Abstract
Background: Glaucomatous neuropathy is a type of cell death by apoptosis. The p53 gene is one of the regulatory genes of apoptosis. Recently, p53 codon 72 polymorphism has been extensively studied to determine the risk factors responsible for many diseases. In the p53 gene, a single base change from G to C causes the alternation of amino acid residue 72 from arginine to proline. In this study the association between p53 codon 72 polymorphism and primary open angle glaucoma (POAG) patients was evaluated.
Methods: 58 POAG patients and 59 healthy volunteers were enrolled in this study. Polymerase chain reaction based analysis was used to resolve the p53 codon 72 polymorphism.
Results: There were significant differences in the distribution of the polymorphism between the control subjects and the POAG patients (p = 0.00782) The proline form of p53 gene codon 72 appears to be a significant risk factor in the development of POAG (odds ratio 2.389, 95% confidence interval: 1.14 to 5.01).
Conclusions: Retinal ganglion cells die during POAG by apoptosis. The tumour suppressor protein, p53, is one of the primary regulators steps of apoptosis, and the results of our study are compatible with this concept.
Keywords: p53 codon, polymorphism, primary open angle glaucoma
Glaucoma is a complex disease. Both mechanical and vascular hypotheses have been proposed as the mechanism. Regardless of the mechanism, the ganglion cells ultimately die, which represents the final common pathway of glaucomatous vision loss.1,2 The major risk factors for open angle glaucoma include the level of intraocular pressure (IOP), ethnicity, some aspects of vascular function, high refractive errors, and a family history of glaucoma.3 Those with a first degree relative with glaucoma have up to four times the risk of developing the disease. At least one gene, located on chromosome 1q, is known to be associated with a form of open angle glaucoma.4 It is likely that several candidate genes influencing glaucoma will be detected. Nevertheless, there are many people with IOP above the statistically normal range who suffer no clinical damage in their lifetimes. Others have glaucoma within the normal IOP range. Hence, IOP level is not used in the definition of glaucoma. IOP is simply a risk factor for glaucoma and is not the only mechanism causing ganglion cell loss in glaucoma. Rather, elevated IOP damages ganglion cells that have a genetic susceptibility to this form of stress.5,6
Several studies demonstrated that retinal ganglion cells die during development and as a result of a variety of optic nerve diseases by a form of cell death known as apoptosis.3–6 Apoptosis is a genetically controlled form of cell death. This mechanism of cell death is controlled by specific genes and their products that are activated by the dying cell.7–11 One of the primary regulatory steps of apoptosis is the activation of the tumour suppressor protein, p53. p53 is one of the major guardians of the genome, and can arrest the cell cycle in response to DNA damage or direct the damaged cell to an apoptotic pathway. Cell cycle arrest can be achieved either by a non-transcriptional route or more commonly by the transcriptional activation of p53.8–10 p53 functions as a transcription factor that can upregulate the expression of the pro-apoptotic gene bax and downregulate the expression of the antiapoptotic gene bcl-2.8–10 Changes in the concentrations of these gene products can stimulate apoptotic events, including changes in the mitochondria, and ultimately lead to the activation of cysteine proteases (caspases) which digest the dying cell from within. To date, the mechanism of ganglion cell apoptosis is not well understood, but the genomic instability of p53 in other areas has provided a blueprint for the study of glaucomatous neuropathy in humans.7–13
Many cancers are related to the abnormal p53 presentation including bladder cancer,11 prostate cancer,12 lung cancer,14 lymphoma,16 cervical carcinoma,9 hepatoma,9 and nasopharyngeal carcinoma.15 The tumour suppressor gene p53 controls the expression of two key genes, bc1-2 and bax, and the products of the two genes act as antagonists.16 The functions of p53 include the upregulation of proapoptotic gene bax expression and the downregulation of the antiapoptotic gene bc1-2 expression.17,18 The change in expression of these genes can evoke changes in mitochondria and ultimately activate the family of proteases called caspases which induce the death of cells.
All structural features of p53 (residues 61–94) have been well resolved except residue 72, which has been recognised as an arginine (Arg) to proline (Pro) polymorphism. The single base change from CGC to CCC caused the alteration of amino acid residue 72 from Arg to Pro.19 Some findings indicate that the p53 codon 72 polymorphism has been associated with many tumour formations in Chinese patients.10,20–22
The p53 gene is suspected of having a role in glaucomatous neuropathy. To resolve this issue, we aimed to investigate the role of p53 codon 72 polymorphism in Chinese patients with primary open angle glaucoma.
MATERIALS AND METHODS
We collected POAG patients from the department of ophthalmology at the China Medical College Hospital from May 2000 to July 2000. All patients in this study received serial ophthalmic examinations included IOP, visual acuity, automated perimetry, gonioscopy, optic disc examination, and retinal examination. The volunteers in the control group were examined by the same ophthalmologist. If there were any doubts about glaucoma the patients would be excluded from the study. Patients with ocular diseases other than POAG were also excluded from our study. The patients included in this study were POAG patients who fulfilled one of following criteria from both the visual field and the optic nerve categories.
Visual field criteria
At least two abnormal visual field tests by Humphrey automated perimetry, as defined by computer based objective criteria
The presence of one or more absolute defects in the central visual field 30°, with ophthalmological interpretation as glaucomatous visual field loss.
Optic disc criteria (optic disc damage present in fundus photographs)
Either a horizontal or vertical cup to disc ratio 0.6 or more
Narrowest remaining neuroretinal rim was 20% or fewer disc diameters.
Ophthalmological criteria
Patients with other possible causes for disc and field changes other than POAG were excluded.
In our study, we investigated the p53 gene codon 72 polymorphism in all subjects. The prevalence of the polymorphism was compared between the control group and patient groups. Odds ratio was used to calculate the frequencies of the different alleles.
This study was carried out with approval from the human study committee of the China Medical College Hospital. Informed consent was obtained from all patients who participated in this study. The genomic DNA was prepared from peripheral blood by use of a DNA Extractor WB kit (Wako, Japan). Polymerase chain reactions (PCRs) were carried out in a total volume of 25 μl, containing genomic DNA; 2–6 pmol of each primer; IX Taq polymerase buffer (1.5 mM MgCl2); and 0.25 units of AmpliTaq DNA polymerase (Perkin Elmer). The primer Pro 72 was designed for p53 codon 72 in proline form and Arg 72 for arginine form, according to the procedure described by Storey et al.16 PCR amplification was performed in a programmable thermal cycler gene Amp PCR System 2400 (Perkin Elmer). Cycling conditions for Pro 72 were set as follows: one cycle at 94°C for 5 minutes, 35 cycles at 94°C for 15 seconds, 52°C for 20 seconds, and 72°C for 30 seconds. One final cycle of extension at 92°C for 7 minutes; conditions for Arg 72 were the same as for Pro 72, except 50°C for annealing.
The PCR products from Arg 72 and Pro 72 from the same individual were mixed together and 10 μl of this solution was loaded into 3% agarose gel containing ethidium bromide for electrophoresis. The molecular analyses of patients and controls were performed in the same laboratory at the same time and the gels were inspected by investigators who were masked to the clinical phenotype of the individuals being studied.
Statistical analysis for the relative risk of p53 codon 72 in the control group and POAG groups were compared using the χ2 test. Results were considered statistically significant when the probability of findings occurring by chance was less than 5% (p <0.05). The frequencies of different alleles were rechecked by means and odds ratios with 95% confidence intervals (CI).
RESULTS
Fifty nine healthy volunteers and 58 patients with POAG were enrolled in this study. The POAG patients ranged in age from 20 to 70 years old (mean 55 years old) and were unrelated. There were 29 females and 29 males. The volunteers ranged in age from 52 to 71 years old (mean age 50 years) and were free from any ophthalmic diseases. The volunteers were all also Chinese and unrelated. There were 30 females and 29 males. All patients were followed up for 2–8 years, with an average period of follow up being 5 years. Ten of the patients received trabeculectomy; two of the 10 patients received trabeculectomy twice at different sites. Fifty patients in the POAG group controlled IOP by topical drugs. Each patient used an average of 1.3 types of antiglaucomatous drug. Nine patients did not require drugs to control IOP following trabeculectomy.
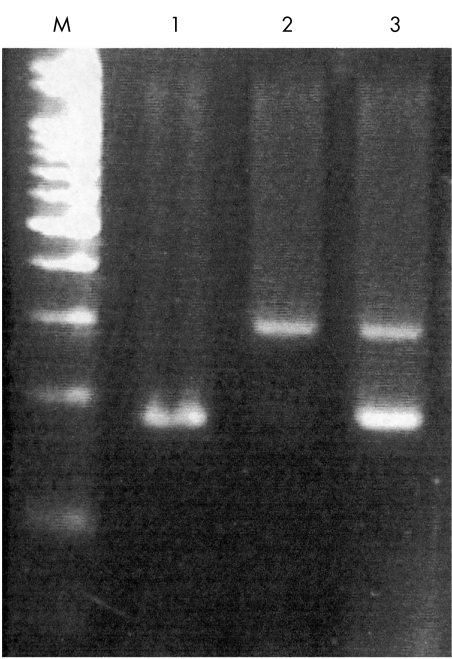
The PCR products of Arg and Pro were 141 and 177 bp, respectively. Those bands revealing Pro or Arg homozygotes and heterozygotes are shown in Figure 1. The frequencies of the genotypes in the control and POAG groups are shown in Table 1. Using the χ2 test, we compared the distribution of p53 codon 72 polymorphism. There were significant differences between the control group and the POAG patients (p = 0.00782). The genotype distribution in the control group revealed 42.3% Arg homozygote, 44% heterozygote, and 13.6% Pro homozygote. The distribution in the POAG group revealed 20.6% Arg homozygote, 44.8% heterozygote, and 34.4% Pro homozygote. The allelic frequencies in the control group were 0.64 Arg and 0.36 Pro. The allelic frequencies in the POAG group were 0.43 Arg and 0.57 Pro (Table 2). The odds ratio of Pro was 2.389 (95% confidence interval: 1.14 to 5.01) in comparison with the Arg and Pro alleles in the POAG group. The frequency of the Pro allele was significantly higher in the POAG group. In our study, the Pro form of p53 codon 72 increases the risk of developing POAG in Chinese people.
Figure 1.
p53 codon 72 genotype panel shows on a 3% agarose gel with ethidium bromide after PCR amplification. M: marker, DNA ladder 100 bp, lane 1: Arg-Arg homozygote, lane 2: Pro-Pro homozygote, and lane 3: Arg-Pro heterozygote. The Arg allele: 141 bp and the Pro allele: 177 bp.
Table 1.
The distribution of p53 codon 72 polymorphism in healthy control subjects and POAG patients
| Arg-Arg | Arg-Pro | Pro-Pro | Total | |
| POAG | 12 (20.7%) | 26 (44.8%) | 20 (34.5%) | 58 (100%) |
| Control | 25 (42.3%) | 26 (44.0%) | 8 (13.56%) | 59 (100%) |
| Total | 37 (31.6%) | 52 (44.4%) | 28 (23.9%) | 117 |
χ2 test: p=0.00782.
Table 2.
The allelic frequencies in healthy subjects and POAG patients
| Arg | Pro | |
| POAG | 0.43 | 0.57 |
| Control group | 0.64 | 0.36 |
Odds ratio of Pro form is 2.38857 of POAG group (95% CI: 1.14 to 5.01).
We also calculated “power” for a test of the null hypothesis by spss. There is a power of 74% to yield a statistically significant result in this sample size. Odds ratio for per copy of the “Pro” allele showing 2.1 (95% confidence interval: 1.2 to 3.4) before being adjusted for age and 2.0 (95% confidence interval: 1.2 to 3.4) after adjustment for age (Table 3) This meant that age did not influence the result.
Table 3.
Age adjusted tests for the genotypes of p53 codon 72 gene polymorphism
| Odds ratio (95% CI)† | ||
| Genotype | Unadjusted | Age adjusted |
| Arg-Arg | 1 | 1 |
| Arg-Pro | 2.3 (0.9 to 5.5) | 2.2 (0.9 to 5.5) |
| Pro-Pro | 4.1*(1.5 to 11.6) | 4.1*(1.4 to 11.6) |
| Per copy of the Pro allele | 2.1*(1.2 to 3.4) | 2.0*(1.2 to 3.4) |
†CI denotes confidence interval. *p<0.05.
DISCUSSION
The pathophysiology of POAG is not precisely known. POAG is a multifactorial disease,23,24 and the assumption that a single gene is involved is not reasonable. POAG may be the result of multiple and interactive genetic and environmental effects. There is a well recognised increased risk of developing the disease in family members of patients with POAG. Currently, there is a lack of information regarding the genetics of the disease and, hence, the molecular biology of glaucoma is currently under close investigation.
Studies of ganglion cell death in animals and human models demonstrated many similarities with the morphological changes seen in apoptosis, such as chromatin condensation, formation of apoptotic bodies, and DNA fragmentation.6,7 POAG is death of optic nerves and the death of glaucomatous optic nerves has been proved to be way of apoptosis.3–6 The p53 gene is located on the short arm of chromosome 17 and its protein product is related to the regulation of the cell cycle. The functional p53 can either arrest cell cycle progression in the late G1 phase,16 thus allowing the DNA to be repaired before its replication, or induce apoptosis, leading to cell death. Genes that are turned on by p53 include p21, bax, and bcl-2.23,24 p53 participates in arresting the cell cycle at G1 or G2. Our study has shown that the distribution of the p53 gene codon 72 polymorphism in Chinese POAG patients and the healthy control group is significantly different (p = 0.00782) (Table 1), a result compatible with the concept of the relation between apoptosis and neuropathy.
We have also shown that the Pro form allele was prevalent in high frequencies in Chinese POAG patients (odds ratio: 2.389, 95% CI: 1.14 to 5.01) (Table 2). Some reports have revealed that the Pro allele homozygote is a risk factor of lung and hepatocellular carcinoma.17 Chen et al noted a significant association between Pro homozygotes and invasive bladder cancer in Chinese people.24 Furthermore, researchers have found that lung carcinoma patients with either p53 Arg or Pro homozygotes had worse prognoses when compared with patients with the heterozygous form.20 This diversity may be due to racial variation. The studies mentioned above might suggest that the dominant p53 Pro form is a risk factor for disease in the Chinese population. Storey et al reported that when p53 was degraded by HPV (human papilloma virus), the Pro form was seven times longer than the Arg form.16 Therefore, the hypothesis is that the Arg form of p53 in residue 72 may be responsible for the less potent effects when the cell is destined to replicate. The results of our study indicate that the Pro form allele is a significant risk factor for POAG. We suspect that the Pro form of p53 gene codon 72 induces the instability of ocular ganglion cells, and the Pro form allele fails to protect ganglion cell from apoptosis. The Arg form allele was noted to be related to cancer. The exact effect of Pro on POAG may be resolved by “proteomics” in the future.
However, we do not intend to assert that Pro is a direct cause of POAG but there is an association. Single nucleotide polymorphisms (SNPs) have important implications in human genetic studies. The screening for such alleles helps the detection of a genetic predisposition to disease. The presence of a specific SNP allele can be implicated as a causative factor of a genetic disorder. Besides, understanding the associated polymorphism is expected to increase the understanding of the course of disease. Our studies are ongoing in order to determine the relation between SNPs and glaucoma. Knowing the genetic pathway of apoptosis is important for designing new treatments for glaucoma.
Video Reports (www.bjophthalmol.com).
Capsule staining and mature cataracts: a comparison of indocyanine green and trypan blue dyes. D F Chang
Pearls for implanting the Staar toric IOL. D F Chang
An intraocular steroid delivery system for cataract surgery. D F Chang
Evaluation of leucocyte dynamics in mouse retinal circulation with scanning laser ophthalmoscopy. Heping Xu, A Manivannan, Garry Daniels, Janet Liversidge, Peter F Sharp, John V Forrester, Isabel J Crane
Dipetalonema reconditum in the human eye. T Huynh, J Thean, R Maini
Surgical revision of leaking filtering blebs with an autologous conjunctival graft. K Taherian, A Azuara-Blanco
REFERENCES
- 1.Garcia-Valenzuela E, Gorczyca W, Darzynkiewicz Z, et al. Apoptosis in adult retinal ganglion cells after axotomy. J Neurobiol 1994;25:431–8. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Valenzuela E, Shareef S, Walsh J, et al. Programmed cell death of retinal ganglion cells during experimental glucoma. Exp Eye Res 1995;61:33–44. [DOI] [PubMed] [Google Scholar]
- 3.Sommer A, Jielsch JM, Quiqley HA, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 1991;109:1090–5. [DOI] [PubMed] [Google Scholar]
- 4.Stone EM, Fingert JH, Alward WLM, et al. Identification of a gene that causes primary open angle glaucoma. Science 1997;275:668–70. [DOI] [PubMed] [Google Scholar]
- 5.Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol 1999;43:S151–61. [DOI] [PubMed] [Google Scholar]
- 6.Stuart JM. Glaucoma, apoptosis, and neuroprotection. Curr Opin Ophthalmol 1997;8:28–37. [DOI] [PubMed] [Google Scholar]
- 7.Nickells RW. Retinal ganglion cell death in glaucoma: the how, the why, and the maybe. J Glaucoma 1996;5:345–56. [PubMed] [Google Scholar]
- 8.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997;88:323–31. [DOI] [PubMed] [Google Scholar]
- 9.Zehbe I, Voglino G, Wilander E, et al. Codon 72 polymorphism of p53 and its association with cervical cancer. Lancet 1999;354:218–19. [DOI] [PubMed] [Google Scholar]
- 10.Kupryjanczyk J, Bell DA, Yandell DW, et al. p53 expression in overian borderline tumors and stage I carcinomas. Am J Clin Pathol 1994;102:671–6. [DOI] [PubMed] [Google Scholar]
- 11.Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 1994;331:1259–64. [DOI] [PubMed] [Google Scholar]
- 12.Steiner MS, Zhang X, Wang Y, et al. Growth inhibition of prostate cancer by an adenovirus expressing a novel tumor suppressor gene, pHyde. Cancer Res 2000;60:4419–25. [PubMed] [Google Scholar]
- 13.Wang NM, Tsai CH, Yeh KT, et al. p53 codon 72 arg polymorphism is not a risk factor for carcinogenesis in Chinese. Int J Mol Med 1999;4:249–52. [PubMed] [Google Scholar]
- 14.Wang YC, Lee HS, Chen SK, et al. Prognostic significance of p53 codon 72 polymorphism in lung carcinomas. Eur J Cancer 1999;35:226–30. [DOI] [PubMed] [Google Scholar]
- 15.Crook T, Nicholls JM, Brooks L, et al. High level expression of deltaN-p63: mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC)? Oncogene 2000;19:3439–44. [DOI] [PubMed] [Google Scholar]
- 16.Nork TM, Poulsen G, Millecchia LL, et al. p53 regulates apoptosis in human retinoblastoma. Arch Ophthalmol 1997;115:213–19. [DOI] [PubMed] [Google Scholar]
- 17.Yu MW, Wang SY, Chiu YH, et al. A p53 genetic polymorphism as a modulator of hepatocellular carcinoma in relation to chronic liver disease, familial tendency, and cigarette smoking in hepatitis B carriers. Hepatology 1999;29:697–702. [DOI] [PubMed] [Google Scholar]
- 18.Storey A, Thomas M, Kalita A, et al. Role of a p53 polymorphism in the development of human papilloma virus-associated cancer. Nature 1998;393:229–34. [DOI] [PubMed] [Google Scholar]
- 19.Ara S, Lee PSY, Hansen MF, et al. Codon 72 polymorphism of the TP53 gene. Nucleic Acids Res 1990;18:4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YC, Chen CY, Chen SK, et al. p53 codon 72 polymorphism in Taiwanese lung cancer patients: association with lung cancer susceptibility and prognosis. Clinical Cancer Res 1999;5:129–34. [PubMed] [Google Scholar]
- 21.Wang YC, Lee HS, Chen SK, et al. Prognostic significance of p53 codon 72 polymorphism in lung carcinomas. Eur J Cancer 1999;35:226–30. [DOI] [PubMed] [Google Scholar]
- 22.Chen WC, Tsai FJ, Wu JY, et al. Distribution of p53 codon 72 polymorphism in bladder cancer-proline form is prominent in invasive tumor. Urol Res 2000;28:239–6. [DOI] [PubMed] [Google Scholar]
- 23.Drance SM, Schulzer M, Thomas B, et al. Multivariate analysis in glaucoma: use of discriminant analysis in predicting glaucomatous visual field damage. Arch Ophthalmol 1981;6:1019–22. [DOI] [PubMed] [Google Scholar]
- 24.Leske MC, Connell AMS, Wu S, et al. Risk factors for open-angle glaucoma. Arch Ophthalmol 1995;113:918–24 [DOI] [PubMed] [Google Scholar]
- 25.E1-Deiry WS, Kern SE, Pietenpol JA, et al. Definition of a consensus binding site for p53. Nat Genet 1992;1:45–9. [DOI] [PubMed] [Google Scholar]
- 26.Harper JW, Adami GR, Wei N, et al. The p21 Cdk-interacting protein Cip 1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993;75:805–16. [DOI] [PubMed] [Google Scholar]