Abstract
Aims: To compare trabeculectomy with viscocanalostomy for the control of intraocular pressure (IOP) in open angle glaucoma (OAG) uncontrolled by medical therapy.
Methods: 48 patients (50 eyes) with uncontrolled OAG were randomised to either trabeculectomy (25 eyes) or a viscocanalostomy technique (25 eyes). Preoperatively, eyes were graded in terms of risk factors for drainage failure. Those undergoing trabeculectomy were given intraoperative antimetabolites (5-fluorouracil 25 mg/ml (5-FU), mitomycin C (MMC) 0.2 mg/ml and 0.4 mg/ml) according to a standard protocol. Antimetabolites were not used intraoperatively in eyes undergoing viscocanalostomy, but they were randomised to the use of viscoelastic (Healonid GV) for intraoperative intracanalicular injection.
Results: There were no significant differences between the groups in age, sex, type of OAG, preoperative medications, risk factors for drainage failure, and preoperative IOP. Mean follow up was 19 months (range 6–24 months). It was 12 months or longer in all eyes, except one lost to follow up at 6 months. At 12 months, complete success (IOP <21 mm Hg without antiglaucoma medications) was seen in all eyes undergoing trabeculectomy (100%), but in only 64% of eyes undergoing viscocanalostomy (p<0.001). The mean IOP was lower at 12 months (p<0.001) with trabeculectomy and the number of eyes with IOPs of 15 mm Hg or less was greater (p<0.05). The mean IOP at 12 months was lower in eyes that had undergone viscocanalostomy using intraoperative intracanalicular Healonid GV injection compared to those where only balanced saline solution had been used (p<0.01). However, in terms of complete success there was no difference between the viscocanalostomy groups (p<0.1). With the exception of measurements at 1 week, visual recovery (logMAR acuity) was similar and laser flare and cell values showed little differences between the groups. Corneal topography and keratometry at 12 months were little different from preoperative values. Postoperative interventions (subconjunctival 5-FU and needling procedures) were similar between the groups. Transient complications such as early bleb leak and hyphaema were more common in the trabeculectomy group (p<0.05). Postoperative cataract formation was more common after trabeculectomy (p<0.05).
Conclusions: IOP control appears to be better with trabeculectomy. Viscocanalostomy is associated with fewer postoperative complications, although significant complications permanently impairing vision did not occur with either technique.
Keywords: trabeculectomy, viscocanalostomy
Surgical trabeculectomy has been the filtering procedure of choice for the management of glaucoma for the past 30 years.1,2 It is quick, technically easy to perform, has fewer complications than full thickness procedures, and high reported success rates.3–9 It is the standard by which all other filtering procedures should be judged. However, results are not always ideal. Early and late filtration failures are not infrequent, especially in eyes with risk factors for drainage failure10–14 and sight threatening complications, such as endophthalmitis, suprachoroidal haemorrhage, hypotony, and cataract, occasionally occur.1–9 To optimise results, frequent and careful outpatient monitoring is required, interventions such as suture release, lysis, and needling are not uncommon, and the adjunctive use of antimetabolites, with their associated problems, is now standard practice especially in eyes with risk factors for failure.15–17
Such limitations have led many investigators to explore other approaches to filtration surgery, including “non-penetrating trabecular” surgery. Epstein and Krasnov were the first to report such procedures, describing techniques in which Schlemm's canal was deroofed and conjunctiva sutured over the externalised canal.18,19 Both reported short term success but poor long term results. In 1984 Zimmerman and colleagues reported “non-penetrating trabeculectomy,” in which Schlemm's canal was deroofed under a partial thickness scleral flap.20,21 In 1989 Fyodorov and Koslov described a modification of this procedure, utilising a collagen implant placed under the scleral flap, in an attempt to maintain drainage. They termed this “deep sclerectomy.”22,23 More recently, Stegmann has developed “viscocanalostomy,” where following deroofing of Schlemm's canal and creation of a Descemet's window, viscoelastic is injected into the canal, in an attempt to bypass the trabecular meshwork while maintaining and opening the normal anatomical drainage channels.24
The potential advantages of such techniques are that by avoiding penetration into the anterior chamber, intraocular complications such as overdrainage or endophthalmitis may be limited. In addition, as no iridectomy is required, the breakdown of the blood-aqueous barrier may be reduced, resulting in less anterior chamber inflammation with perhaps fewer cataracts, synechiae, and bleb failure and possibly a reduced need for antimetabolites. Finally, such surgery may not rely entirely on subconjunctival drainage, but allow aqueous to exit via Schlemm's canal and the normal anatomical outflow pathways and/or increase uveoscleral outflow.
Recent reports of such techniques have been encouraging. Using deep sclerectomy, Demailly reported a 76% success rate at 16 months,25 but found no difference with the use of collagen implants.26 While Sanchez reported a 70% success rate at 9 months, but found better results with collagen implants.27 Similarly, Welsh reported an 87% success rate at 12 months28 and Massey, in a study excluding eyes with risk factors for failure, an 81% success rate at 14 months.29 However, Karlen, with longer term follow up of 36 months, reported a success rate of only 45% and used neodymium:yttrium-aluminium-garnet (Nd:YAG) laser goniopuncture in 40% to augment drainage.30 Similarly, Hamard reported limited success of 60% and utilised goniopuncture in a third.31 With viscocanalostomy, Stegmann reported encouraging results with a success rate of 83% at 35 months in black African patients24 and Carassa found similar success rates albeit with very limited follow up.32 In all these studies, sight threatening complications were very infrequent.
Thus far there have been few studies comparing these techniques with trabeculectomy. Mermoud, in a retrospective study of deep sclerectomy with collagen implants versus trabeculectomy, found little difference in terms of overall success33 and similar results were reported by El Sayyad in a prospective study of deep sclerectomy without collagen implants.34 In both studies, complications were less in the deep sclerectomy groups. However, Nd:YAG goniopuncture was utilised in some eyes to augment drainage after deep sclerectomy, converting it to a penetrating technique. In addition, no antimetabolites were used intraoperatively, which is now considered standard practice in eyes with risk factors for failure.10–17 Indeed, both studies appeared to exclude eyes with risk factors for failure such as young age and previous ocular surgery.
In view of the paucity of the current literature, we designed a prospective, randomised study to compare the efficacy of trabeculectomy with a viscocanalostomy technique. Intraoperative antimetabolites are routinely used by most glaucoma surgeons as an adjunct for trabeculectomy in eyes with risk factors for failure.10–14 Therefore they were used intraoperatively according to a standardised protocol for eyes within the study undergoing trabeculectomy. As in previous studies, they were not used in eyes undergoing viscocanalostomy, as it has been postulated that such techniques may reduce the need for antimetabolites and may not always rely on the subconjunctival route for aqueous drainage. However, within the viscocanalostomy group eyes were randomised to the use of viscoelastic (Healonid GV) for intraoperative intracanalicular injection in order to examine its importance.
MATERIALS AND METHODS
Following ethics committee approval, 48 patients (50 eyes) with uncontrolled open angle glaucoma (OAG) were randomised, between February 1999 and March 2000, to either trabeculectomy (25 eyes) or a viscocanalostomy technique (25 eyes). Randomisation was performed using a sealed envelope system, where 50 shuffled envelopes designating the surgery to either trabeculectomy or viscocanalostomy, were opened immediately before surgery by the theatre nurse. Patient demographic data are shown in Table 1. Before entry into the study, informed consent was obtained from all patients. Inclusion criteria for the study were primary or secondary OAG uncontrolled on maximally tolerated medical therapy. Exclusion criteria were congenital glaucoma and any type of angle closure glaucoma. Patients were not excluded from the study on the basis of their age, race, previous ocular surgery, or any other risk factor for drainage failure.
Table 1.
Preoperative patient demographics
| Characteristic | Trabeculectomy (n=25) | Viscocanalostomy (n=25) |
| Age (years) | 65.5 (range 24–89) | 64.2 (range 38–80) |
| Sex (male:female) | 14:11 | 18:7 |
| Race: | ||
| White | 9 | 8 |
| Afro-Caribbean/African | 13 | 15 |
| Indian subcontinent | 3 | 2 |
| Preoperative glaucoma medications | 2.7 (range 1–5) | 3 (range 1–5) |
| Risk factors for failure score (see Table 2) | 9.7 (range 3–17) | 9.6 (range 3–17) |
| Type of OAG | ||
| Primary | 21 | 24 |
| Secondary | 4 | 1 |
| Preoperative IOP (mm Hg) | 24.2 (range 18–30) | 24 (18–30) |
Preoperatively, full baseline data were obtained for each patient and included a full ocular and medical history, logMAR visual acuity (Early Treatment of Diabetic Retinopathy Chart), visual field assessment (Humphrey 24-2 computerised perimetry), slit lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, and mydriatic funduscopy. In addition, corneal topography was evaluated using a computerised photokeratoscope, computed anatomy, TMS-1 and anterior chamber flare, and cell counts were measured with the Kowa FC 1000 laser flare meter.
Based on a standardised protocol (Table 2), eyes undergoing trabeculectomy were graded in terms of their risk factors for drainage failure. On the basis of their protocol score, eyes undergoing trabeculectomy were selected to receive intraoperative antimetabolites (5-fluorouracil 25 mg/ml (5-FU), mitomycin C (MMC) 0.2 mg/ml and 0.4 mg/ml) (Table 2). Eyes undergoing viscocanalostomy were not given intraoperative antimetabolites, but were further randomised to the use of viscoelastic (Healonid GV) for intraoperative intracanalicular injection.
Table 2.
Protocol for antimetabolite use and risk factor for failure scoring. (Based on protocol developed by Professor RN Weinreb, Shiley Eye Centre, San Diego)
| Risk factors for failure | Score |
| Race: | |
| Indian, Asian | 3 |
| Afro-Caribbean | 5 |
| Combined cataract and glaucoma surgery | 5 |
| Previous ocular surgery | 5 |
| Secondary glaucoma (uveitis, neovascular) | 5 |
| Topical treatments >2 | 3 |
| Duration of topical treatment >1 year | 3 |
| IOP target <16 mm Hg | 3 |
| Conjunctival cicatrising disease | 5 |
| Age <20 years | 5 |
| Age <40 years | 3 |
| Diabetes | 1 |
| Previous ALT | 1 |
If score: give:
Less than 5: no antimetabolites
Between 6–10: 5-FU (25 mg/ml)
Between 11–15: MMC 0.2 mg/ml
Over 15: MMC 0.4 mg/ml.
Surgical techniques
Immediately preoperatively single applications of prednisolone 0.5%, pilocarpine 4%, amethocaine 1%, and chloramphenicol 0.5% drops were instilled into the operative eye. A single surgeon (DO'B), using retrobulbar anaesthesia with 2% lignocaine, performed all surgeries. Following insertion of a lid speculum, a 7/0 silk traction suture was inserted at the inferior corneoscleral limbus if required.
Trabeculectomy
Trabeculectomy was performed via a fornix based conjunctival flap. If readily apparent, Tenon's capsule was excised. Haemostasis was achieved using bipolar cautery. Antimetabolities were applied using a single cellulose sponge (John Weiss, UK) to the scleral bed and subconjunctival space taking care to avoid exposure to the conjunctival wound edge. Application time was 5 minutes for 5-FU and 2 minutes for MMC. Following irrigation with balanced saline solution, a 3.00 mm, triangular-shaped, one third thickness scleral flap was fashioned to within 0.5 mm of the limbus. A crescent blade was used to tunnel into clear cornea. A paracentesis was performed 90 degrees from the trabeculectomy site. The anterior chamber was entered just beyond the limbus using a 3.2 mm slit blade. A punch trabeculectomy was performed using a crozeform punch (Altomed, UK). A peripheral iridectomy was fashioned and the scleral flap closed with three 10/0 nylon sutures, two at the base near the limbus and one at the apex. Releasable sutures were not used. The conjunctiva was closed with a continuous 8/0 vicryl suture.
Viscocanalostomy
Viscocanalostomy was performed using a procedure similar to that described by Stegmann,24 who had personally instructed the surgeon (DO'B) in the technique. Before the study the surgeon had been performing the procedure for over 12 months. A fornix based conjunctival flap was fashioned and, if apparent, Tenon's capsule was excised. Haemostasis was achieved using bipolar cautery. A 5.00 mm triangular-shaped, one third thickness scleral flap was fashioned and dissected 1.00–2.00 mm into clear cornea. A second triangular flap was dissected 0.5 mm inside the border of the first. This deeper flap constituted approximately two thirds of scleral thickness, leaving only a thin translucent layer overlying the choroid. With forward dissection of this flap, Schlemm's canal could be identified approximately 1.00 mm posterior to the limbus and deroofed. A moistened cellulose sponge was used to apply gentle pressure on Schwalbe's line to separate Descemet's membrane from the overlying stroma and create an intact Descemet's “window,” at least 1.00 mm in width, though which aqueous could diffuse. The deep scleral flap was excised at its base using Vannas scissors. A specially designed cannula (Grieshaber, Switzerland), with an outer diameter of 150 μm, was introduced into the ostia of Schlemm's canal, left and right, to inject fluid into the canal in an attempt to widen its diameter. Patients were randomised preoperatively to the use of high viscosity sodium hyaluronate (Healonid GV, Pharmacia, UK) for intraoperative intracanalicular injection or balanced saline solution (BSS, Alcon, TX, USA) alone. The superficial scleral flap was closed with three 10/0 nylon sutures, two at the base near the limbus and one at the apex. Healonid GV was injected under this flap into the space created by removal of the deep flap. The conjunctiva was closed with a continuous 8/0 vicryl suture.
Postoperative management
Immediately postoperatively, a subconjunctival injection of Betnesol (betamethasone) and cefuroxime was given, a drop of phenylephrine 10% instilled, and the eye padded overnight. Postoperatively, topical chloramphenicol 0.5% was administered three times a day for 2 weeks and prednisolone 1% 8 times a day for 2 weeks; this was then reduced over the next 3 months.
Postoperatively patients were examined at day 1 and then at 1, 2, 4, and 8 weeks and 3, 6, 12, 18, and 24 months. At each visit during the first year a full ocular examination was performed, including logMAR visual acuity, corneal topography, slit lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, laser flare and cell measurements, and mydriatic funduscopy. In addition, visual field assessment was performed at 6, 12, 18, and 24 months.
Needling procedures with subsequent subconjunctival injections of 5-FU were performed on eyes in both operative groups with drainage failure due to encysted blebs, where just the conjunctiva was penetrated, or excessive subconjunctival fibrosis, where an attempt was made to lift the scleral flap. Such procedures were generally performed during the first 3 months after surgery. Great care was taken in eyes after viscocanalostomy to ensure that penetration into the anterior chamber, converting the procedure to full thickness, did not occur during needling.
In contrast with previous studies, no eyes undergoing viscocanalostomy underwent Nd:YAG goniotomy during the first 18 months after surgery, as such interventions clearly convert a “non-penetrating” technique into a penetrating, full thickness procedure. After this time, however, in selected cases with drainage failure and unsatisfactory intraocular pressure control, Nd:YAG goniotomy was attempted.
Statistical methods
Student's t tests were used to compare continuous variables between the groups such as IOP differences. χ2 analysis was used to compare qualitative data. Results with p<0.05 were considered statistically significant.
RESULTS
Patient demographics
There were no significant differences in terms of age, sex, race, preoperative IOP, numbers of preoperative glaucoma medications, type of open angle glaucoma, and risk factors for failure score between eyes undergoing trabeculectomy and viscocanalostomy (Table 1).
Operative data
Viscocanolostomy generally took longer to perform, average time of first incision to closure 28.2 minutes (range 18–40 minutes), than trabeculectomy, average time of first incision to closure 21.6 minutes (range 12–35 minutes) (p<0.001).
One eye (4%) undergoing viscocanalostomy was converted to a trabeculectomy at the time of surgery, owing to failure to deroof Schlemm's canal. In no eyes undergoing viscocanalostomy was Descemet's membrane ruptured during surgery with exposure or prolapse of iris tissue and in every case the Descemet's window appeared to be grossly intact. However, careful observation of loss of convexity and an increase in the egress of fluid through the Descemet's window suggested the presence of small perforations in 12 eyes (48%). In one eye (4%) undergoing viscocanalostomy, an iris prolapse occurred at 10 days following an episode of eye rubbing and necessitated further surgery and conversion to trabeculectomy.
In one eye viscoelastic was noted to enter the anterior chamber following intracanalicular injection. This patient was subsequently found to have an intraocular pressure in excess of 40 mm Hg on the first day postoperatively, which settled within a few hours. This eye has since maintained excellent drainage with over 18 months of follow up.
Postoperative interventions
Postoperative interventions such as needling and subconjunctival injections of 5-FU were similar between the two operative groups. After viscocanalostomy, eight eyes (35%) underwent bleb needling with subconjunctival injections of 5-FU. Similarly, 11 eyes (44%) underwent needling after trabeculectomy. The mean number of needling procedures was 0.65 (range 0–4) for viscocanalostomy and 0.68 for trabeculectomy (range 0–3). The mean number of subconjunctival injections of 5-FU given postoperatively was 0.87 after viscocanalostomy and 0.68 after trabeculectomy.
At 18 months, three eyes with drainage failure after viscocanalostomy, underwent Nd:YAG goniotomy. In two of these, little effect was seen. In one eye, following the laser procedure, IOP has thus far been maintained below 21 mm Hg without antiglaucomatous medications.
Intraocular pressure control
The mean follow up was 19 months (range 6–24 months) and was 12 months or longer in all eyes, except one lost to follow up at 6 months.
In all groups immediately postoperatively intraocular pressure (IOP) was significantly reduced, with a number of eyes being hypotonous (IOP <6 mm Hg). The mean IOP in the trabeculectomy group was 7.3 mm Hg (range 1–24 mm Hg, median 4 mm Hg) at 1 day and 8.3 mm Hg (range 1–25 mm Hg, median 8 mm Hg) at 1 week, compared to 9.2 mm Hg (range 2–48 mm Hg, median 6 mm Hg) at 1 day and 9.7 mm Hg (range 2–40 mm Hg, mean 7 mm Hg) at 1 week in the viscocanalostomy group. Thereafter, IOP returned to more normal levels.
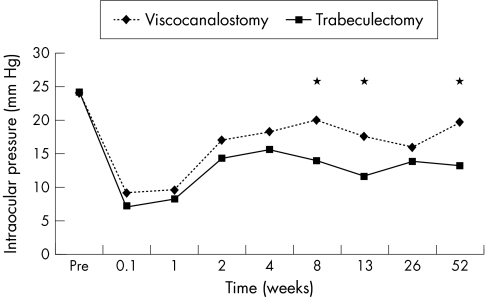
At 6 months the percentage of eyes with successful drainage, defined as an IOP of 21 mm Hg or less without antiglaucomatous medications, was 95% with viscocanalostomy and 100% with trabeculectomy. At 12 months, however, the complete success rate for viscocanalostomy had fallen to 64% and was significantly lower than that for trabeculectomy which was still 100% (p<0.01). In all viscocanalostomy eyes with successful drainage at 6 and 12 months a subconjunctival drainage bleb with conjunctival epithelial microcysts was evident. After this time period drainage blebs were still present in such eyes, although epithelial microcysts were less evident (Fig 1).
Figure 1.
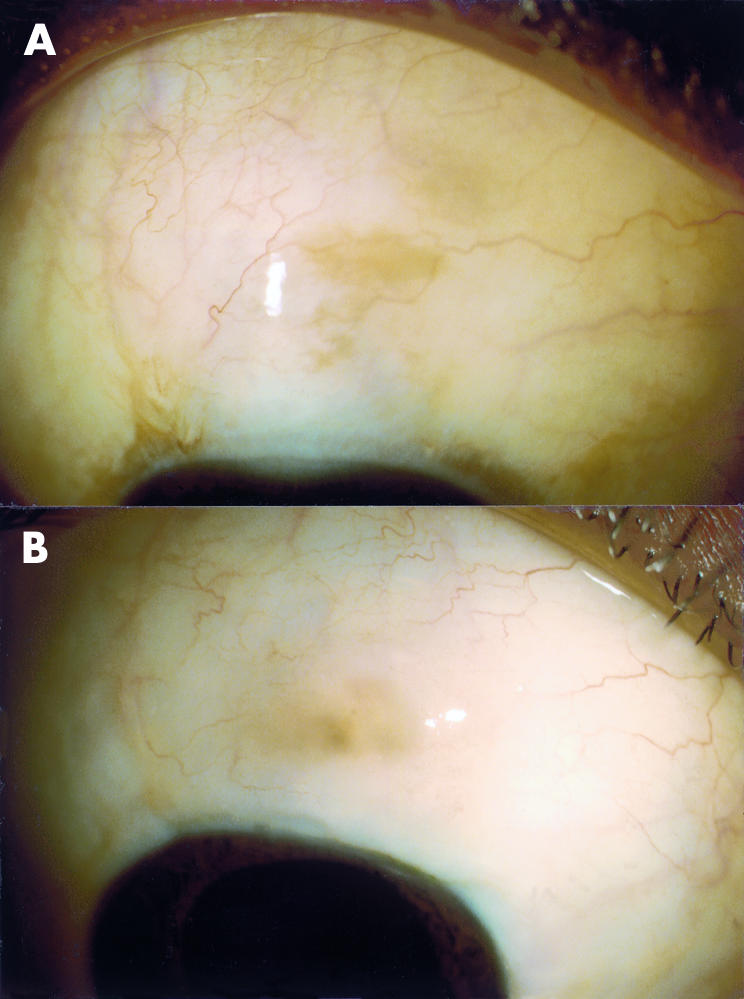
(A) and (B) Photographs of two eyes 18 months after viscocanalostomy with successful drainage, showing evidence of a small drainage blebs.
The percentage of eyes achieving an IOP of 15 mm Hg or less without antiglaucomatous medications was similar between the groups at 6 months, 52% with viscocanalostomy and 60% with trabeculectomy. However, at 12 months only 26% of viscocanalostomy eyes achieved an IOP of 15 mm Hg or less, compared to a significantly higher rate of 76% in the trabeculectomy group (p<0.001).
The IOP results for the first 12 months are summarised in Figure 2. The mean IOP was significantly lower in eyes that had undergone trabeculectomy compared to viscocanalostomy at 2, 3, and 12 months (p<0.01). This trend has continued in those eyes that have reached 18 and 24 months of follow up, with mean IOP being significantly lower in the trabeculectomy group (p<0.02), despite a number of eyes in the viscocanalostomy group receiving antiglaucomatous medications.
Figure 2.
Mean intraocular pressure with time. Comparison of trabeculectomy (n=25) with viscocanalostomy (n=23). *Denotes time points where significant differences between the groups were found.
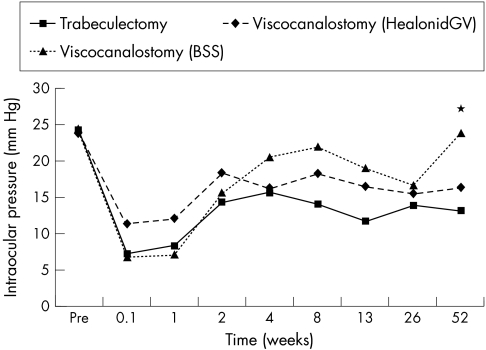
Within the viscocanalostomy group, the mean IOP at 12 months was lower in eyes where viscoelastic had been used for intraoperative intracanalicular injection compared to balanced saline (p<0.01). At other times there were no differences (Fig 3) between these subgroups. At 12 months, the complete success rate was 75% in viscoelastic eyes compared to 50% where saline only had been used. This difference was not statistically significant, although this should be taken in context with the small numbers in each group.
Figure 3.
Mean intraocular pressure with time. Comparison of trabeculectomy (n=25) with viscocanalostomy where only balanced saline solution (BSS) had been used for intracanalicular injection (n=11) and viscocanalostomy where viscoelastic (Healonid GV) had been used (n=12). *Denotes times where significant differences between the three groups were found.
At 2, 3, and 12 months the mean IOP was lower in the trabeculectomy group compared to eyes in the viscocanalostomy group where intracanalicular viscoelastic injection had been used (p<0.03). The differences in complete success rates at 12 months, 100% with trabeculectomy compared to 75% with the viscocanalostomy subgroup, were statistically significant (p<0.02).
There were no differences in terms of mean IOP or success rates in eyes undergoing viscocanalostomy where small perforations of Descemet's membrane had possibly occurred, compared to those where it was deemed that Descemet's membrane was completely intact. There were also no differences in mean IOP or success rates within the viscocanalostomy group in eyes with relatively low “risk factors for failure” score (9 or less) and those with higher scores (10 or greater).
With a mean follow up of 19 months, only one eye (4%) in the trabeculectomy group is thus far receiving antiglaucomatous medication to maintain an intraocular pressure below 21 mm Hg compared to nine (39%) in the viscocanalostomy group (p<0.01). At the last follow up visit the average number of antiglaucomatous medications per treated eye was 0.04 for the trabeculectomy and 0.65 for the viscocanalostomy patients. No cases of hypotony have occurred with all eyes maintaining IOPs of 8 mm Hg or greater. One eye in the viscocanalostomy group has undergone further glaucoma drainage surgery (trabeculectomy) at 18 months owing to complete failure of drainage and inadequate IOP control of antiglaucomatous medications. Following this second procedure, intraocular pressure control in this eye has been satisfactory without the need for antiglaucomatous medications. A further eye in the viscocanalostomy group in a poorly controlled diabetic with hypertension developed vitreous haemorrhage and rubeotic glaucoma at 14 months and underwent cyclodiode laser ablation. The two eyes in the viscocanalostomy group, which were converted to trabeculectomy, have successful drainage with good IOP control, requiring no antiglaucomatous medications thus far, with a follow up of 24 months.
Visual acuity
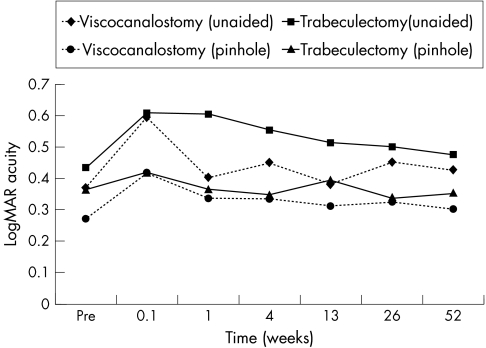
With exception of unaided logMAR visual acuity at 1 week, when eyes undergoing viscocanalostomy had slightly better vision than those undergoing trabeculectomy (p<0.02), visual recovery was similar, with no differences either in unaided or pinhole acuity between the groups (Fig 4). At the last postoperative visit logMAR unaided and pinhole visual acuity was either improved or unchanged in 74% of eyes undergoing viscocanalostomy and 84% after trabeculectomy. One eye in the viscocanalostomy group and one in the trabeculectomy group had lost more than two lines of logMAR acuity.
Figure 4.
Unaided and pinhole logMAR visual acuity with time.
Laser flare and cell measurements
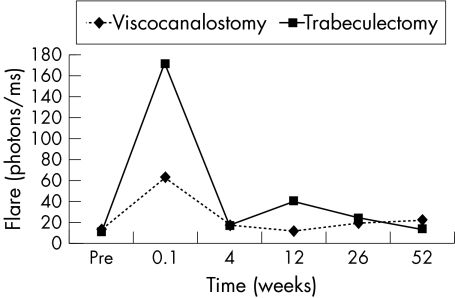
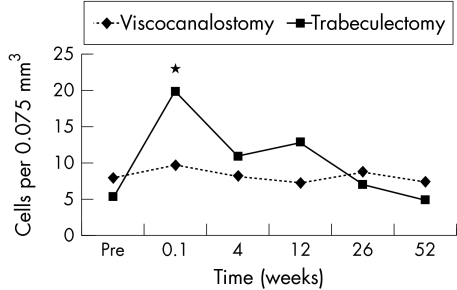
With the exception of laser cell values at 1 week, when eyes after trabeculectomy had higher values than those after viscocanalostomy (p<0.03), the were no differences in flare or cell values between the groups (Figs 5 and 6).
Figure 5.
Mean laser flare values with time for trabeculectomy (n=25) and viscocanalostomy (n=23).
Figure 6.
Mean laser cell values with time for trabeculectomy (n=25) and viscocanalostomy (n=23). *Denotes times where significant differences between the groups were found.
Keratometry and corneal topography
There were no significant changes in keratometry measurements between preoperative values and those at 12 months in either treatment group. Corneal topographic regularity and astigmatic indices35,36 had generally returned to normal values by 6 months.
Complications
Postoperative complications are detailed in Table 3. Early complications such as transient bleb leaks and transient hyphaema were more common after trabeculectomy (p<0.05). The presence or not of cystic areas within the drainage blebs was more common after trabeculectomy (p<0.02), although in neither group was there any occurrence of overhanging and persistent overlarge blebs, with their associated complications of ocular discomfort and Dellen.
Table 3.
Early and late complications after trabeculectomy and viscocanalostomy
| Trabeculectomy | Viscocanalostomy | |
| Early complications | ||
| Transient bleb leak | 5 (20%) | 0 |
| Transient hyphaemia | 7 (28%) | 2 (9%) |
| Transient anterior chamber shallowing | 5 (20%) | 1 (4%) |
| Injected blebs | 3 (12%) | 4 (17%) |
| Encysted blebs | 4 (16%) | 3 (13%) |
| Transient overlarge blebs | 2 (8%) | 3 (13%) |
| IOP spike | 0 | 1 (4%) |
| Peripheral anterior synechiae | 1 (4%) | 1 (4%) |
| Posterior synechiae | 3 (12%) | 0 |
| Late complications | ||
| Cystic area within blebs | 8 (32%) | 1 (4%) |
| Overlarge/overhanging blebs | 0 | 0 |
| Hypotony (IOP <8 mm Hg) | 0 | 0 |
| Late bleb leak | 1 (4%) | 0 |
| Cataract | 5 (20%) | 0 |
Cataract formation, occurring between 4–18 months postoperatively, was more common after trabeculectomy (p<0.05). In the five eyes in which cataract developed, three had shallowing of the anterior chamber, associated with a relatively low intraocular pressure, in the early (first 3 months) postoperative period. All eyes with cataract have undergone successful phacoemulsification cataract surgery with complete return of preoperative logMAR visual acuity and maintenance of successful drainage.
DISCUSSION
The results of this randomised, prospective study strongly suggest that trabeculectomy, augmented with the use of intraoperative antimetabolites in cases at risk of drainage failure, is superior to non-penetrating techniques, such as viscocanalostomy, for the control of IOP in open angle glaucoma. Success rates at 12 months, in terms of maintenance of IOP both below 21 mm Hg and 15 mm Hg, were significantly better with trabeculectomy, and mean IOP at 2, 3, 12, 18, and 24 months postoperatively was significantly lower compared with viscocanalostomy (Fig 2).
Such findings are in contrast with previous studies comparing trabeculectomy with non-penetrating techniques,33,34 where few differences in success rates were found. However, the use of Nd:YAG goniopuncture to augment drainage (converting eyes to a fully penetrating technique), the avoidance of intraoperative antimetabolites (considered standard practice in trabeculectomy in eyes with risk factors for failure),10–17 and the exclusion of eyes with risk factors for drainage failure may have biased the results in these studies.
The mechanism of aqueous drainage in non-penetrating trabecular techniques is uncertain. It has been postulated that drainage may be either subconjunctival, through Schlemm's canal, via increased uveoscleral outflow or by a combination of these pathways.22–24 In our patients with successful drainage at 6 and 12 months following viscocanalostomy, there was evidence of subconjunctival drainage of aqueous, confirmed by the presence of conjunctival epithelial microcysts and drainage blebs (Fig 1). In eyes without successful drainage these changes were not evident. Such findings suggest that with our viscocanalostomy technique, the subconjunctival route is the main drainage pathway. Interestingly, when trabeculectomy was first described it was postulated that aqueous might drain through the cut ends of Schlemm's canal under the scleral flap.2,3 Subsequently, it was recognised that aqueous drained into the subconjunctival space.37
It is important to note that we found no difference in IOP control in eyes undergoing viscocanalostomy where small perforations of Descemet's membrane had possibly occurred, compared to those where it was deemed that Descemet's membrane was intact. This is somewhat confusing if we postulate that subconjunctival drainage is likely to be the main source of aqueous outflow. However, a difference might not be expected, if the increase in fluid egress caused by tiny perforations is minimal compared to the usual diffusion of aqueous through an intact Descemet's window or if microperforations are present in virtually all cases even when the window appears to be completely intact.
At 6 months the results of viscocanalostomy in our patients were encouraging with successful drainage in all but one case. With further follow up the results became disappointing, with success rates of 64% at 12 months and 61% at the last follow up visit (mean 19 months). While such results are poorer than in some previous studies of non-penetrating techniques, such as those of Stegmann,24 Demailly,25 Welsh,28 and Massey,29 they are similar to those of other investigators, such as Sanchez,27 Hamard,31 and Karlen.30 While it is difficult to explain the mechanisms responsible for the deterioration in successful drainage after 6 months, other investigators have documented significant late drainage failure with non-penetrating techniques.18,19,30 The observation of the disappearance of subconjunctival blebs in our patients with drainage failure after viscocanalostomy appears to suggest that subconjunctival fibrosis is responsible. Certainly, late drainage failure has been well documented after trabeculectomy with subconjunctival fibrosis being strongly implicated.38 Stegmann has postulated that failure of drainage after viscocanalostomy might occur because of peripheral anterior synechiae (PAS) forming over the Descemet's window or excessive fibrosis reducing drainage through the window or closing the ostia of Schlemm's canal.24 In our series, however, careful postoperative gonioscopy revealed PAS in only one eye after viscocanalostomy, and eyes showed few signs of intraocular inflammation, with laser cell and flare values at 3–12 months little different from preoperative levels (Figs 5 and 6).
Within the viscocanalostomy group, the use of viscoelastics for intraoperative intracanalicular injection appeared to improve long term drainage. The precise role of viscoelastics in this procedure is unknown. It has been postulated that they are necessary to open and widen Schlemm's canal to allow aqueous to drain through the ostia of the canal and into the normal outflow pathways.24 While our results might support this, and perhaps even suggest that viscoelastic use might have a role in reducing fibrosis and drainage failure, it might be that in addition to widening Schlemm's canal, viscoelastic injection simply improves drainage by actually rupturing the canal into the anterior chamber. Certainly, in one eye in our series, viscoelastic was clearly seen to enter the canal during intracanalicular injection, resulting in a postoperative IOP spike. It is important to note, however, that even with the use of viscoelastics, viscocanalostomy in our study was still inferior to trabeculectomy in terms of IOP control.
With the exception of laser cell values at 1 week, viscocanalostomy did not significantly reduce postoperative intraocular inflammation (Figs 5 and 6), despite its theoretical advantages. These findings are in contrast with those of Chiou et al who found much more prolonged laser flare readings with trabeculectomy compared to deep sclerectomy.39 It is of note, however, that our flare results were very similar to those of Siriwardena et al who did not find prolonged inflammation after trabeculectomy when compared to phacoemulsification cataract extraction.40
When designing this study, a great deal of thought was given to the use of intraoperative antimetabolites. They are routinely used as an adjunct for trabeculectomy in eyes with risk factors for failure10–14 and were therefore used according to a standardised protocol for eyes within the study undergoing trabeculectomy. In previous investigations of non-penetrating techniques, intraoperative antimetabolites have generally not been used because of the supposition that these techniques reduce the need for antimetabolites and might not entirely rely on subconjunctival drainage. Therefore we decided not to use them in eyes undergoing viscocanalostomy. However, evidence of subconjunctival blebs in our patients with successful drainage after viscocanalostomy suggests a possible role for antimetabolites in maintaining long term drainage in these procedures. This merits further investigation and is the subject of an ongoing study. Interestingly, no differences in long term IOP control in the viscocanalostomy group, were found between eyes with low “risk factors for failure” scores (nine or less) and high “risk factors for failure” scores (10 or more).
Despite the potential advantages of viscocanalostomy, patients did not appear to benefit greatly from its less invasive nature. Visual recovery was similar between the two operative groups (Fig 4). Viscocanalostomy took longer to perform and required the same degree of postoperative interventions. It did not therefore appear to offer any economic advantages in terms of reduced postoperative follow up requirements. Early complications such as hyphaema and bleb leaks were less common after viscocanalostomy, but such problems were transient and not sight threatening. The only notable advantage of viscocanalostomy over trabeculectomy was the reduced incidence of postoperative cataract formation, which necessitated cataract extraction in five eyes after trabeculectomy. Importantly, in all these cases, following cataract surgery there was complete return of preoperative logMAR visual acuity and maintenance of successful drainage.
Although viscocanalostomy offers some theoretical advantages, in terms of its less invasive nature and possible reduced reliance on subconjunctival drainage, intraocular pressure control appears to be far superior with trabeculectomy and it continues to be the filtering procedure of choice for the management of glaucoma. Viscocanalostomy is generally associated with fewer postoperative complications, especially cataract formation, but significant problems permanently impairing vision did not occur with either technique. The possible role of intraoperative antimetabolites to perhaps improve and maintain the encouraging results seen in the early postoperative phase after viscocanalostomy merits further investigation.
Acknowledgments
The authors would like to thank Mr W Meacock, FRCOphth, and Mr L Bender, FRCOphth, for their assistance with this study.
REFERENCES
- 1.Sugar HS. Experimental trabeculectomy in glaucoma. Am J Ophthalmol 1961;51:623. [Google Scholar]
- 2.Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 1968;66:673–9. [PubMed] [Google Scholar]
- 3.Cairns JE. Surgical treatment of primary open-angle glaucoma. Trans Ophthalmol Soc UK 1972;92:745–56. [PubMed] [Google Scholar]
- 4.Watson PG. Surgery of the glaucomas. Br J Ophthalmol 1972;56:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson PG, Barrett F. Effectiveness of trabeculectomy in glaucoma. Am J Ophthalmol 1975;79:831–45. [DOI] [PubMed] [Google Scholar]
- 6.Wilson P. Trabeculectomy: long-term follow-up. Br J Ophthalmol 1977;61:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields MB. Trabeculectomy versus full-thickness filtering operations for control of glaucoma. Ophthalmic Surg 1980;11:498–505. [PubMed] [Google Scholar]
- 8.Zaidi AA. Trabeculectomy: a review and 4-year follow-up. Br J Ophthalmol 1980;64:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills KB. Trabeculectomy: a retrospective long-term follow-up of 444 cases. Br J Ophthalmol 1981;65:790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egbert PR, Williams AS, Singh K, et al. A prospective trial of intraoperative fluorouracil during trabeculectomy in a black population. Am J Ophthalmol 1993;116:612–6. [DOI] [PubMed] [Google Scholar]
- 11.Gressel MG, Heuer DK, Parrish RK. Trabeculectomy in young patients. Ophthalmology 1984;91:1242–6. [DOI] [PubMed] [Google Scholar]
- 12.Broadway DC, Grierson I, Hitchings RA. Local effects of previous conjunctival incisional surgery and the subsequent outcome of filtration surgery. Am J Ophthalmol 1998;125:805–8. [DOI] [PubMed] [Google Scholar]
- 13.Gross RL, Feldman RM, Spaeth GL, et al. Surgical therapy of chronic glaucoma in aphakia and pseusdophakia. Ophthalmology 1988;95:1195–201. [DOI] [PubMed] [Google Scholar]
- 14.Allen RC, Bellows AR, Hutchinson BR, et al. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology 1982;89:1181–7. [DOI] [PubMed] [Google Scholar]
- 15.The Fluorouracil Filtering Surgery Study Group. Fluorouracil filtering surgery study one year follow up. Am J Ophthalmol 1989;108:625–35. [DOI] [PubMed] [Google Scholar]
- 16.Chen C-W, Huang H-T, Bair JS, et al. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J Ocul Pharmacol 1990;6:175–82. [DOI] [PubMed] [Google Scholar]
- 17.Khaw PT. Antimetabolites in glaucoma filtration surgery. Current medical literature, the Royal Society of Medicine 1996;6:71–7. [Google Scholar]
- 18.Epstein E. Fibrosing response to aqueous. Its relation to glaucoma. Br J Ophthalmol 1959;43:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasnov MM. Sinusotomy: foundations, results, prospects. Trans Am Ophthalmol Otolaryngol 1972;76:369–74. [PubMed] [Google Scholar]
- 20.Zimmerman TJ, Kooner KS, Ford VJ, et al. Effectiveness of non-penetrating trabeculectomy in aphakic patients with glaucoma. Ophthalmic Surg 1984;15:44–50. [PubMed] [Google Scholar]
- 21.Zimmerman TJ, Kooner KS, Ford VJ, et al. Trabeculectomy vs non-penetrating trabeculectomy: a retrospective study of two procedures in phakic patients with glaucoma. Ophthalmic Surg 1984;15:734–40. [PubMed] [Google Scholar]
- 22.Fyodorov SN, Kozlov VI, Timoshkina NT, et al. Non-penetrating deep sclerectomy in open angle glaucoma. IRTC Eye Microsurgery. Moscow: RSFSR Ministry of Public Health, 1989:52–5.
- 23.Kozlov VI, Bagrov SN, Anisimova SY, et al. Non-penetrating deep sclerectomy with collagen. IRTC Eye Microsurgery. Moscow: RSFSR Ministry of Public Health, 1989:44–6.
- 24.Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999;25:316–22. [DOI] [PubMed] [Google Scholar]
- 25.Demailly P, Jeanteur-Lunel MN, Berkani M, et al. La sclerectomie profonde non perforante associee a la pose d'un implant de collagene dans le glaucoma primitif a angle ouvert. J Fr Ophtalmol 1996;19:659–66. [PubMed] [Google Scholar]
- 26.Demailly P, Lavat P, Kretz G, et al. Non-penetrating deep sclerectomy (NPDS) with or without collagen device (CD) in primary open-angle glaucoma: middle-term retrospective study. Int Ophthalmol 1997;20:131–40. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez E, Schnyder CC, Sickenberg M, et al. Deep sclerectomy: results with and without collagen implant. Int Ophthalmol 1997;20:157–62. [DOI] [PubMed] [Google Scholar]
- 28.Welsh NH, DeLange J, Wasserman P, et al. The “de-roofing” of Schlemm's canal in pateints with open angle glaucoma through placement of a collagen drainage device. Ophthalmic Surg Lasers 1998; 29:216–26. [PubMed] [Google Scholar]
- 29.Massey J, Gruber D, Muraine M, et al. La sclerectomie profonde non perforante dans le traitement chirurgical du glaucome chrinique a angle ouvert. Resultats a moyen terme. J Fr Ophtalmol 1999;22:292–8. [PubMed] [Google Scholar]
- 30.Karlen ME, Sanchez E Schnyder CC, et al. Deep sclerectomy with collagen implant: medium term results. Br J Ophthalmol 1999;83:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamard P, Plaza L, Kopel J, et al. Sclerectomie profonde non perforante (SPNP) et glaucome a angle ouvert. Resultants a moyen terme des premiers patients operes. J Fr Ophtalmol 1999;22:25–31. [PubMed] [Google Scholar]
- 32.Carassa RG, Bettin P, Fiori M, et al. Viscocanalostomy: a pilot study. Eur J Ophthalmol 1998;8:57–61. [DOI] [PubMed] [Google Scholar]
- 33.Mermoud, Schnyder CC, Sickenberg M, et al. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open angle glaucoma. J Cataract Refract Surg 1999;25:323–31. [DOI] [PubMed] [Google Scholar]
- 34.El Sayyad F, Helal M, El-Kholify H, et al. Non-penetrating deep sclerectomy versus trabeculectomy in bilateral primary open angle glaucoma. Ophthalmology 2000;107:1671–74. [DOI] [PubMed] [Google Scholar]
- 35.Dingeldein SA, Klyce SD, Wilson SE. Quantitative descriptors of corneal shape derived from computer assisted analysis of photokeratographs. Refract Corneal Surg 1989;5:372–8. [PubMed] [Google Scholar]
- 36.Wilson SE, Klyce SD. Quantitative descriptors of corneal topography. A prospective clinical study. Arch Ophthalmol 1991;109:349–53. [DOI] [PubMed] [Google Scholar]
- 37.Skuta GL, Parrish RK. Wound healing in glaucoma after filtering surgery. Surv Ophthalmol 1987;32:149–70. [DOI] [PubMed] [Google Scholar]
- 38.Hitchings RA, Grierson I. Clino pathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc UK 1983;103:84–8. [PubMed] [Google Scholar]
- 39.Chiou AG, Mermoud A, Jewelewicz DA. Post-operative inflammation following deep sclerectomy with collagen implant versus standard trabeculectomy. Graefes Arch Clin Exp Ophthalmol 1998;236:593–6. [DOI] [PubMed] [Google Scholar]
- 40.Siriwardena D, Kotecha A, Minassian D, et al. Anterior chamber flare after trabeculectomy and after phacoemulsification. Br J Ophthalmol 2000;84:1056–7. [DOI] [PMC free article] [PubMed] [Google Scholar]