Abstract
Aim: To determine the relation between alterations in the retinal topography and thickness, visual acuity, and retinal pigment epithelium hypopigmentation in atrophic age related macular degeneration (AMD).
Methods: 22 patients, mean age 74 (SD 8) years, with atrophic AMD were recruited. An optical imaging system based on the retinal thickness analyser (RTA) was applied to generate a series of 20 optical section images that encompass 2 mm × 2 mm retinal areas. The optical section images were digitised and analysed to provide topographic maps of the vitreoretinal and chorioretinal surfaces and the retinal thickness. Vitreoretinal and chorioretinal surface elevations and retinal thickness were determined.
Results: Variation in the vitreoretinal surface height was moderately correlated with visual acuity (r = −0.4; p = 0.03; n = 22). Increase in variation of chorioretinal surface height was correlated with decrease in visual acuity (r = −0.5; p = 0.01; n = 22). The retinal thickness was not associated with visual acuity (r = 0.2; p = 0.2; n=22). Relative height of the vitreoretinal surface in eyes with retinal pigment epithelium (RPE) hypopigmentation was significantly less than eyes without RPE hypopigmentation (p = 0.005). Eyes with and without RPE hypopigmentation had a similar relative height of the chorioretinal surface (p = 0.4). Retinal thickness in eyes with RPE hypopigmentation was less than in eyes without RPE hypopigmentation (p = 0.04).
Conclusion: Mapping of chorioretinal and vitreoretinal topography and retinal thickness provides objective and quantitative measurements of retinal structural abnormalities and shows promise as an adjunct for the evaluation of retinal structural changes due to AMD.
Keywords: age related macular degeneration, atrophy, chorioretinal topography, retinal thickness
Age related macular degeneration (AMD) is the most common cause of vision loss in people over 50 years of age.1–5 In non-exudative AMD, loss of vision is caused by atrophy of the retinal pigment epithelium (RPE), outer retina, and choriocapillaris. In the exudative type of AMD, ingrowth of new vessels from the choroid into the subretina and/or sub-RPE space results in vision loss.
The survival of the photoreceptors cells is critically dependent on the normal function of the RPE cells. The accumulation of extracellular deposits (drusen) between the RPE and the choroid is known to occur in AMD.6 The location, composition, and structure of drusen associated with ageing and AMD have been studied.7,8 Moreover, the RPE overlying drusen have altered lipofuscin autofluorescence, suggestive of additional changes in RPE metabolism due to AMD.9 Furthermore, clinicopathological studies have demonstrated thinning of the retina related to atrophy of retinal photoreceptors.10 Therefore, documentation of topographic changes of the chorioretinal surface and quantitative assessment of retinal thickness may be helpful in identifying and monitoring the presence of abnormalities of the RPE.
Scanning laser ophthalmoscopy (SLO) has been used to map the topography of the retinal surface.11–13 The technique of optical coherence tomography (OCT) has been used to provide high resolution cross sectional images of the retina and permit visualisation of abnormalities in the retinal structure.14–18 The retinal thickness analyser (RTA) has been used to map the retinal thickness in the posterior pole.19–21 A prototype version of the RTA has been applied to map the topography of the retinal surfaces, in addition to mapping the thickness of the retina.22,23
In the present study, a prototype version of the RTA was applied to patients with atrophic AMD to assess pathological alterations in the vitreoretinal and chorioretinal surface heights and the retinal thickness and determine their relation to visual acuity and RPE hypopigmentation.
METHODS
The system for retinal topography and thickness mapping is based on a prototype version of the commercially available RTA and has been described previously.22 The system generates an optical section image of the retina by projecting a laser onto the retina at an angle and viewing the reflection and/or scattering of the laser light from the vitreoretinal and chorioretinal interfaces. The vitreoretinal and chorioretinal interfaces correspond to layers close to the inner limiting membrane and retinal pigment epithelium, respectively.24,25 By scanning the laser beam, a series of 20 optical section images encompassing a 2 mm × 2 mm retinal area was acquired in 330 ms. The digitised images were analysed to determine retinal elevations and thickness by a dedicated software algorithm, as described in detail previously.22 Two two dimensional arrays containing the topographic data for the vitreoretinal and chorioretinal surfaces were created. Since retinal thickness is defined as the depth separation between the vitreoretinal and chorioretinal interfaces, a two dimensional array containing retinal thickness values was produced by subtracting the two corresponding arrays. The data in the arrays were plotted to map the topography of the vitreoretinal and chorioretinal surfaces and the retinal thickness. The system has a reproducibility of 13 μm, a depth resolution of 50 μm,19 and spatial resolution of 100 μm in the horizontal and vertical directions. The resolution for topography can be estimated by a fraction of the depth resolution.26
The retinal thickness was averaged over a 500 × 500 μm area centred on fixation. From the topographic maps obtained in the same area, the variations of the heights of the vitreoretinal and chorioretinal surfaces (SD/mean) were determined in each eye. Pearson correlation was performed to determine the correlation coefficient and significance level of the association between the topographic variations, retinal thickness, and visual acuity of the retinal area that was used for fixation.
The presence of RPE hypopigmentation, excluding drusen, was established by inspection of colour fundus photographs of the eyes. In eyes with RPE hypopigmentation, the relative chorioretinal and vitreoretinal surface height was determined by averaging surface heights in the area of RPE hypopigmentation and subtracting the 10th percentile height measurements in the surrounding 2 × 2 mm retinal area. Similarly, in eyes without RPE hypopigmentation, relative surface heights were also determined in areas comparable in size to hypopigmented areas. The 10th percentile height measurement was used as baseline rather than minimum height in order to eliminate outliers that represent errors that may occur due to single point noise related artefacts. Statistical t test (unpaired) was applied to determine the significance level of the difference in relative surface height and thickness in eyes with and without RPE hypopigmentation.
Subjects
Patients diagnosed with non-exudative AMD were referred from the vitreoretinal service at the University of Illinois at Chicago (UIC). Approval for use of human subjects was obtained from the institutional review board of the UIC. Patients with clear media, pupil dilatation of 5 mm or more, and visual acuities of 20/200 or better were included in the study. The study was explained to the patients, and signed consent forms were obtained. Imaging was performed in 22 white patients, 12 females and 10 males, 10 left and 12 right eyes. The patients' mean age was 74 (SD 8) years and the best corrected visual acuity was 0.5 (0.3) in logarithm of minimum angle of resolution (logMAR) units (20/90 (20/68) Snellen).
The eye was dilated with 2.5% phenylephrine hydrochloride and 1% tropicamide. Fundus examination was performed using direct or indirect ophthalmoscopy and slit lamp biomicroscopy with a 78 dioptre precorneal, 90 dioptre precorneal, or Goldmann contact lens. The patient was seated in front of the modified slit lamp biomicroscope, and an internal fixation target was presented for viewing during optical section imaging. Imaging was performed at nine contiguous retinal areas, covering a 20 degree field centred on fixation. After optical section imaging, a colour fundus photograph of the eye was obtained using a clinical fundus camera.
The patients were classified in two groups based on slit lamp examination of the fundus and evaluation of colour fundus photographs of a 30 degree field centred on the fovea and according to a previously published study.27 Group 1 (n = 11) included patients with multiple drusen, areas of increased pigment or hyperpigmentation associated with drusen, areas of depigmentation or hypopigmentation of the RPE, and presence of multiple foci of atrophy less than 175 μm in diameter. Group 2 (n = 11) included patients with areas of hypopigmentation or depigmentation or apparent absence of RPE that were more than 175 μm in diameter, in which choroidal vessels were more visible than in surrounding areas.
RESULTS
An example of imaging performed in the left eye of a 75 year old patient with atrophic AMD is presented (Fig 1). A box overlaid on the fundus photograph indicates the area of imaging. Optical section images were analysed to map the topography of the vitreoretinal and chorioretinal surfaces and the thickness of the retina. The vitreoretinal surface displayed a depression corresponding to the area of reduced thickness. The chorioretinal surface showed topographic variations.
Figure 1.
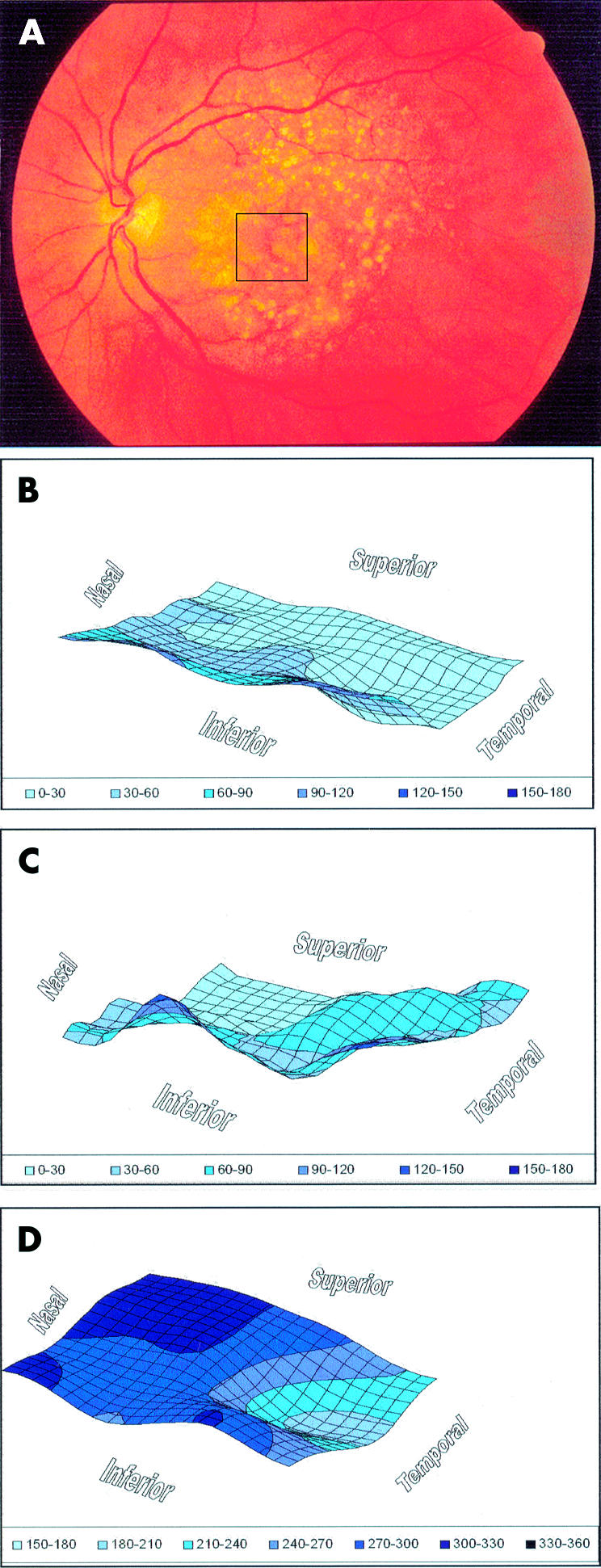
Imaging was performed in the left eye of a patient with atrophic age related macular degeneration. (A) Optical section images were obtained from a scan through the retinal area indicated by the box overlaid on the fundus photograph. Optical section images were analysed to generate three dimensional topographic maps of the (B) vitreoretinal surface, (C) chorioretinal surface, and (D) retinal thickness. The relative surface height and retinal thickness are indicated in μm and pseudocolour coded for display. The vitreoretinal surface displayed a depression corresponding to the area of reduced thickness. The chorioretinal surface showed topographic variations.
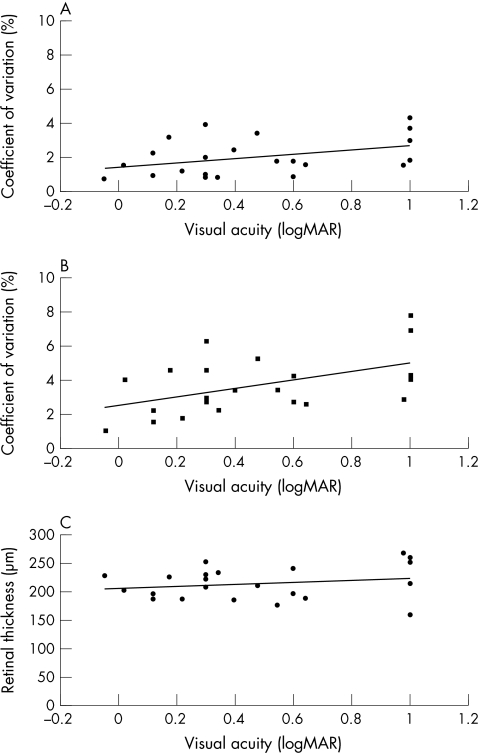
The relation between the topographic variations, retinal thickness, and visual acuity is shown (Fig 2). Variation in the vitreoretinal surface height was moderately correlated with visual acuity (r = −0.4; p = 0.03; n = 22). Increase in variation of chorioretinal surface height was correlated with decrease in visual acuity (r = −0.5; p = 0.01; n = 22). The retinal thickness was not associated with visual acuity (r = 0.2; p = 0.2; n =22).
Figure 2.
The relation between topographic variations (SD/mean) of the heights of the vitreoretinal (A) and chorioretinal (B) surfaces and the visual acuity is shown. (C) The relation between retinal thickness and visual acuity is shown.
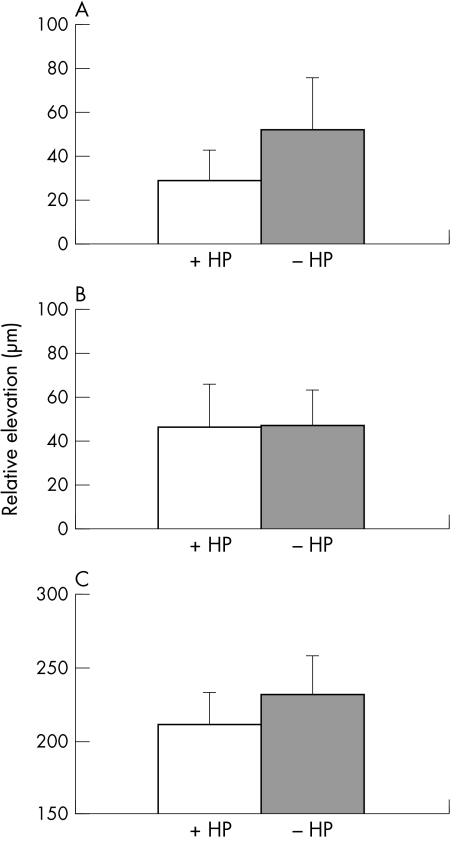
The average relative height of the vitreoretinal and chorioretinal surfaces and thickness of the retina in eyes with and without RPE hypopigmentation is shown (Fig 3). Relative height of the vitreoretinal surface in eyes with RPE hypopigmentation was significantly less than eyes without RPE hypopigmentation (p = 0.005). Relative height of the chorioretinal surface was similar in eyes with and without RPE hypopigmentation (p = 0.4). Retinal thickness in eyes with RPE hypopigmentation was less than eyes without RPE hypopigmentation (p = 0.04).
Figure 3.
The average relative height of the vitreoretinal (A) and chorioretinal (B) surfaces in eyes with RPE hypopigmentation (+HP) and without RPE hypopigmentation (−HP) is shown. (C) The average retinal thickness in eyes with and without RPE hypopigmentation is shown.
DISCUSSION
Since loss of vision in AMD is attributed in part to anatomical changes in retinal and subretinal tissues, the ability to quantitatively document topographic and thickness variations in these tissues may improve the diagnosis, monitoring, and management of this disease. The clinical method of slit lamp biomicroscopy is subjective and has limited resolution for evaluation of fine details in retinal structures. Slit lamp biomicroscopy provides information on retinal structural abnormalities primarily by comparison with adjacent locations and does not allow quantification or documentation of retinal thickness. Additionally, elevations of the chorioretinal surface are often difficult to appreciate on slit lamp biomicroscopy. While fluorescein angiography is useful for delineating areas of geographic atrophy due to the hypopigmentation of the RPE, it doe not provide quantitative evaluation of the associated changes in the overlying retinal tissue.
Scanning laser ophthalmoscopy is inadequate for mapping of the chorioretinal surface and retinal thickness in absolute units, because of its limited depth resolution.28 Optical coherence tomography allows high resolution cross sectional retinal imaging and visualisation of intraretinal structures.15,17 In contrast, our system provides in only 330 ms, 20 serial optical section images encompassing a 2 mm × 2 mm retinal area, which are analysed to generate maps of the vitreoretinal and chorioretinal surface height and retinal thickness with spatial resolution of 100 μm. Since the analysis algorithm detects the peak locations corresponding to the retinal interfaces, changes in the reflection and scatter of light due to media opacities that result in broadening of the lines, do not significantly affect the measurements. In the presence of severe media opacities, the peak corresponding to the vitreoretinal interface may not be identified. Likewise, in the presence of subretinal fluid or blood, the imaging of the chorioretinal interface may be limited. However, these factors did not limit the interpretation of the data that was obtained in the current study. Since a significant reduction in RPE pigmentation will result in an indistinguishable chorioretinal interface, the image analysis algorithm identified indistinct peaks as artefacts and excluded such data from the thickness maps.
Progressive vision loss due to geographic atrophy, the advanced form of atrophic AMD, occurs in 3.5% of people aged 75 years and over in the United States.29 Enlargement of areas of atrophy and visual acuity loss have been associated in the geographic atrophy form of AMD.30 Additionally, increased fundus autofluorescence has been shown to be present in areas of geographic atrophy of RPE associated with AMD.31 We expected decreased thickness at the fovea to be correlated with reduced visual acuity, based on the premise that abnormalities in the subfoveal pigment epithelium would result in roughly proportionate changes in the foveal thickness and visual acuity. However, we found a lack of association between thickness and visual acuity in the present study. One explanation for this finding is that since patients with subfoveal atrophy might prefer to fixate with extrafoveal sites, we would be correlating thickness with acuity of extrafoveal location. Some of these extrafoveal sites tend to be less involved in the atrophic process and have better visual acuity than the diseased fovea, thus weakening the correlation between thickness and visual acuity. Additionally, decreased visual acuity in these patients is attributed to the combined effects of pathological loss of photoreceptor function and the use of extrafoveal fixation. Furthermore, the reduction in the retinal thickness due to partial photoreceptor cell layer atrophy, undetectable by our technique, may have contributed to the lack of correlation between thickness and visual acuity. While, in the current study, we did not determine the precise fixation location of the patients, methods such as SLO perimetry are available that can be used in future studies. The findings further imply that in atrophic AMD, changes in the height of the chorioretinal surface that may be attributed to changes in the retinal pigment epithelium and Bruch's membrane are related to the visual acuity loss. Future prospective studies will be required to establish the value of assessment of chorioretinal topographic variations in prediction of visual acuity loss.
It is hypothesised that RPE dysfunction leads to accumulation of intracellular material in the RPE and extracellular material in the form of basal laminar and linear deposit, thus changing the composition of Bruch's membrane and its permeability to nutrients. In the areas with excessive accumulated extracellular debris, one response to the metabolic distress may be RPE atrophy, followed by choriocapillaris and photoreceptor atrophy.32 In the present study, we found that eyes with areas of RPE hypopigmentation had significantly less elevated vitreoretinal surface and lower retinal thickness when compared to eyes without RPE hypopigmentation. The depression on the vitreoretinal surface was found to be larger than the chorioretinal surface, suggesting atrophy of the photoreceptor layer and associated intraretinal layers as the result of RPE loss. This result is in accord with findings on histopathology of geographic atrophy of the RPE that demonstrated a much greater alteration of the contour of the vitreoretinal interface, due to loss of rods and cones, outer nuclear layer, and overall retinal thickness, than that of the chorioretinal interface.33 Overall, mapping of retinal topography and thickness provides objective and quantitative measurements of retinal structural abnormalities and shows promise as an adjunct for the evaluation of retinal structural changes caused by AMD.
Acknowledgments
This study was supported by the National Eye Institute, Bethesda, MD, EY10314 (MS) and EY1792 (UIC), and an unrestricted fund from Research to Prevent Blindness, New York, NY, USA.
Proprietary interest: None.
Abbreviations
AMD, age related macular degeneration
OCT, optical coherence tomography
RPE, retinal pigment epithelium
RTA, retinal thickness analyser
SLO, scanning laser ophthalmoscopy
REFERENCES
- 1.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham eye study monograph; an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol (suppl) 1980;24:335–610. [PubMed] [Google Scholar]
- 2.Sorsby A. The incidence and causes of blindness in England and Wales 1963–1968. Ministry of Health Reports on Public Health and Medical Subjects. London: Stationery Office, 1972:128. [PubMed]
- 3.Ferris FLI. Senile macular degeneration:review of epidemiologic features. Am J Epidemiol 1983;118:132–51. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy; the Beaver Dam Eye Study. Ophthalmology 1992;99:993–43. [DOI] [PubMed] [Google Scholar]
- 5.Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 1995;102:205–10. [DOI] [PubMed] [Google Scholar]
- 6.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration:pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol 1999;44:1–29. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RF, Russell SR, Anderson DH, et al. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J 2000;14:835–46. [PubMed] [Google Scholar]
- 8.Russell SR, Mullins RF, Schneider BL, et al. Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. Am J Ophthalmol 2000;129:205–14. [DOI] [PubMed] [Google Scholar]
- 9.Delori FC, Fleckner MR, Goger DG, et al. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci 2000;41:496–504. [PubMed] [Google Scholar]
- 10.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E Zimmerman Lecture. Ophthalmology 1993;100:1519–35. [DOI] [PubMed] [Google Scholar]
- 11.Fitzke IW, Woon H, Timberlake G, et al. Optical modifications to a scanning laser ophthalmoscope for high magnification, narrow optical section imaging. Lasers Light Ophthalmol 1991;4:7–14. [Google Scholar]
- 12.Bartsch D-U, Intaglietta M, Bille JF, et al. Confocal laser tomographic analysis of the retina in eyes with macular hole formation and other focal macular diseases. Am J Ophthalmol 1989;108:277–87. [DOI] [PubMed] [Google Scholar]
- 13.Woon WH, Fitzke FW, Bird AC, et al. Confocal imaging of the fundus using a scanning laser ophthalmoscope. Br J Ophthalmol 1992;76:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991;254:1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol 1995;113:325–32. [DOI] [PubMed] [Google Scholar]
- 16.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995;102:217–29. [DOI] [PubMed] [Google Scholar]
- 17.Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology 1996;103:1260–70. [DOI] [PubMed] [Google Scholar]
- 18.Spraul CW, Lang GE, Lang GK. [Value of optical coherence tomography in diagnosis of age-related macular degeneration. Correlation of fluorescein angiography and OCT findings.Klin Monatsbl Augenheilkd 1998;212:141–8. [DOI] [PubMed] [Google Scholar]
- 19.Zeimer R, Shahidi M, Mori M, et al. A new method for rapid mapping of the retinal thickness at the posterior pole. Invest Ophthalmol Vis Sci 1996;37:1994–2001. [PubMed] [Google Scholar]
- 20.Asrani S, Zeimer R, Goldberg MF, et al. Application of rapid scanning retinal thickness analysis in retinal diseases. Ophthalmology 1997;104:1145–51. [DOI] [PubMed] [Google Scholar]
- 21.Asrani S, Zou S, D'Anna SA, et al. Noninvasive mapping of the normal retinal thickness at the posterior pole. Ophthalmology 1999;106: 269–73. [DOI] [PubMed] [Google Scholar]
- 22.Gieser JP, Rusin MM, Mori M, et al. Clinical assessment of the macula by retinal topography and thickness mapping. Am J Ophthalmol 1997;124:648–60. [DOI] [PubMed] [Google Scholar]
- 23.Gieser JP, Mori M, Blair NP, et al. Findings on retinal topography and thickness mapping in age-related macular degeneration. Retina 2001;21:352–60. [DOI] [PubMed] [Google Scholar]
- 24.Ogura Y, Shahidi M, Mori MT, et al. Improved visualization of macular hole lesions with laser biomicroscopy. Arch Ophthalmol 1991;109:957–61. [DOI] [PubMed] [Google Scholar]
- 25.Kiryu J, Ogura Y, Shahidi M, et al. Enhanced visualization of vitreoretinal interface by laser biomicroscopy. Ophthalmology 1993;100:1040–3. [DOI] [PubMed] [Google Scholar]
- 26.Born M, Wolf E. Principles of optics: electromagnetic theory of propagation interference and diffraction of light. New York: Pergamon Press, 1980:6.
- 27.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39:367–74. [DOI] [PubMed] [Google Scholar]
- 28.Bille JF, Dreher AW, Zinser G. Noninvasive diagnostic techniques in ophthalmology. New York: Springer-Verlag, 1990:528–47.
- 29.Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis 1999;5:25. [PubMed] [Google Scholar]
- 30.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 1999;106:1768–79. [DOI] [PubMed] [Google Scholar]
- 31.Holz FG, Bellmann C, Margaritidis M, et al. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 1999;237:145–52. [DOI] [PubMed] [Google Scholar]
- 32.Zarbin MA. Age-related macular degeneration:review of pathogenesis. Eur J Ophthalmol 1998;8:199–206. [DOI] [PubMed] [Google Scholar]
- 33.Bressler NM, Silva JC, Bressler SB, et al. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina 1994;14:130–42. [PubMed] [Google Scholar]