Abstract
Aim: To evaluate the clinical outcome of patients in whom ocular surface reconstruction was performed using amniotic membrane transplantation (AMT) after the excision of large (>20 mm square) ocular surface neoplasias (OSN).
Methods: A non-comparative interventional case series. In 16 eyes of 16 patients, excision of large OSN including conjunctival intraepithelial neoplasia (CIN), primary acquired melanosis, and malignant melanoma was followed by adjunctive cryotherapy and suturing of a single layer of amniotic membrane (AM) with the basement membrane side facing up to the healthy bordering tissue. Epithelial healing, complications, and tumour recurrences were analysed.
Results: During a mean follow up of 23.7 (SD 11, range 11–43) months, ocular surface healing was rapid and complete in all cases. One complication of pyogenic granuloma was noted. Tumour recurrence occurred in one out of 10 CIN cases (10%), no recurrences were observed in the patients with melanotic lesions.
Conclusions: AMT in lieu of conjunctival or mucosal autograft is an effective substrate for reconstructing the ocular surface following excision of large OSN. AMT is effective in managing large OSN by avoiding the complications that may be associated with conventional removal, specifically in cases where the limbal architecture is destroyed by surgical resection or adjuvant therapies.
Keywords: amniotic membrane transplantation, excision, ocular surface neoplasias
The “gold standard” treatment in cases of ocular surface neoplasia (OSN) rests mainly on the complete surgical eradication in the form of excisional biopsy, accompanied by resection of a wide tumour free margin of 3–4 mm. Adjuvant therapeutic measures, including cryotherapy,1 alcohol epitheliectomy,2,3 radiation,4,5 and/or other medical therapies including mitomycin C and interferon are also used in the management of OSN, especially those with malignant potentials. Regrettably, residual tumour cells left in the bordering tissues carry the risk of frustrating recurrences. In cases with conjunctival malignant melanoma, the pigmented margin is a common guide for resection extent and non-pigmented infiltration can be unfortunately missed increasing the chances of recurrences. A successful treatment depends principally on the absolute eradication of tumour cells from the ocular surface.6,7
A major concern for surgeons in these challenging situations is to attain an optimal technique for reconstructing the excised areas. In cases where extensive resections are performed, reconstruction may not be viable with either conjunctival and/or mucosal autograft8,9 or lamellar keratoplasty. To avoid such extensive surface reconstruction, there may be a tendency to create relatively smaller defects, which in some instances may even contribute to the increase of recurrences. Furthermore, large tissue removal and the accompanying inflammation secondary to adjunctive cryotherapy and/or other conventional therapies might result in a myriad of fearsome collateral damages, among them partial limbal stem cell deficiency,10 corneal pannus and scarring, symblepharon, pseudopterygium,11–13 cataract, uveitis, and retinal breaks.2
The amniotic membrane (AM) is the innermost layer of the placenta and consists of a thick basement membrane and avascular stroma. Most notable for our purposes is the finding that amniotic membrane transplantation (AMT) as a single therapy may be enough to reconstruct the corneal surface in eyes with partial limbal stem cell deficiency—that is, part of the limbal circumference is involved.14 Previously, we published the utility of AM to reconstruct surgical defects created during removal of conjunctival lesions or symblepharolysis.15 The purpose of this clinical study is to report our experience using AMT for ocular surface reconstruction after excision of large OSN that may involve the limbus without invading intraocular structures.
PATIENT AND METHODS
Patients
After obtaining the informed consent, 10 patients with a clinical diagnosis of conjunctival intraepithelial neoplasia (CIN), two eyes with conjunctival malignant melanoma (CMM) and four patients with primary acquired melanosis (PAM) underwent meticulous excision and adjuvant perilesional cryotherapy with a cryoprobe in contact with the tissue for 10–20 seconds until an ice ball formed, followed by AMT. Patients were enrolled in the study from January 1996 to October 2000. Surgery was performed in two centres by SCGT and PP, respectively, and approved by the medical science subcommittee for the protection of human subjects in research of the University of Miami School of Medicine and Mahidol University. Clinical data concerning age, sex, visual acuity (VA), tumour size and location, epithelial healing, complications, and recurrence rates were stored in a computer database for further analysis. Patients younger than 50 years old were tested for HIV. Preoperative and postoperative pictures were taken of every eye. Dye staining with rose bengal and fluorescein was performed during the follow up.
Tumour removal and AMT
Under retrobulbar block and/or topical anaesthesia, lesions were resected with a 3–4 mm lesion free margin at the conjunctiva and the limbus. A superficial keratectomy was performed if there was corneal infiltration. Amniotic membrane14 used in this study was obtained from Bio-Tissue (Miami, FL, USA), and prepared and preserved as described previously.15,16 After thawing, AM was removed from the filter paper and placed over the surgical defect with the basement membrane side facing up. The basement membrane side of the membrane was distinguished from the stromal side by touching it with a dry sponge; the former being non-sticky, while the latter being sticky. AM was secured to the adjacent conjunctiva and episclera by interrupted or continuous 10-0 Vicryl sutures, making sure that its borders were placed under the conjunctival margin. Postoperatively, all patients were treated with topical 1% prednisolone acetate (Pred-Forte, Allergan, Irvine, CA, USA) four times a day for a period of 4 weeks. Topical antibiotics were given for the first 2 weeks or until complete epithelialisation. Sutures were removed after 2–3 weeks. No contact lenses were used.
Pathology
All surgically resected specimens were sent for pathology diagnosis at the pathology department of Mahidol University and eye pathology department of Bascom Palmer Eye Institute. Ocular pathologists at their respective institutions made the diagnosis of CIN, CMM, and PAM.
RESULTS
These 16 patients (10 men and six women) with a pathological diagnosis of ocular surface neoplasia had a mean age of 58.6 (SD 20.2) years (range 20–95 years) (Table 1). The following patient complaints' were recorded: (1) foreign body sensation in nine patients (56%), (2) cosmetic disturbance in seven patients (43%), (3) decreased visual acuity in four patients (25%), (4) pain in one patient (0.6%). Two patients were asymptomatic. The mean duration of symptoms before surgery was 17.7 (18.7) months. The mean area of tumour involvement was 35 (15.3) mm2 (range 20–60 mm2). The mean circumference involvement was 4.6 (2) hours (range 2–8 hours) (Table 1). The limbal area was not reached by tumour only in two cases (12%), conjunctival location in two PAM is, and a combination of limbal, corneal, and conjunctival involvement was present in the remaining 14 patients (88%) as determined clinically by detailed slit lamp examination. During the follow up, complete conjunctival epithelial healing assessed by fluorescein staining and corneal smoothness was achieved in all cases. No persistent defect, ulcer, or signs of partial or total limbal deficiency were noted. The healed corneal surface did not have blood vessels, nor did it show any late staining with fluorescein. Both superficial vascularisation and late fluorescein staining are signs of limbal stem cell deficiency.17 Visual improvement was evident in five patients (31%) who had corneal involvement before the surgery.
Table 1.
Demographic data, tumour size, and recurrences
| Patient | Area (hours) | Area (mm2) | Diagnosis | Recurrence | Follow up (months) |
| 1 | 3 | 25 | CIN | No | 12 |
| 2 | 3 | 25 | CIN | No | 11 |
| 3 | 4 | 40 | CIN | No | 14 |
| 4 | 2 | 25 | CIN | No | 20 |
| 5 | 7 | 60 | CIN | No | 43 |
| 6 | 3 | 20 | CIN | No | 12 |
| 7 | 6 | 35 | CIN | No | 14 |
| 8 | 8 | 60 | CIN | No | 30 |
| 9 | 6 | 20 | CIN | No | 36 |
| 10 | 3 | 20 | CIN | Yes | 12 |
| 11 | 5 | 48 | CMM | No | 30 |
| 12 | 8 | 60 | CMM | No | 14 |
| 13 | 4 | 35 | PAM | No | 36 |
| 14 | 7 | 45 | PAM | No | 34 |
| 15 | 2 | 20 | PAM | No | 32 |
| 16 | 4 | 25 | PAM | No | 30 |
Inflammatory signs characterised by conjunctival and/or episcleral injection surrounding the lesion or the entire ocular surface were a typical finding in many of these cases before surgery (for example, see Fig 1). However, no sign of inflammation was observed in early and late postoperative periods during the entire length of follow up. The cosmetic appearance assessed by clinical photography and patients' satisfaction were good in those cases without recurrence. Except for one case that developed pyogenic granuloma, there were no complications such as infection, scarring, motility restriction, or symblepharon. All specimens were sent to the pathology department for histological evaluation. None of these cases was invasive to the surrounding structures. The recurrence rate was 10% for CIN cases. No CMM has recurred after a mean follow up of 23.7 (11) months (range 11–43 months) (Table 1). The recurrent case was treated with a second surgical resection. The site of recurrence was located at the border of resection.
Figure 1.
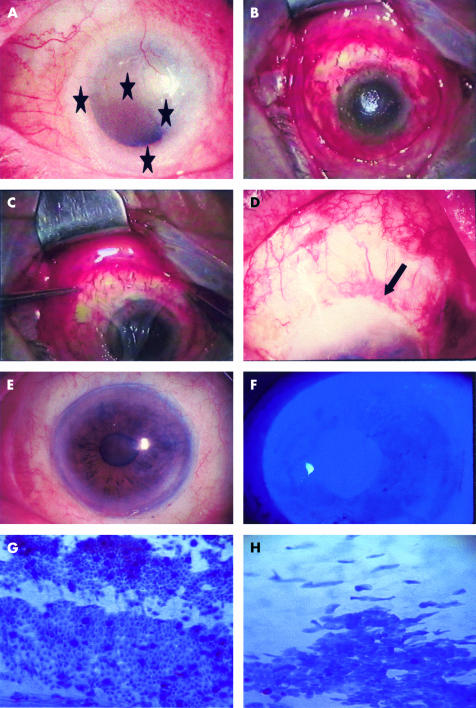
(A) A 77 year old man presented with a large papillomatous lesion involving the superior temporal bulbar conjunctiva and an elevated flat lesion involving the entire limbus except at the 6 o'clock position extending to the conjunctiva and cornea (indicated by stars) in the left eye. (B) Intraoperative view of the defect created with limbal removal after resection of the lesion with a free border. (C) Placing the amniotic membrane stromal side down. (D) First day after surgery with AMT (arrow indicates the suture). (E) A stable ocular surface without inflammation noted at 18 months after AMT. (F) Negative fluorescein staining noted at 2 weeks after surgery. (G) Impression cytology displaying epithelial conjunctival cells an goblet cell in the reconstructed conjunctival surface. (H) Impression cytology in the limbal area.
CASE REPORTS
Case 8
A 77 year old man had complained of redness in the left eye for 2 years. The visual acuity had deteriorated to 5/200 with a dense cataract in the involved eye. Slit lamp biomicroscopy showed an elevated lesion with corkscrewed vessels involving the temporal conjunctiva (Fig 1A) and nearly 360 degrees of the limbus except at the 6 o'clock region, and a contiguous flat lesion involving three quarters of the cornea with superficial neovascularisation (indicated by stars, Fig 1A) and positive rose bengal staining (not shown). Under the clinical diagnosis of CIN, the tumour involved conjunctival and corneal tissue was removed (Fig 1B). AM was applied with the basement membrane side facing up to cover the entire denuded conjunctival and corneal surface (Fig 1C), and sutured to the surrounding conjunctiva with a 10-0 Vicryl running suture, and perilimbally with a 10-0 nylon running suture in a purse-string fashion (Fig 1D, indicated by an arrow). Complete corneal and conjunctival epithelialisation over the AM was noted in 2 weeks (Fig 1E). The ocular surface was stable without any inflammation or recurrence at 18 months after surgery (Fig 1F). Impression cytology showing normal epithelial and goblet cells in the reconstructed conjunctival surface (Fig 1G) and in the limbal area (Fig 1H).
Case 11
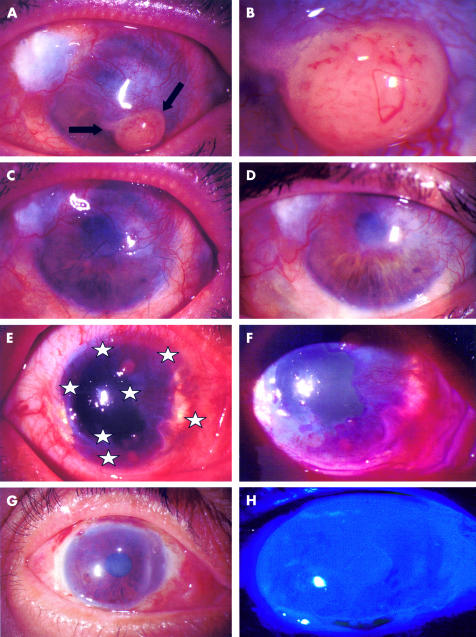
A 60 year old woman with a history of conjunctival recurrent melanoma returned for routine follow up. She complained of decreased visual acuity, foreign body sensation, and the presence of a yellowish mass with increasing size in her left cornea for the past month. She was treated previously on three occasions with surgical excision and cryotherapy of a pathology proved conjunctival malignant melanoma. Her visual acuity with the Snellen chart was 20/20 in the right eye and 2/200 in the left eye. Slit lamp biomicroscopy showed an elevated yellowish lesion with superior and inferior feeder vessels in the inferior half of the cornea (indicated by arrows, Fig 2A, and 2B under higher magnification) and contiguous vascularisation of the cornea. A clinical diagnosis of recurrent melanoma was made and the patient underwent excisional biopsy, cryotherapy, and AMT. A crescent blade was used to mark 3 mm from the tumour border, and a lamellar keratoplasty involving the tumour was performed. AM was applied with the basement membrane side facing up to cover the corneal and conjunctival surface (Fig 2C), and sutured to the surrounding conjunctiva and cornea with a 10-0 nylon running suture. Pathology results confirmed the presence of atypical hyperchromatic cells with pleomorphic nuclei in the specimen with positive immunoperoxidase staining for S-100 and negative for keratins. Corneal and conjunctival epithelialisation over AM was evident 3 weeks postoperatively. The ocular surface was stable without any inflammation or recurrence for 30 months of follow up (Fig 2D).
Figure 2.
(A) A 60 year old woman with a three times recurrent melanoma presented with a large yellowish lesion with superior and inferior feeder vessels (mass indicated by arrows). (B) Tumour under higher magnification. (C) Postoperative view after AMT covering the corneal and conjunctival surface. (D) 30 month follow up appearance. (E) A conjunctival intraepithelial neoplasia covering 300 degrees of the limbus, indicated by stars. (F) Rose bengal staining of the lesion. (G) Postoperative appearance 6 months after surgery. (H) Fluorescein staining showing a smooth healed surface.
DISCUSSION
Free or rotational conjunctival grafts or other mucosal grafts are standard techniques to cover the resultant defects after extensive resections involving the ocular surface are carried out. All these surgical techniques carry risks of compromised wound healing, scarring, granulation tissue formation, motility restriction, and partial or total limbal stem cell deficiency. This study shows the benefits of AMT for reconstructing the ocular surface of 16 patients in which the limbal zone was compromised by large OSN or the surgical removal with a clear margin and the use of adjuvant therapy would have destroyed the limbus because of the close vicinity (see the two cases with pure corneal involvement—for example, Fig 1). Consistent with our previous reports,15,16,18 here we confirmed that AMT facilitates epithelialisation and restores such surfaces without inflammation or scarring, and showed the value of AM in reconstructing the corneal surface in eyes with partial limbal stem cell deficiency.14,19
AMT offers an advantage over buccal or nasal mucosal autografts, which invariably result in a non-conjunctival epithelial morphology. The AMT reconstructed conjunctival surface retains a normal conjunctival epithelial phenotype as shown by impression cytology,20 a finding also supported by in vitro data.21,22 More importantly, as illustrated in Figures 1 and 2, AMT can restore the corneal surface without being complicated with limbal stem cell deficiency.14,19 This result is in agreement with the finding that AMT alone without concomitant transplantation of autologous or allogeneic limbal epithelial stem cells is amenable to treat pre-existing partial limbal stem cell deficiency.14,22 Lately, in vitro23–25 and in vivo26–29 (Koizumi et al, Grueterich et al, submitted) studies have shown that AM serves as a suitable substrate for expanding ocular surface epithelia and subsequent transplantation. Many clinical reports26,27,29(Koizumi et al, Grueterich et al, submitted) strongly support the fact that AM is a suitable tissue for the expansion and survival of epithelial cells.30
In vitro experiments have shown that AM stromal matrix suppresses signalling of transforming growth factor β activity and myofibroblast differentiation in normal human corneal and limbal fibroblasts,31 conjunctival fibroblasts, and pterygium body fibroblasts.32 Rabbit studies have also demonstrated that AM stromal matrix trapped leucocytes and rendered them into a state of rapid apoptosis.33,34 Furthermore, AM contains a number of protease inhibitors such as α1 antitrypsin, α2 macroglobulin, inter-α trypsin inhibitor, α2 plasmin inhibitor, and α2 antichymotrypsin.35 All these studies explain why AM may help suppress inflammation following surgical excision, possibly making AM an ideal tissue substitute for reconstruction in cases of extensive resection. The lack of inflammation and scarring is particularly important when extensive resections are performed in conjunction with cryotherapy because the increased inflammation may even compromise the ocular motility or cause symblepharon. We did not note any such cicatricial problems in this series. As a result, the final cosmetic appearance met patients' satisfaction in all cases.
The recurrence rate of ocular surface neoplasia may not be related to the cell type or degree of atypia, but rather the presence of remaining neoplastic cells in the resected margin.2,3 In our series, we noted a recurrence rate of 10% in the CIN cases, which is a comparable number to previous recurrence reports for CIN. An additional number of cases and a longer follow up should be emphasised. One likely reason for recurrence is the incomplete resection of the lesion, a problem that can be overcome by adopting the dermatological Mohs' micrographic technique to assure tumour free border.18
Recently, several new therapies have been reported in the management of ocular surface neoplasia. These include topical and subconjunctival interferon,36,37 mitomycin C,38–41 5-fluorouracil,42 and amniotic membrane transplantation for reconstruction.43,44 Our study did not compare with these new therapies. Chemotherapy with topical mitomycin C has important advantages over surgical excision. Firstly, the rapid cycling tumour cells are treated while the slow cycling stem cells are probably left unaffected. Secondly, topical mitocmycin C can treat satellite and multifocal lesions and the entire ocular surface, obviating the need to establish margins of excision.
New studies including an additional number of patients and a longer follow up are needed for further conclusions. As a summary, our method of surgical reconstruction with AMT proved to be effective, accelerating the epithelialisation of the ocular surface. Anti-scarring properties of AM may avoid serious complications when extensive resections are performed. Also, we believe that AM is useful for reconstruction because of its unique properties as an ideal substrate for expansion of progenitor cells in cases of partial limbal deficiency after resection of extensive ocular surface neoplasia.
Acknowledgments
Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc, New York, USA.
Proprietary interest statement: the senior author (S C G Tseng) has obtained a patent for the preparation and clinical uses of human amniotic membrane.
Abbreviations
AM, amniotic membrane
AMT, amniotic membrane transplantation
CIN, conjunctival intraepithelial neoplasia
CMM, conjunctival malignant melanoma
PAM, primary acquired melanosis
VA, visual acuity
REFERENCES
- 1.Peksayar G, Soyturk MK, Demiryont M. Long-term results of cryotherapy on malignant epithelial tumors of the conjunctiva. Am J Ophthalmol 1989;107:337–40. [DOI] [PubMed] [Google Scholar]
- 2.Seregard S. Conjunctival melanoma. Surv Ophthalmol 1998;42:321–50. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc 2000;98:471–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman M, Wybar K, Busby E. Malignant epibulbar melanoma: natural history and treatment by radiotherapy. Br J Ophthalmol 1984;68:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lommatzsch PK, Lommatzsch RE, Kirsch I, et al. Therapeutic outcome of patients suffering from malignant melanomas of the conjunctiva. Br J Ophthalmol 1990;74:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B McMahan Lecture. Arch Ophthalmol 1997;115:808–15. [DOI] [PubMed] [Google Scholar]
- 7.Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg 1998;14:208–15. [DOI] [PubMed] [Google Scholar]
- 8.Kenyon KR, Rapoza PA. Limbal allograft transplantation for ocular surface disorders. Ophthalmology 1995;102(Suppl):101–2.7831023 [Google Scholar]
- 9.Copeland RA, Char DH. Limbal autograft reconstruction after conjunctival squamous cell carcinoma. Am J Ophthalmol 1990;110:412–5. [DOI] [PubMed] [Google Scholar]
- 10.Puangsricharern V, Tseng SCG. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 1995;102:1476–85. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GS, Holland EJ. Iatrogenic limbal stem cell deficiency. Cornea 1998;7:31–7. [DOI] [PubMed] [Google Scholar]
- 12.Manche EE, Afshari MA, Singh K. Delayed corneal epitheliopathy after antimetabolite-augmented trabeculectomy. J Glaucoma 1998;7:237–9. [PubMed] [Google Scholar]
- 13.Pires RTF, Chokshi A, Tseng SCG. Amniotic membrane transplantation or limbal conjunctival autograft for limbal stem cell deficiency induced by 5- fluorouracil in glaucoma surgeries. Cornea 2000;19:284–7. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SCG, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998;116:431–41. [DOI] [PubMed] [Google Scholar]
- 15.Tseng SCG, Prabhasawat P, Lee S-H. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol 1997;124:765–74. [DOI] [PubMed] [Google Scholar]
- 16.Tseng SCG, Kim JC, Meller D, et al. Amniotic membrane transplantation for ocular surface reconstruction. Hong Kong J Ophthalmol 1998;2:26–34. [Google Scholar]
- 17.Tseng SCG, Sun T-T. In: Brightbill FS, ed. Corneal surgery: theory, technique, and tissue. 3rd ed. St Louis: Mosby, 1999:9–18.
- 18.Buus DR, Tse DT, Folberg R, et al. Microscopically controlled excision of conjunctival squamous cell carcinoma. Am J Ophthalmol 1994;117:97–102. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DF, Ellies P, Pires RT, et al. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol 2001;85:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhasawat P, Tseng SCG. Impression cytology study of epithelial phenotype of ocular surface reconstructed by preserved human amniotic membrane. Arch Ophthalmol 1997;115:1360–7. [DOI] [PubMed] [Google Scholar]
- 21.Cho B-J, Djalilian AR, Obritsch WF, et al. Conjunctival epithelial cells cultured on human amniotic membrane fail to transdifferentiate into corneal epithelial-type cells. Cornea 1999;18:216–24. [DOI] [PubMed] [Google Scholar]
- 22.Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci 1999;40:878–86. [PubMed] [Google Scholar]
- 23.Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial progenitor cells by amniotic membrane. Invest Ophthalmol Vis Sci 1999;40:S329. [Google Scholar]
- 24.Koizumi N, Fullwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci 2000;41:2506–13. [PubMed] [Google Scholar]
- 25.Grueterich M, Tseng SCG. Connexin 43 expression and proliferative activity of limbal and corneal epithelial cells expanded on intact and epithelially-denuded amniotic membrane. Invest Ophthalmol Vis Sci 2001;42:S303. [Google Scholar]
- 26.Tsai RJF, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 2000;343:86–93. [DOI] [PubMed] [Google Scholar]
- 27.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000;19:421–6. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 2000;19:65–71. [DOI] [PubMed] [Google Scholar]
- 29.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol 2001;119:298–300. [PubMed] [Google Scholar]
- 30.Anderson DF, Ellies P, Pires RTF, et al. Amniotic membrane transplantation for partial limbal stem cell deficiency: long term outcomes. Br J Ophthalmol 2001;85:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng SCG, Li D-Q, Ma X. Suppression of transforming growth factor isoforms, TGF-β receptor II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999;179:325–35. [DOI] [PubMed] [Google Scholar]
- 32.Lee S-B, Li D-Q, Tan DTH, et al. Suppression of TGF-β signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res 2000;20:325–34. [PubMed] [Google Scholar]
- 33.Park WC, Tseng SCG. Modulation of acute inflammation and keratocyte death by suturing, blood and amniotic membrane in PRK. Invest Ophthalmol Vis Sci 2000;41:2906–14. [PubMed] [Google Scholar]
- 34.Wang X-F, Lin HY, Ng-Eaton E, et al. Expression cloning and characterization of the TGF-β type III receptor. Cell 1991;67:797–805. [DOI] [PubMed] [Google Scholar]
- 35.Na BK, Hwang JH, Kim JC, et al. Analysis of human amniotic membrane components as proteinase inhibitors for development of therapeutic agent of recalcitrant keratitis. Trophoblast Res 1999;13:459–66. [Google Scholar]
- 36.Maskin SL. Regression of limbal epithelial dysplasia with topical interferon. Arch Ophthalmol 1994;112:1145–6. [DOI] [PubMed] [Google Scholar]
- 37.Vann R, Karp C. Perilesional and topical interferon alfa-2b for conjunctival and corneal neoplasia. Ophthalmology 1999;106:91–6. [DOI] [PubMed] [Google Scholar]
- 38.Frucht-Pery J, Sugar J, Baum J, et al. Mitomycin C treatment for conjunctival-corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology 1997;104:2085–93. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MW, Hungerford JL, George SM, et al. Topical mitomycin C for the treatment of conjunctival and corneal epithelial dysplasia and neoplasia. Am J Ophthalmol 1997;124:303–11. [DOI] [PubMed] [Google Scholar]
- 40.Rozenman Y, Frucht-Pery J. Treatment of conjunctival intraepithelial neoplasia with topical drops of mitomycin C. Cornea 2000;19:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Wilson MW, Hungerford JL, George SM, et al. Topical mitomycin C for the treatment of conjunctival and corneal epithelial dysplasia and neoplasia. Am J Ophthalmol 1997;124:303–11. [DOI] [PubMed] [Google Scholar]
- 42.Yeatts RP, Engelbrecht NE, Curry CD, et al. 5-Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology 2000;107:2190–5. [DOI] [PubMed] [Google Scholar]
- 43.Paridaens D, Beekhuis H, van Den BW, et al. Amniotic membrane transplantation in the management of conjunctival malignant melanoma and primary acquired melanosis with atypia. Br J Ophthalmol 2001;85:658–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields CL, Shields JA, Armstrong T. Management of conjunctival and corneal melanoma with surgical excision, amniotic membrane allograft, and topical chemotherapy. Am J Ophthalmol 2001;132:576–8. [DOI] [PubMed] [Google Scholar]