Abstract
Aim: To evaluate the outcome of corneal grafting in patients with stromal keratitis of herpetic (HSK) and non-herpetic origin, using predefined diagnostic criteria and standardised postoperative therapeutic strategies.
Methods: 384 adult immunocompetent recipients of a corneal graft for herpetic (n = 186) or non-herpetic (n = 198) keratitis were followed up prospectively for up to 5 years.
Results: The herpetic group displayed significantly more corneal vascularisation (p = 0.013), more epithelial defects (p = 0.049), lower corneal sensitivity (p <0.001), more graft rejection episodes (p = 0.002), and required larger grafts (p<0.001). However, the postoperative course of visual acuity, endothelial cell numerical density, and rate of graft failures were similar in both groups. After 5 years, cumulative probability of graft survival in HSK patients (40.85%) was similar to that observed in individuals with non-herpetic keratitis (50.15%; log rank = 0.874; relative risk: 1.04).
Conclusion: Despite a markedly higher preoperative risk profile in herpetic eyes, the functional outcomes of grafts in individuals with keratitis of herpetic or non-herpetic origin were similar. Probably the most important contribution is a consequent close follow up and a therapeutic strategy including systemic prophylaxis of viral recurrence and of graft rejection by well adopted local steroid therapy.
Keywords: keratitis, herpes simplex virus, corneal transplantation, risk factors
Recurrent herpetic infection of the eye can lead to stromal keratitis with corneal scaring and eventually loss of vision. Indeed, the herpes simplex virus (HSV) is a leading cause of infectious corneal blindness in humans, with 1.5 per 1000 people being so stricken.1 And herpetic stromal keratitis (HSK) is the most common infectious condition requiring penetrating keratoplasty.2–4 According to accepted concepts, recurrences of herpetic stromal disease are attributable to reactivation of the latent virus within the trigeminal ganglion or its periphery and its transneuronal spread into the cornea.5–8 However, in cases of chronic and recurrent inflammation, HSK is the result of an immunopathological process,9–12 and the relative contributions of reactivation and immune response in this scenario are still a matter of controversy. Consequently, it has not been possible to define a clearcut therapeutic strategy according to either an antiviral or an anti-inflammatory approach. In corneal transplantation for HSK, the postoperative course may be complicated by recurrence of herpetic disease which compromises the grafts and results in a significant increase in their failure rate.13
Before the advent of systemic antiviral therapy, the survival rate of corneal grafts in HSK patients ranged between 14% and 61%, failure being due to a recurrence of the active herpetic disease and to consequent allograft rejection.3,14,15 A recent prospective study has demonstrated that the postoperative risk of herpetic epithelial and stromal keratitic recurrences after corneal grafting could be reduced if patients underwent systemic antiviral therapy with aciclovir.16 Indeed, earlier indications of this particular beneficial influence of systemic aciclovir have already been garnered from several case studies.2,17–19 Nevertheless, it has proved to be well nigh impossible to rationalise the observations and recommendations of the various investigators owing to differences in clinical diagnostic criteria, statistical analyses, and intraoperative and postoperative graft management. Not even the beneficial effects on visual acuity have been clearly analysed.
Since reactivation of herpetic disease leads to the expression of HLA class I and II antigens,20–22 graft rejection is a potential risk in all individuals with HSK. Although this risk can be reduced by administering high local doses of steroids, the resulting immunosuppressive response renders the individuals more susceptible to reactivated herpetic disease. We thus adopted a standardised therapeutic approach which respected both of these considerations. The aim of the present controlled clinical study was to compare the postoperative course after corneal grafting in patients with herpetic and non-herpetic keratitis using predefined and clearcut diagnostic criteria as well as a standardised follow up schedule and post-transplantation therapeutic strategy in a prospective way.
METHODS
Study population
In this prospective study, we evaluated the outcome of corneal allografting following penetrating keratoplasty in 394 consecutive adult immunocompetent patients presenting with keratitis of herpetic (group 1; n = 186) or non-herpetic (group 2; n = 198) origin. Data acquisition was performed at regular intervals during and accomplished at the end of the study using a computer based data documentation system (custom made database, programmed by M Böhnke) on the basis of a predefined prospective protocol for diagnosis and postoperative management. This was not changed throughout the study period except for the introduction of systemic antivirals which had been realised only after 1987. Surgery was performed between 1981 and 1993 at the university departments of ophthalmology, in Hamburg (Germany) and Bern (Switzerland), and patients followed up for 1–5 years.
Diagnostic criteria
Patients included in this study were selected from a total of 1483 who had undergone corneal grafting and manifested evidence of active or past keratitis of any origin except trauma or chemical burning. Eligible individuals were assigned to herpetic or non-herpetic groups according to predefined diagnostic criteria (Table 1). Patients were diagnosed as having HSK or herpetic keratouveitis (group 1) due to herpes simplex or varicella zoster virus if at least five of the nine relevant criteria were fulfilled, one of which had to be the absence of systemic granulomatous disease. Individuals in whom these criteria were not satisfied were assigned to the non-herpetic keratitis group.
Table 1.
Clinical criteria for stromal herpetic keratitis or keratouveitis caused by herpes simplex or varicella zoster virus (group 1) and non-herpetic keratitis (group 2)
| Stromal herpetic keratitis and keratouveitis (group 1) (the diagnosis was made if at least five of the criteria were satisfied, one had to be the exclusion of systemic granulomatous disease) |
| • Exclusion of systemic granulomatous disease |
| • Documented recurrent stromal keratitis |
| • Interstitial keratitis with deep stromal vascularisation or acute stromal necrosis |
| • Sector granulomatous iritis, iris stroma haemorrhage, or sector atrophy of the iris |
| • History of dendritic keratitis |
| • Documented stromal disciform keratitis with endothelial precipitates |
| • Marked corneal hypoaesthesia |
| • Secondary glaucoma |
| • Documented response to combined systemic aciclovir and local steroid treatment |
| Non-herpetic keratitis and keratouveitis (group 2) (inflammatory corneal disease with no evidence of herpetic keratitis, that is) |
| • Interstitial keratitis without a history of recurrent inflammatory episodes |
| • Viral keratitis of known other origin (varicella zoster, adenovirus) |
| • Keratitis associated with systemic rheumatic or autoimmune disease |
| • Bacterial or mycotic keratitis without recurrence |
Patient assessment
Preoperative, intraoperative, and postoperative findings pertaining to each patient were documented using standardised protocols, and included information on medical history, clinical diagnosis, therapy, age, sex, decimal visual acuity (corrected), Goldmann applanation tonometry, aesthesiometry, lacrimation (Schirmer II), numerical density of corneal endothelial cells, epithelial lesions (as revealed by routine fluorescein staining), and the number of pretransplantation rejection episodes. Neovascularisation of the recipient cornea was quantitatively assessed by discriminating between superficial and deep vascularisation, and by documenting the number of quadrants involved and the degree of progression towards the centre of the cornea. This condition was thus classified as low risk (0 quadrants), intermediate risk (1–4 superficial peripheral or 0–2 deep peripheral quadrants involved) or high risk (central involvement and/or 3–4 quadrants of deep vascularisation). The corneal sensitivity of each individual was quantitatively assessed using a Draeger aesthesiometer (Rodenstock Instruments Inc, Heidelberg, Germany).
Intraoperative data included graft size, suturing technique, and intraoperative complications. All transplanted tissue had been processed in organ culture for at least 48 hours before grafting, and its quality assessed as previously reported.23
In all cases, surgery was required for the treatment of visually impairing or threatening corneal ulcers and scars, attributable to the underlying keratitis. Generally, the smallest possible graft diameter was chosen to cover the scar and thinning stromal zone. The recipient's corneal button—with a diameter 0.25 mm smaller than that of the graft—was removed using a motor trephine. Grafts were attached with approximately 16 interrupted sutures of 10-0 monofilament nylon thread. The surgical technique adopted and the experience of the surgeon were similar in all cases. Except when the separation of anterior or posterior synechiae was necessary, combined surgery was not performed. Postoperatively, gentamicin (10 mg) and dexamethasone-21-acetate (4 mg) were deposited within the parabulbar space. Single sutures were withdrawn immediately if they became loosened, but otherwise the process of stitch removal was not usually complete until a year had elapsed.
Operated eyes were subjected to a thorough examination on postoperative days 1 and 6, and then after 1, 4, and 12 months, and 2, 3, and 5 years. On each occasion, the information respecting the following parameters was recorded—corrected visual acuity, intraocular pressure (IOP), corneal sensitivity, numerical density of corneal endothelial cells (using a specular microscope SP100 (Topcon Inc, Tokyo, Japan)), corneal epithelial changes—like persistent epithelial defects (PED), stromal haze, therapeutic course, and time of onset and outcome of graft disease. Additional examinations were performed as clinically requested, and if new ocular symptoms became manifest. A graft disease was considered to have developed if stromal dullness was apparent, if signs of stromal or endothelial inflammation (precipitates) became manifest, or if endothelial decompensation occurred, irrespective of the probable pathogenesis. If the duration of graft disease persisted for 3 months or more without signs of respite, irreversible graft failure was considered to have occurred. For treatment purposes, we clinically distinguished between an immune reaction and a recurrence of herpetic corneal disease involving the graft. Hence, as it is not always possible to distinguish between both clinical appearances we grouped them together as graft disease for statistical analysis. Immune graft rejection episodes were recognised clinically by stromal oedema (within cell infiltration of the graft), cell infiltration of the anterior chamber, endothelial precipitates exclusively at the graft site, or the presence of an endothelial rejection line.24 Herpetic recurrence was diagnosed if the graft stroma had become infiltrated with cells or if endothelial precipitates were not confined to the graft margins. In cases of graft rejection, an imminent herpes keratitis reactivation was assumed to be operative. Likewise, graft rejection was deemed to be imminent if a herpetic recurrence occurred, owing to the expression of HLA class I and II antigens,20–22 with the necessity of administering high local doses of steroids.
Postoperative therapeutic management
During the first few postoperative days, all patients received the same topical treatment, which consisted of steroids, antibiotics, and cycloplegics (Table 2).
Table 2.
Early postoperative treatment (group 1 and 2) and postoperative antiviral prophylaxis (group 1)
| Standard early postoperative treatment (group 1 and 2) | Daily dose | Duration of treatment |
| Prednisolone acetate (2.5%) | three times | first 7 days |
| Prednisolone acetate (1.0%) | three times | from day 7, until the removal of the last sutures (>1 year after surgery) |
| Atropine (1%) | once | first 7 days |
| Gentamicin (0.3%) or tobramycin (0.5%) | three times | until closure of the corneal epithelium |
| Postoperative antiviral prophylaxis (group 1) | ||
| Indication for surgery | Daily dose by mouth* | Duration of treatment |
| *Maximum dose; modifications necessitated by differences in renal function and body weight were as recommended by the manufacturer. | ||
| (aciclovir) | ||
| Active herpetic keratouveitis and herpetic stromal keratitis | 5 × 800 mg | 2–4 weeks |
| 3 × 400 mg | 2–12 months | |
| Herpetic ulcerate stromal keratitis; no activity, recurrence free <1 year | 3 × 800 mg | 4–8 weeks |
| 3 × 400 mg | 2–12 months | |
| Herpetic corneal scars; no activity; recurrence free <3 years | 3 × 400 mg | 3 months |
Systemic antiviral recurrence prophylaxis had been performed in individuals with HSK since 1987 except when no signs of active inflammation were apparent or when a recurrence free interval of more than 1 year had elapsed before surgery. Systemic treatment with aciclovir was instigated postoperatively if herpes activity was patent clinically (Table 3). The administered dose of aciclovir accorded with the degree of viral activity.14,16–18,25 Individuals who received grafts before the advent of systemic aciclovir therapy in 1987 were treated topically with this drug.
Table 3.
Intensified postoperative treatment in cases of herpetic recurrence and in instances of graft rejection
| Drug | Daily dose | Duration | |
| Intensified postoperative treatment in cases of herpetic recurrence (based on clinical judgement, irrespective of group affiliation) | |||
| I Systemic treatment | |||
| Keratouveitis | Aciclovir | 3 × 10 mg/kg bw* IV | 7–10 days |
| 3 × 800 mg* by mouth | 2–4 weeks | ||
| Endotheliitis | Aciclovir | 5 × 800 mg* by mouth | 2 weeks |
| 3 × 800 mg* by mouth | 2 weeks | ||
| Stromal keratitis | Aciclovir | 3 × 800 mg* by mouth | 2 weeks |
| Subsequent prophylaxis | Aciclovir | 3 × 400 mg* by mouth | 4–12 months |
| II Topical treatment | |||
| Epithelial keratitis | Aciclovir | 5 × 3% ointment | 10–30 days |
| Herpetic recurrence | Prednisolone | 5 × 1% eyedrops | whole episode |
| Accompanying uveitis | Cycloplegics | According to clinical symptoms and signs | |
| Intensified postoperative treatment in instances of graft rejection (aciclovir only in cases of herpetic stromal keratitis (group 1)) | |||
| I Systemic treatment | Prednisolone | 1–2 mg/kg bw* | 3 days; subsequent dose tapering |
| Aciclovir | 3 × 400 mg* by mouth | during high dose local steroid therapy | |
| II Topical treatment | Prednisolone acetate | 2.5% eyedrops | every 30–60 minutes; subsequent dose tapering |
| Cycloplegics | according to clinical symptoms and signs | ||
*Maximum dose; modifications necessitated by differences in renal function and body weight (bw) were as recommended by the manufacturer.
Episodes of graft rejection were treated by administering systemic and more patent topical steroids. A prophylactic regimen using aciclovir was adopted to stave off herpes recurrences caused by steroid induced immunosuppression (Table 3).
Data analysis
Statistical analyses were performed using Student's t test (quantitative data), the χ2 test (qualitative data), and Wilcoxon's rank test (quantitative, non-parametric data). Distribution profiles were described in terms of skewness and kurtosis.
Survival analysis
The cumulative probability of graft failure during the 5 year observation period was calculated for each group with the Kaplan-Meier product limit method and compared by the Mantel log rank test. Cox proportional hazards regression was used to assess correlations between potential risk factors and cumulative graft failure. The relative risk of graft failure for each value of the factor was compared to the risk for a specified reference value.
Correlation between corneal sensitivity and persisting epithelial defects (PEDs) was calculated using Spearman's rho. Only PEDs present on day six postoperatively were considered for statistical analysis.
Differences between sets of data were considered to be statistically significant if p values were ≤0.05 (on the basis of two tailed tests).
RESULTS
Perioperative data
Patients within the herpetic group were significantly younger (mean age 56.4 (SD 19.0) years) than those in the non-herpetic one (61.8 (17.5) years, p = 0.04) and were more frequently of the male sex (56% v 33%, p <0.001), both of which results accord with the findings of previous epidemiological surveys (age26; sex27). Patients with HSK had lower corneal sensitivities (430 (SD 450) v 150 (320) N-5/mm3, p = 0.008) and a higher incidence of pre-existing corneal surface problems (20.2% v 15.3%, p = 0.049) than did individuals with non-herpetic keratitis. But the lacrimation in the herpetic group (13.9 (17.5) mm) was similar to the non-herpetic group (14.6 (4.4) mm, p = 0.188). The number of previous graft rejection episodes on either one eye or the other was comparable in each group (herpetic (n=28) v non-herpetic (n= 67), p= 0.289). There were fewer eyes without corneal neovascularisation in the HSK group (17.7% v 51.5%; p = 0.009), but a similar proportion with stromal neovascularisation of superficial layers (18.8% v 21.7%; p = 0.480) in both groups. The herpetic group displayed a higher number of cases with deep (27.9% v 15.2%; p = 0.003) and central corneal vascularisation (35.4% v 11.6%; p <0.001) than the non-herpetic group. The more advanced central corneal scarring observed in individuals with HSK was also evidenced by their poorer preoperative corrected visual acuities (0.10 (0.16)) compared to the non-herpetic individuals (0.15 (0.16); p = 0.038). The non-herpetic group displayed a higher intraocular pressure (14.6 (44) mm Hg) than the herpetic group (13.8 (9.3) mm Hg, p = 0.035). Individuals in the herpetic group required larger graft diameters (78.6 (5.6) mm than their counterparts in the non-herpetic group (75.8 (8.64) mm; p < 0.001).
Postoperative data
The falling away of individuals from postoperative consultations was comparable in each group (p= 0.524), the average follow up time being 2.6 years in group 1 and 2.4 years in group 2.
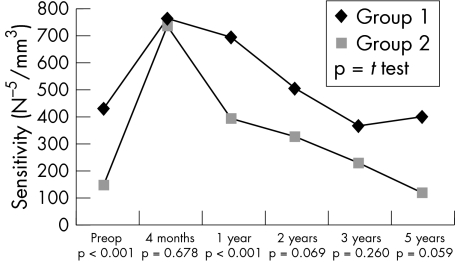
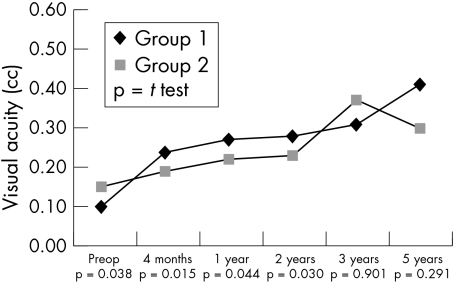
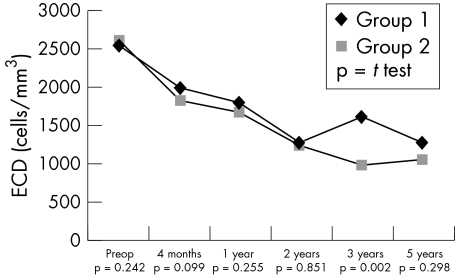
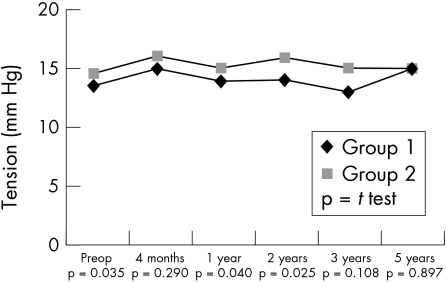
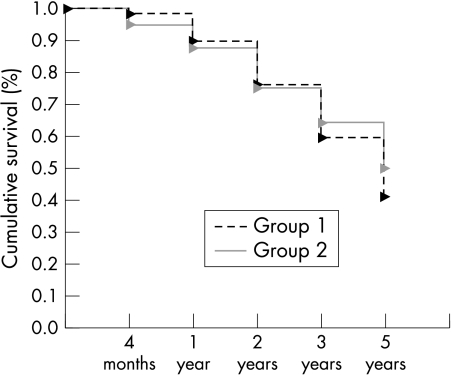
Consistent with the preoperative situation, postoperative data revealed a higher incidence of PEDs in group 1 individuals (21 v 9; p = 0.027). Also a slower and less complete recovery of corneal sensitivity in HSK patients could be observed (Fig 1). No correlation was observed between corneal sensitivity and the presence of PEDs (r = 0.018, p = 0.853) in the herpetic group. The non-herpetic group revealed a weak correlation (r = 0.185, p = 0.034) between sensitivity and PEDs. In contrast, visual rehabilitation was more rapid in HSK individuals (Fig 2), which might be accounted for by their being, on average, younger. The progressive reduction in the numerical density of corneal endothelial cells with time was comparable in both groups (Fig 3). The preoperative and postoperative (1 year and 2 years) IOP was higher in the non-herpetic group (Fig 4). The observed cumulative probability of graft survival in both groups (Fig 5) was not significantly different after 5 years (50.15% v 40.85%; log rank = 0.874; relative risk: 1.04). There was a higher incidence of graft disease in HSK patients (p = 0.002), although the portion of definitely failed grafts (p = 0.126) and the time to graft failure (p = 0.3419) did not differ between the two groups (Table 4).
Figure 1.
Course of corneal sensitivity.
Figure 2.
Course of visual acuity.
Figure 3.
Course of endothelial cell numerical density.
Figure 4.
Course of intraocular pressure.
Figure 5.
Cumulative graft survival analysis (Kaplan-Meier).
Table 4.
Overall graft disease and recovery after rejection
| Group 1 | Group 2 | p Value | |
| Graft disease (n (%)) Of these | 43 (23.1%) | 13 (6.3%) | 0.002* |
| Recovery from disease (n (%)) | 19 (44.0%) | 4 (31.0%) | 0.133* |
| Definitive graft failure (n (%)) | 24 (56.0%) | 9 (69.0%) | 0.126* |
| Time to graft failure (weeks (SD)) | 38 (35) | 44 (44) | 0.319* |
*χ2 test.
Cox proportional hazards regression displayed significantly higher rates for graft failure considering corneal sensitivity (p < 0.0003) in group 1 and graft size (p < 0.001) in group 2. Interestingly, we were not able to demonstrate an association for the risk factors vascularisation (p = 0.123) and PEDs (p = 0.264) in association with graft failure. The relative risk of graft failure for each factor in groups 1 and 2 is summarised in Table 5.
Table 5.
Analysis of risk factors
| Herpetic keratitis | Non-herpetic keratitis | |||
| Risk factor | Cumulative* probability of graft failure (%) | Relative risk (confidence interval) | Cumulative* probability of graft failure (%) | Relative risk (confidence interval) |
| Vascularisation | ||||
| No† | 27.13 | 33.49 | ||
| Superficial (low risk) | 48.82 | 1.78 (0.93 to 3.42) | 32.49 | 0.989 (0.75 to 1.30) |
| Additional deep (intermediate risk) | 44.18 | 1.26 (0.92 to 1.72) | 35.15 | 1.08 (0.81 to 1.43) |
| Additional central (high risk) | 48.85 | 1.78 (0.96 to 3.27) | 49.1 | 1.44 (0.82 to 2.50) |
| PEDs (persistent epithelial defects) | ||||
| No† | 57.60 | 46.41 | ||
| Present | 67.60 | 1.40 (0.92 to 2.12) | 67.74 | 1.40 (1.00 to 1.97) |
| Corneal sensitivity (10−5N/mm2) | ||||
| 1–250† | 38.4 | 36.54 | ||
| 300–750 | 66.09 | 1.98 (0.86 to 4.60) | 24.00 | 0.91 (0.52 to 1.60) |
| 800–1000 | 67.74 | 1.73 (1.19 to 2.50) | 61.55 | 1.38 (1.00 to 1.89) |
| Graft size (mm) | ||||
| 7.0–7.5† | 51.67 | 37.12 | ||
| 7.6–8.0 | 56.77 | 1.12 (0.73 to 1.72) | 52.43 | 1.43 (0.85 to 2.39) |
| 8.1–9.0 | 58.76 | 1.12 (0.74 to 1.70) | 60.47 | 1.75 (1.07 to 2.86) |
*Kaplan-Meier estimates; †reference value for estimation of relative risk.
DISCUSSION
Data presented in this prospective study reveal the rate of graft failure and functional outcome (cumulative survival, visual acuity) of corneal grafts to be similar in herpetic and non-herpetic keratitis groups for up to 5 years. Graft failure in individuals with HSK is most commonly attributable to allograft rejection and herpetic disease recurrence,28 and our favourable results may reflect the instigation of combined antiviral and local immunosuppressive therapy immediately after transplantation, as recommended by Ficker.2,29 He reported graft survival to be improved by the prompt removal of loose sutures, concurrent topical antiviral and immunosuppressive treatment during rejection episodes, and timely handling of recrudescent HSV disease. The functional outcome of corneal grafts in herpetic eyes has also been reported to be markedly improved by the systemic administration of aciclovir.30 The prophylactic working of this drug was first demonstrated in cases of non-ocular (genital and labial) herpetic infections,31,32 which generated a surge of interest in its potential for the treatment of HSK and management of corneal grafting in herpetic eyes.16–19,33–36 Aciclovir penetrates the eye well and, when administered at a dosage of 400 mg five times per day,37 furnishes the aqueous humour with levels that are likely to reduce corneal pools of the virus. Despite its widespread use it is extremely rare for clinical isolates to exhibit resistance.38 Aciclovir is not capable of preventing recurrences, but may prolong the recurrence free interval and eventually reduce the duration of herpetic disease.39 This drug may thus have contributed to our results. However, our data do not allow a comparison of the outcome of grafts using topical versus systemic aciclovir because during the follow up a large number of cases were treated only topically before 1987 and with systemic antivirals after their introduction into the protocol. Numerous additional cases had to be excluded from stratification owing to intermittent topical aciclovir application prescribed by their local doctors in the years after 1987. The number of remaining cases was too low to allow clearcut conclusions about the time dependent impact on graft outcome using topical versus systemic aciclovir. Before the introduction of systemic aciclovir, raised IOP with insufficient response to treatment had been a usual observation.40 The average IOP in both groups in our series was, however, below 18 mm Hg. Thus, the observed statistical difference had no significant clinical impact. Obviously, with sufficient antiviral treatment, the main risk factor for postoperatively increased IOP seems to be increased IOP before surgery and eventually the surgery itself which may induce peripheral anterior synechiae formation.41,42
Since viral replication is known to be effectively inhibited by aciclovir,30 attempts have been made to combine its topical delivery with topical steroid therapy. Success with this combined treatment was first achieved by the Herpetic Eye Disease Study,43 the strategy being found to hinder the progression of disease and curtail its duration without increasing the risk of HSV recurrence. Postponing the initiation of topical steroid therapy delayed the healing of HSK but did not adversely affect the visual outcome at 6 months.43 Although other studies support our own findings of a combined therapeutic regimen with systemic aciclovir and topical steroids, it is difficult to critically compare the results owing to the adoption of different therapeutic regimens and clinical criteria.17–19,34 In order to avoid the undesired side effects associated with non-specific generalised immune suppression we chose not to use systemic steroids19 but preferred instead topical treatment. This is known to be very effective; and it is remarkable that even to this day, the advent of topical corticosteroids half a century ago remains the most significant contribution to corneal transplant immunotherapy.44 A situation commonly encountered in the management of herpetic disease is the underdosage of steroids for the immunological component. Many clinicians repeatedly attempt to taper topical steroid treatment, being fearful of side effects such as cataracts and glaucoma, and of enhancing the susceptibility of keratocytes to viral infection, secondary bacterial or fungal infection, as well as of augmenting viral proliferation.45,46 These fears are not, however, borne out by clinical evidence.43
Within non-vascularised host beds, high graft survival rates can be achieved without regard to MHC antigens47 owing to the immune privileged status of the eye.48 But as soon as vascular ingrowth into the corneal stroma occurs, graft rejection rates shoot up dramatically to 50% or more even when immune responses are maximally suppressed with systemic or topical steroids.2,28,49 Many authors have reported on the existence of a positive correlation between graft failure rate and corneal vascularisation49 or graft diameter.29,47 The larger corneal button size required in our HSK patients, in conjunction with the more extensive neovascularisation, may have contributed to the higher rate of graft disease in these individuals. Most of the graft diseases in our patients occurred during the first year, and could therefore be a consequence of surgical trauma and/or an unquiet corneal situation in the host tissue.50,51 Such an interpretation would be consistent with the observation of Moyes et al.52
Reported incidences of graft epithelial defects in patients with recurrent epithelial herpetic keratitis vary considerably between 3% and 44%,3,53 which may reflect the proportion of cases manifesting an active disease state at the time of surgery.52 Such defects might be associated with a reduced tear film or be attributable to neurotrophic deficiencies. However, preoperative tear film production did not differ between our two groups of patients, although the proportion of individuals with epithelial defects was significantly higher in HSK subjects. As another explanation, reduced corneal sensitivity may serve to explain reduced epithelial growth and differentiation. And indeed, there was a significant association between decreasing corneal sensitivity and cumulative probability of graft failure. However, for the herpetic group with the lowest corneal sensitivity we did not find any correlation between corneal sensitivity and PEDs. Until 2 years after surgery the corneal sensitivity in the herpetic group remained significantly lower than in the non-herpetic group. During those 2 years grafts in herpetic individuals may be more susceptible to neurotrophic keratitis of epithelial and deeper layers.
Episodes of endothelial rejection are treatable, and the results are better if medication is initiated early, preferably before the formation of a classic Khodadoust rejection line,13 which is a pathomorphological indicator of excessive endothelial cell loss. This timing can be best gauged by examining the corneal endothelium on a regular basis, independently of clinical complaints. On the other hand, this strategy leads to a more frequent diagnosis of rejection episodes and a higher local steroid load. Regular monitoring of the endothelial cell numerical density is deemed to be a sensitive indicator of the graft's functional reserve capacity.54 In our patients, endothelial cell loss was similar in both groups and continued for 3 years after keratoplasty. This finding is in accord with data reported by Bourne.55 Although the reason for this continuous depletion eludes us, the high initial loss of cells (which peaked at 4 months in our study) is probably attributable to surgical trauma and in part also to the biological quality of the grafted tissue.50
Although HSK is initiated by a viral process, this probably evolves into an immune mediated reaction, which is believed to be the main motor of tissue destruction and functional loss.9 In a recent study, we demonstrated that the detection of viral presence either directly by DNA amplification and polymerase chain reaction (PCR) or indirectly, by ELISA and immunohistochemistry of antigens, corresponded well with the clinical group affiliation (herpetic keratitis or non-herpetic keratitis) according to the diagnostic criteria used in the present study.56 However, somewhat surprisingly, a substantial proportion of samples from patients with non-herpetic keratitis also yielded evidence of herpetic presence. The detection of viral particles in patients who have been clinically diagnosed as having non-herpetic keratitis does not necessarily imply that HSV caused the disease, but it affords strong evidence that it contributes to the keratitis, perhaps by inducing the shift from a local immune to an immunopathological process. Hence, one has always to bear in mind the possibility of a herpetic background and recurrence in keratitis of unknown origin. The underdiagnosis of underlying herpetic disease in keratitis may thus be an important predictor of the outcome after corneal grafting.
Thus, there exists a body of evidence for a herpes participation even in patients with stromal keratitis of unknown origin.57 Further studies will have to address the question whether this serves as the basis for a broader use of a prophylactic treatment with oral aciclovir and topical steroids in keratitis of unknown origin and in recurrent disease. Therapeutic decisions should not be related to the detection of viral presence. During keratitis pro-inflammatory cytokines and chemokines lead to an expression of adhesion molecules like HLA class I and II antigens20–22 and may also attract non-HSV specific T cells, which would further promote inflammation, even in the absence of living virus.58 Thus, local environmental disturbances support the recurrence of keratitis even in the absence of detectable viral replication at the time of clinical presentation.58,59 Clearly, clinically or anamnestically detectable herpetic eye disease is excluded for corneal donor tissue. Nevertheless, a distinct portion of clinically healthy tissues may harbour viral antigen or DNA,60 but not likely active replicative virus.61–63 In case of an undetected active donor corneal infection, the likelihood of reactivation in organ culture resulting in a total endothelial necrosis would lead in the most cases to secondary exclusion of the tissue from transplantation.61–63 Although the presence of HSV in donor corneas57 revealed by combined DNA and antigen detection is high (∼10%), the development of HSV related eye disease is rare.61,62 Consistent with this, we did observe primary graft failure in only very singular cases. Thus, the keratitis with its expression of cellular adhesion molecules may have a critical role in facilitating the recruitment of immune regulatory cells and contribute to the observed similarity in graft outcome in both groups.20–22 Therefore, a central prognostic factor may be the keratitis itself—with no regard to its suggested background, but unlikely a donor transmitted viral infection.
After all, the herpetic group displayed a markedly higher risk profile for graft failure. The graft outcome was, however, comparable to the non-herpetic group. If the patient compliance allows an appropriate postoperative follow up and treatment schedule, there is no restriction to go forward to penetrating keratoplasty for visual rehabilitation in stromal keratitis of herpetic and non-herpetic origin.
REFERENCES
- 1.Liesegang TJ, Melton LJ 3rd, Daly PJ, et al. Epidemiology of ocular herpes simplex: incidence in Rochester. Minnesota, 1950 through 1982. Arch Ophthalmol 1989;107:1155–9. [DOI] [PubMed] [Google Scholar]
- 2.Ficker LA, Kirkness CM, Rice NSC, et al. The changing mangement and improved prognosis for corneal grafting in herpes simplex keratitis. Ophthalmology 1989;96:1587–96. [DOI] [PubMed] [Google Scholar]
- 3.Langston RHS, Pavan-Langston D, Dohlmann CH. Penetrating keratoplasty for herpetic keratitis: prognosis and therapeutic determinants. Trans Am Acad Ophthalmol 1975;79:577–83. [Google Scholar]
- 4.Cook SD. Herpes simplex virus in the eye. Br J Ophthalmol 1992;76:365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodpasture EW. Herpetic infections with special reference to involvement of nervous system. Medicine (Baltimore) 1929;8:223–43. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JG, Cook ML. Latent herpes simplex virus in spinal ganglia of mice. Science 1971;73:843–5. [DOI] [PubMed] [Google Scholar]
- 7.Dawson C, Togni B, Moore TE. Structural changes in chronic herpetic keratitis. Arch Ophthalmol 1968;79:740–7. [DOI] [PubMed] [Google Scholar]
- 8.Nichols SM, Benylles A, Shimeld C, et al. Recurrent herpes simplex after corneal transplantation in rats. Invest Ophthalmol Vis Sci 1994;35(Suppl):1681. [PubMed] [Google Scholar]
- 9.Pepose JS. Herpes simplex keratitis: role of viral infection versus immune response. Surv Ophthalmol 1991;35:345–52. [DOI] [PubMed] [Google Scholar]
- 10.Missotten L. Immunology and herpetic keratitis. Eye 1994;8:12–21. [DOI] [PubMed] [Google Scholar]
- 11.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today 1997;18:443–9. [DOI] [PubMed] [Google Scholar]
- 12.Zhao ZS, Granucci F, Yeh L, et al. Molecular mimicry by herpes simplex virus type-1: autoimmune disease after viral infection. Science 1998;279:1344–47. [DOI] [PubMed] [Google Scholar]
- 13.Epstein RJ, Seedor JA, Dreizehn NG. Penetrating keratoplasty for herpes simplex keratitis and keratokonus: allografts rejection and survival. Ophthalmol 1987;94:935–44. [DOI] [PubMed] [Google Scholar]
- 14.Foster CS, Barney NP. Systemic acyclovir and penetrating keratoplasty for herpes simplex keratitis. Doc Ophthalmol 1992;80:363–9. [DOI] [PubMed] [Google Scholar]
- 15.Lomholt JA, Baggesen K, Ehlers N. Recurrence and rejection rates following corneal transplantation for herpetic keratitis. Acta Ophthalmol Scand 1995;73:29–32 [DOI] [PubMed] [Google Scholar]
- 16.Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease. Arch Ophthalmol 2000;118:1030–6. [PubMed] [Google Scholar]
- 17.Barney NP, Foster SF. A prospective randomised trial of oral acyclovir after penetrating keraoplasty for herpes simplex keratitis. Cornea 1994;13:232–6. [DOI] [PubMed] [Google Scholar]
- 18.Tambasco FP, Cohen EJ, Nguyen LH, et al. Oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Arch Ophthalmol 1999;117:445–9. [DOI] [PubMed] [Google Scholar]
- 19.Kersten A, Sundmacher R, Reinhard T. Postoperative Komplikationen nach perforierender Keratoplastik in Herpesaugen. Ophthalmolge 1997;94:889–96. [DOI] [PubMed] [Google Scholar]
- 20.Tang S, Scheiffarth OF, Stefani FH. Clinical and immunohistochemical correlation of herpetic keratitis with the expression of HLA-DR antigen. Graefes Arch Clin Exp Ophthalmol 1993;231:162–5. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg MF, Ferguson TA, Pepose JS. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmology 1994;101:161–8. [DOI] [PubMed] [Google Scholar]
- 22.Pepose JS, Gardner KM, Nestor MS, et al. Detection of HLA class I and II antigens in rejected human corneal allografts. Ophthalmology 1985;92:1480–84. [DOI] [PubMed] [Google Scholar]
- 23.Böhnke M. Corneal preservation in organ culture. Curr Opin Ophthalmol 1991;2:432–42. [Google Scholar]
- 24.Khodadoust AA, Silverstein AN. Transplantation and rejection of individual cell layers of the cornea. Invest Ophthalmol 1969,8:180–94. [PubMed] [Google Scholar]
- 25.Mannis JM, Plotnik RD, Schwab IR, et al. Herpes simplex keratitis after keratoplasty. Am J Ophthalmol 1991;111:480–4. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelmus KR, Coster DJ, Donovan HC, et al. Prognostic indicators of herpeic keratitis: analysis of a five-year observation period after corneal ulceration. Arch Ophthalmol 1981;99:1578–82. [DOI] [PubMed] [Google Scholar]
- 27.Ribarić V. The incidence of herpetic keratitis among population. Ophthalmologica 1976;173:19–22. [DOI] [PubMed] [Google Scholar]
- 28.Cobo LM, Coster DJ, Rice NS, et al. Prognosis and management of corneal transplantation for herpetic keratitis. Arch Ophthalmol 1980;98:1755–9. [DOI] [PubMed] [Google Scholar]
- 29.Ficker LA, Kirkness CM, Rice NSC, et al. Longterm prognosis for corneal grafting in herpes simplex keratitis. Eye 1988;2:400–8. [DOI] [PubMed] [Google Scholar]
- 30.Jones BR, Fison PN, Cobo ML, et al. Efficacy of acycloguanosine (Wellcome 248u) against herpes simplex ulceration. Lancet 1979;i:243–4. [DOI] [PubMed]
- 31.Gold D, Corey L. Acyclovir prophylaxis for herpes simplex virus infection. Antimicrob Agents Chemother 1987;95:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz GJ, Jones CC, Mills J, et al. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection: a multicentered doubleblind trial. JAMA 1988;260:201–6. [PubMed] [Google Scholar]
- 33.Wilhelmus KR. Diagnosis and management of herpes simplex stromal keratitis. Cornea 1987;6:286–91. [DOI] [PubMed] [Google Scholar]
- 34.Simon AL, Pavan-Langston D. Long-term oral acyclovir therapy. Ophthalmology 1996;103:1399–405. [DOI] [PubMed] [Google Scholar]
- 35.Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. Arch Ophthalmol 1997;115:703–12. [DOI] [PubMed] [Google Scholar]
- 36.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med 1998;339:300–6. [DOI] [PubMed] [Google Scholar]
- 37.Hung SO, Patterson A, Rees PJ. Pharmacokinetics of oral acyclovir (Zovirax) in the eye. Br J Ophthalmol 1984;68:192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menage MJ, de Clercq E, van Lierde A, et al. Antiviral drug sensitivity in ocular herpes simplex infection. Br J Ophthalmol 1990;74:532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brik D, Dunkel E, Pavan-Langstone D. Herpetic keratitis: persistence of viral particles despite topical and systemic antiviral therapy. Arch Ophthalmol 1993;111:522–7. [DOI] [PubMed] [Google Scholar]
- 40.Falcon MG, Williams HP. Herpes simplex kerato-uveitis and glaucoma. Trans Ophthalmol Soc UK 1978;98:101–4. [PubMed] [Google Scholar]
- 41.Sihota R, Sharma N, Panda A, et al. Post-penetrating keratoplasty glaucoma: risk factors, management and visual outcome. Aust N Z J Ophthalmol 1998;26:305–9. [DOI] [PubMed] [Google Scholar]
- 42.Jonas JB, Rank RM, Hayler JK, et al. Intraocular pressure after homologous penetrating keratoplasty. J Glaucoma 2001;10:32–7. [DOI] [PubMed] [Google Scholar]
- 43.Wilhelmus KR, Gee L, Hauk WW, et al. Herpetic eye disease study:a controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology 1994;101:1883–96. [DOI] [PubMed] [Google Scholar]
- 44.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology. Cornea 2000;5:625–43. [DOI] [PubMed] [Google Scholar]
- 45.Dawson C, Togni B, Moore TE, et al. Herpes virus infection of human mesodermal tissue (cornea) detected by electron microscopy. Nature 1968:217:460–2. [DOI] [PubMed] [Google Scholar]
- 46.Thygeson P, Hogan MJ, Kimura SJ. The unfavourable effect of topical steroid therapy on herpetic keratitis. Trans Am Ophthalmol Soc 1960;58:245–62. [PMC free article] [PubMed] [Google Scholar]
- 47.The Collaborative Transplantation Studies Research Group (CCTS). Effectiness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol 1992;110:1392–403. [PubMed] [Google Scholar]
- 48.Niederkorn JY. Immune privilege and immune regulation in the eye. Adv Immunol 1990;48:191–226. [DOI] [PubMed] [Google Scholar]
- 49.Mader TH, Stulting RD. The high-risk penetrating keratoplasty. Ophthamol Clin North Am 1991;4:411–26. [Google Scholar]
- 50.Hagenah M, Carstens D, Böhnke M, et al. Entwicklung der Endothelzelldichte bei Verwendung von frischem und organkultiviertem Gewebe. Ophthalmologe 1997;94:90–3. [DOI] [PubMed] [Google Scholar]
- 51.Shimeld C, Tullo AB, Easty DL, et al. Isolation of herpes simplex virus from the cornea in chronic stromal keratitis. Br J Ophthalmol 1982;66:643–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moyes AL, Sugar A, Mush DC, et al. Antiviral therapy after penetrating keratoplasty for herpes simplex keratitis. Arch Ophthalmol 1994;112:601–7. [DOI] [PubMed] [Google Scholar]
- 53.Foster CS, Duncan J. Penetrating keratoplasty for herpes simplex keratitis. Am J Ophthalmol 1981;92:336–43. [DOI] [PubMed] [Google Scholar]
- 54.Musch DC, Meyer RF, Sugar A. Predictive factors for endothelial cell loss after keratoplasty. Arch Ophthalmol 1993;111:80–3. [DOI] [PubMed] [Google Scholar]
- 55.Bourne WM. Morphologic and functional evaluation of the endothelium of transplanted human corneas. Trans Am Ophthalmol 1983;81:403–50. [PMC free article] [PubMed] [Google Scholar]
- 56.Garweg JG, Schacher S, Schellhorn M. Detection of viral antigen and DNA in patients with granulomatous keratitis and keratouveitis in and ex vivo. Ocul Immunol Inflammation 1998;6(suppl):29. [Google Scholar]
- 57.Kaye SB, Baker K, Bonshek R, et al. Human herpesviruses in the cornea. Br J Ophthalmol 2000;84:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gangappa S, Babu JS, Thomas J, et al. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol 1998;161:4289–300. [PubMed] [Google Scholar]
- 59.Thomas J, Rousse BT. Immunopathogenisis of herpetic ocular disease. Immologic Res 1997;16:375–86. [DOI] [PubMed] [Google Scholar]
- 60.Remeijer L, Maertzdorf J, Doornenbal P, et al. Herpes simplex virus 1 transmission through corneal transmission. Lancet 2001;357:442. [DOI] [PubMed] [Google Scholar]
- 61.Biswas S, Suresh P, Bonshek RE, et al. Graft failure in human donor corneas due to transmission of herpes simplex virus. Br J Ophthalmol 2000;84:701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cockerham GC, Bijwaard K, Sheng ZM, et al. Primary graft failure a clincopathologic and molecular analysis. Arch Ophthalmol 2000;107:2083–91. [DOI] [PubMed] [Google Scholar]
- 63.Garweg JG, Boehnke M. Low rate shedding of HSV-1 DNA, but not infectious virus from human donor corneae into culture media. J Med Virol 1997;52:320–5. [DOI] [PubMed] [Google Scholar]