google Scholar]
- 2.Mishima S, Gasset A, Klyce SD, et al. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci 1966;5:264–75. [PubMed] [Google Scholar]
- 3.Webber WRS, Jones DP, Wright P. Fluorophotometric measurements of tear turnover rate in normal healthy persons: evidence for a circadian rhythm. Eye 1987;1:615–20. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa RE, Polse KA. Changes in tear flow accompanying aging. Am J Optom Phys Optics 1978;55:69–74. [DOI] [PubMed] [Google Scholar]
- 5.Mathers WD, Lane JA, Zimmermann MB. Tear film changes associated with normal aging. Cornea 1996;15:229–34. [DOI] [PubMed] [Google Scholar]
- 6.Puffer MJ, Neault RW, Brubaker RF. Basal precorneal tear turnover in the human eye. Am J Ophthalmol 1980;89:369–76. [DOI] [PubMed] [Google Scholar]
- 7.Göbbels M, Achten C, Spitznas M. Einfluβ vasokonstruktiver Augentropfen auf Tränenvolumen und Tränensekretion. Fortschr Ophthalmol 1991;88:173–5. [PubMed] [Google Scholar]
- 8.Göbbels M, Achten C, Spitznas M. Effect of topically applied oxymetazoline on tear volume and tear flow in humans. Graefes Arch Clin Exp Ophthalmol 1991;229:147–9. [DOI] [PubMed] [Google Scholar]
- 9.Göbbels M, Selbach J, Spitznas M. Effect of eledoisin on tear volume and tear flow in humans as assessed by fluorophotometry. Graefes Arch Clin Exp Ophthalmol 1991;229:549–52. [DOI] [PubMed] [Google Scholar]
- 10.Göbbels M, Goebels G, Breitbach R, et al. Tear secretion in dry eyes as assessed by objective fluorophotometry. German J Ophthalmol 1992;1:350–3. [PubMed] [Google Scholar]
- 11.Göbbels M. Tear secretion and tear function in insulin dependent diabetics. Br J Ophthalmol 2000;84:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetto DA, Clinch TE, Laibson PR. In vivo observation of tear dynamics using fluorophotometry. Arch Ophthalmol 1984;102:410–12. [DOI] [PubMed] [Google Scholar]
- 13.Stolwijk TR, van Best JA, Lemkes HHPJ, et al. Determination of basal tear turnover in insulin-dependent diabetes mellitus patients by fluorophotometry. Int Ophthalmol 1991;15:377–82. [DOI] [PubMed] [Google Scholar]
- 14.Xu K-P, Tsubota K. Correlation of tear clearance rate and fluorophotometric assessment of tear turnover. Br J Ophthalmol 1995;79:1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afonso AA, Monroy D, Stern ME, et al. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology 1999;106:803–10. [DOI] [PubMed] [Google Scholar]
- 16.Van Best JA, Benitez del Castillo JM, Coulangeon LM. Measurement of basal tear turnover using a standardized protocol. European concerted action on ocular fluorometry. Graefes Arch Clin Exp Ophthalmol 1995;233:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Gray JR, Mosier MA, Ishimoto BM. Optimized protocol for Fluorotron Master. Graefes Arch Clin Exp Ophthalmol 1985;222:225–9. [DOI] [PubMed] [Google Scholar]
- 18.Munnerlyn CR, Gray JR, Hennings DR. Design considerations for a fluorophotometer for ocular research. Graefes Arch Clin Exp Ophthalmol 1985;222:209–11. [DOI] [PubMed] [Google Scholar]
- 19.Maurice DM. The use of fluorescein in ophthalmological research. Invest Ophthalmol. 1967;6:464–77. [PubMed] [Google Scholar]
- 20.De Kruijf EJFM, Boot JP, van Best JA, et al. A simple method for determination of corneal epithelial permeability in humans. Curr Eye Res 1987;6:1327–34. [DOI] [PubMed] [Google Scholar]
- 21.Holly FJ. Formation and rupture of the tear film. Exp Eye Res 1973;15:515–25. [DOI] [PubMed] [Google Scholar]
- 22.Tang NEM, Zuure PL, Pardo RD, et al. Reflex lacrimation in patients with glaucoma and healthy control subjects by fluorophotometry. Invest Ophthalmol Vis Sci 2000;41:709–14. [PubMed] [Google Scholar]
- 23.Jordan A, Baum J. Basic tear flow—does it exist? Ophthalmology 1980;87:920–30. [DOI] [PubMed] [Google Scholar]
- 24.Webber WRS, Jones DP. Continuous fluorophotometric method of measuring tear turnover rate in humans and analysis of factors affecting accuracy. Med Biol Eng Comput 1986;24:386–92. [DOI] [PubMed] [Google Scholar]